Abstract
Persistent infection with hepatitis B virus (HBV) is a leading cause of human liver disease and is strongly associated with hepatocellular carcinoma, one of the most prevalent forms of human cancer. Apoptosis (programmed cell death) is an important mediator of chronic liver disease caused by HBV infection. It is demonstrated that the HBV HBx protein acutely sensitizes cells to apoptotic killing when expressed during viral replication in cultured cells and in transfected cells independently of other HBV genes. Cells that were resistant to apoptotic killing by high doses of tumor necrosis factor α (TNFα), a cytokine associated with liver damage during HBV infection, were made sensitive to very low doses of TNFα by HBx. HBx induced apoptosis by prolonged stimulation of N-Myc and the stress-mediated mitogen-activated-protein kinase kinase 1 (MEKK1) pathway but not by up-regulating TNF receptors. Cell killing was blocked by inhibiting HBx stimulation of N-Myc or mitogen-activated-protein kinase kinase 1 using dominant-interfering forms or by retargeting HBx from the cytoplasm to the nucleus, which prevents HBx activation of cytoplasmic signal transduction cascades. Treatment of cells with a mitogenic growth factor produced by many virus-induced tumors impaired induction of apoptosis by HBx and TNFα. These results indicate that HBx might be involved in HBV pathogenesis (liver disease) during virus infection and that enhanced apoptotic killing by HBx and TNFα might select for neoplastic hepatocytes that survive by synthesizing mitogenic growth factors.
Hepatitis B virus (HBV) and woodchuck hepatitis B virus (WHV, a model for human HBV infection) are small hepatotropic para-retroviruses (hepadnaviruses) that replicate by reverse transcription and strongly promote liver disease and development of hepatocellular carcinoma (1, 2). Pathogenesis and carcinogenesis by HBV and WHV are poorly understood processes that occur over years of chronic infection and are associated with a process of hepatocyte death and liver regeneration. Death of liver cells is facilitated by the inflammatory response, including elaboration of tumor necrosis factor α (TNFα) (1, 3). Regeneration of liver involves production of mitogenic growth factors, often from preneoplastic cells as disease ensues (4). There is some evidence that the HBx protein of HBV might play an indirect role in liver disease and development of carcinoma during virus infection (5). The HBx protein is highly conserved in mammalian hepadnaviruses and is essential for virus infection in mammals (6, 7). HBx weakly promotes tumorigenesis in certain transgenic mouse lineages (8) and deregulates cell cycle checkpoint controls (9). HBx is therefore an essential viral protein that might be a cofactor in development of hepatocellular carcinoma during chronic hepadnavirus infection.
HBx is a multifunctional protein with a number of reported activities that might participate in viral pathogenesis and carcinogenesis. Several activities of HBx that are relevant to disease include activation of the Ras-Raf-mitogen-activated protein (MAP) kinase and protein kinase C signal transduction pathways (10–13), prolonged stimulation of the MEKK1/JNK stress-mediated signaling pathway (14–16), stimulation of transcription by RNA polymerases II and III (17–19), and binding and activation of several transcription factors (20–23). Additionally, HBx stimulates quiescent cells in culture to cycle by deregulating cell cycle control checkpoints (9), binds in vitro to p53 protein (24) and a DNA repair factor (25), and can activate endogenous c-Myc or N-Myc proto-oncogenes (26–27). There are therefore a number of reported HBx activities that might be expected to promote apoptotic destruction of liver tissue during infection. In particular, inappropriate deregulation of cell cycle control checkpoints, prolonged stimulation of the MEKK1 pathway, induction of Myc genes, and enhanced DNA repair abnormalities could all play a role in viral pathogenesis (28–31). The role of HBx in induction of apoptosis was therefore investigated to determine whether it is a likely cofactor in pathogenic liver disease during HBV infection.
MATERIALS AND METHODS
Transfection of Cells and Quantitation of Killing.
Chang cells were cotransfected by calcium phosphate precipitation for 20 h with 10 μg of HBx, HBxo, HBx-SLN, or HBx-NLS expression plasmid DNAs per 5 × 105 cells (≈20% confluency). Some experiments were performed with or without an equal amount of the E1B-19k protein or dominant-interfering plasmid DNA for MEKK-1 or Myc with or without treatment of cells 20 h posttransfection for 16 h with 1.0 ng/ml TNFα. Cells were grown in DMEM containing 10% (vol/vol) calf serum. Transfection of infectious pregenomic cDNAs of WHV were carried out using Chang cells and a wild-type WHV pregenomic cDNA controlled by the CMV promoter (pWHV) or a mutant that expresses nonfunctional, C-terminal, truncated HBx (pCWHV) (7). Cells were transfected, maintained in DMEM plus 10% calf serum, and passaged when confluent. The amount of cell killing was determined by counting the fraction of cells stained with trypan blue or by Hoechst-stained condensed nuclei. Results represent the average from four independent experiments from which SE were calculated.
Estimate of HBx Protein Number.
Chang cells (40–50%) expressed HBx from transfected pCMV-X based on in situ immunofluorescence staining of fixed cells with anti-HBx-Flag antibodies (IBI) as described (12) and are shown in Fig. 1D. To estimate the number of HBx molecules per cell, 5 × 106 cells were labeled for 5 h with 400 μCi (1 Ci = 37 GBq) of [35S]-methionine to 4 × 105 cpm/μg protein. HBx was cleared from 130 × 106 cpm by immunoprecipitation with anti-Flag antibodies and resolved by SDS/gel electrophoresis, and the HBx band was located by phosphorimaging, excised, and quantitated by liquid scintillation counting at 80% efficiency. The number of HBx molecules per cell was calculated from the cpm per band: (≈200) ÷ mean protein specific activity [HBx contains 2% methionine, close to the 1.7% average] ÷ μg weight of HBx [2.8] 10−14] ÷ number of HBx-expressing cells [2 × 106]. If one to three methionines of HBx are labeled, a transfected Chang cell was calculated to contain 8000–24,000 HBx proteins.
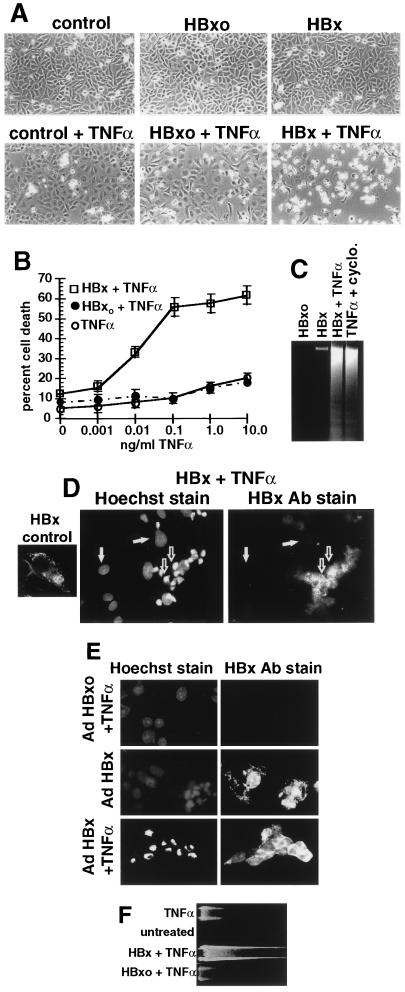
Figure 1.
Induction of apoptosis by expression of HBV HBx protein in TNFα-treated cells. (A) Phase-contrast microscopic examination of apoptosis induced by transfected HBx plus TNFα treatment. Chang cells were photographed at 36 h posttransfection (16 h post-TNFα) by phase-contrast microscopy (magnification, ×100). (B) Titration of TNFα sensitization. Chang cells were transfected for 20 h with HBx plasmids and treated for 16 h with TNFα, and cell death was quantitated by trypan blue exclusion. Results are the mean of four independent experiments. (C) Apoptotic DNA fragmentation in HBx-expressing Chang cells treated with TNFα. Cells were transfected with plasmids as above with or without 1.0 ng/ml TNFα and with or without 5 μg/ml cycloheximide for 16 h, and DNA fragmentation was determined. (D) Immunofluorescence photomicrograph (magnification, ×600) of HBxFlag (14) transfected plus TNFα-treated Chang cells costained with Hoechst 33258 and fluorescein isothiocyanate-conjugated M2 anti-Flag antibodies to visualize HBx. HBx control was not treated with TNFα. Apoptotic cells with condensed nuclei are indicated by open arrows; normal cells are indicated by filled arrows. (E) Immunofluorescence photomicrograph (magnification, ×600) of Ad-HBx- and Ad-HBxo-infected HepG2 cells. HepG2 cells were treated with TNFα and then stained with Hoechst 33258 and anti-Flag antibodies. (F) Apoptotic DNA fragmentation in HBx-expressing HepG2 cells treated with TNFα.
Indirect Immuofluorescence.
Chang or HepG2 cells were grown on coverslips and transfected as above, washed in PBS after transfection or infection with replication-defective recombinant adenovirus (Ad) vectors, then fixed/permeabilized with fresh 70% acetone/30% methanol at −20°C for 7 min as described (14). Cells were incubated with primary antibodies to HBsAg (Dako) or HBx-Flag (IBI), washed, and then incubated with fluorescein isothiocyanate-conjugated secondary antibody as described (14). Stained cells were examined and photographed using a Zeiss Axiophot fluorescence microscope.
Immunoblot Analysis.
Equal amounts of whole cell protein extracts were resolved by SDS/10% PAGE, transferred to nitrocellulose, and immunoblotted with antibodies (Upstate Biotechnology, Lake Placid, NY) to c-myc, N-myc, or c-Jun as described (15). Immunecomplexes were visualized with the enhanced chemiluminescence system (Amersham). Results were quantitated by densitometry, and experiments were repeated at least three times.
Assay of JNK Activity.
JNK activity was measured by the solid-phase kinase assay (32) using glutathione S-transferase–c-Jun (1–223 amino acids) as both ligand and substrate as described (15). Phosphorylated glutathione S-transferase–c-Jun was detected by SDS/12.5% PAGE and then visualized and quantitated by PhosphorImager (Molecular Dynamics) analysis. Results typical of three independent experiments are shown.
RESULTS AND DISCUSSION
HBx Sensitizes Cells to TNFα Killing During Transfection.
The level of HBx protein in HBV- and WHV-infected cells is not known with certainty although it is thought to be moderate (10,000–80,000 molecules) (33). We estimate the level of HBx expressed in transfected Chang cells studied here to be 8000–24,000 copies, although direct comparison with HBx levels during infection is not possible given the different methodologies that were used (see Materials and Methods). Many of the quantitative studies presented were carried out in Chang cells despite the fact that they retain few hepatocyte characteristics because they transfect at high efficiency (≈50%). Key experiments are also presented in a qualitative fashion in HepG2 cells, a differentiated hepatocyte line that transfects poorly. In transfected Chang cells, HBx alone did not induce detectable cell killing (Fig. 1A). To determine whether HBx might instead sensitize cells to induction of apoptosis, cells transfected with a HBx expression plasmid were treated with TNFα, a cytokine mediator of apoptosis that is produced in response to HBV infection (34). Although many tumor cell lines are sensitive to TNFα-mediated killing, nontransformed fibroblasts are typically resistant but can be sensitized by certain viral regulatory proteins (31). Chang cells are normally resistant to TNFα-mediated killing, even when exposed to high levels of the cytokine (Fig. 1B). However, expression of HBx induced sensitivity to killing by very low levels of TNFα (Fig. 1B). Treatment of HBx-transfected Chang cells with 1.0 ng/ml TNFα for 16 h induced killing only in cell cultures transfected with HBx (Fig. 1A), involving ≈40–50% of cells, the same proportion that was transfected (shown in Figs. 1D and 3D). TNFα treatment of control cells was associated with a rounder cell morphology but no detectable increase in killing. Cell death mediated by HBx plus TNFα displayed typical characteristics of apoptosis, such as generation of DNA cleavage ladders (Fig. 1C). Only transfected cells expressing HBx were sensitized to TNFα-mediated killing (Fig. 1D). HBx protein (which contains the Flag epitope) was stained with anti-Flag antibodies and colocalized to the same cells that contained condensed and fragmented nuclei, as observed by Hoechst 33258 staining, which is indicative of apoptosis. The poor imaging of HBx in apoptotic cells was due to the severe morphological changes associated with apoptosis. These results demonstrate that expression of HBx protein acutely sensitizes cells to apoptotic killing by TNFα.
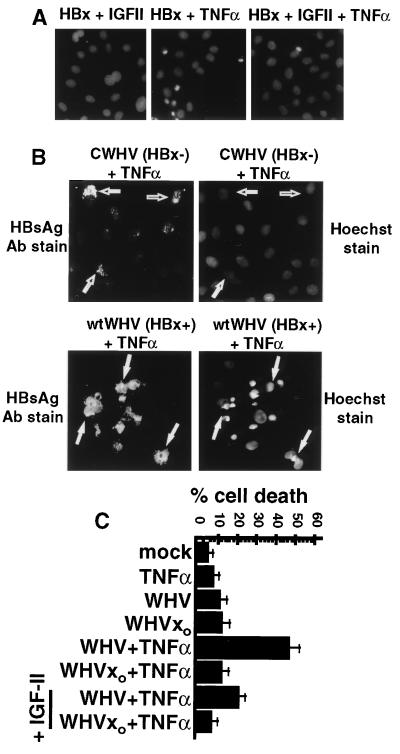
Figure 3.
Effect of IGF-II treatment and WHV replication on HBx-mediated apoptosis. (A) IGF-II blocks HBx sensitization of cells to TNFα-mediated apoptosis. Chang cells transfected with HBx plasmids were treated with TNFα with or without 100 ng/ml IGF-II for 16 h starting 20 h posttransfection. Cells were fixed on coverslips, stained with Hoechst 33258, and photographed. (B) Colocalization of apoptosis to WHV replicating cells. Chang cells were transfected for 3 days with cDNAs encoding wild-type WHV or HBx mutant CWHV, treated with TNFα for 16 h, fixed and stained with Hoechst 33258 and anti-HBsAg fluorescein isothiocyanate antibodies, and then photographed (magnification ×600). HBsAg stained cells with CWHV(HBx−) (open arrows) and with wtWHV (HBx+) (filled arrows). 1(C) HBx expressed during in vitro WHV replication sensitizes cells to TNFα-induced killing. Chang cells were transfected for 3 days with a wild-type WHV pregenomic cDNA (pWHV) or an HBx(−) mutant (pCWHV) with or without 1.0 ng/ml TNFα and with or without 100 ng/ml human IGF-II for 20 h. Cell killing was quantitated by entry of trypan blue dye. Results present average of four independent experiments.
HBx also sensitized HepG2 cells, a differentiated hepatocyte cell line, to apoptotic killing by TNFα. It was not possible to perform HepG2 cell killing studies by transfection of HBx plasmids because the efficiency of transfection was very low. Instead, HBx and HBxo genes were introduced on replication-defective recombinant Ad vectors by infecting HepG2 cells at low multiplicity, as described (9, 12, 14). The Ad vectors were previously shown to express only the HBx trans-gene during the limited time frame of these types of studies (12). Cells were infected for 6 h with Ad vectors expressing HBx or HBxo genes. Treatment of cells for 16 h with 1.0 ng/ml TNFα induced apoptosis only in HepG2 cells expressing HBx, but not in those expressing HBxo, as shown by condensed and fragmented nuclei (Fig. 1E) and generation of a DNA cleavage ladder (Fig. 1F). Expression of HBx in the absence of TNFα treatment did not induce significant cell killing or DNA cleavage. TNFα induced morphological alterations in control HepG2 cells (rounding) but no measurable increase in apoptosis. Noninfected HepG2 cells were identical to HBxo-expressing cells (data not shown). Thus, both a hepatocyte cell line (HepG2 cells) and liver-derived fibroblasts (Chang cells) were sensitized to apoptotic killing by TNFα during HBx expression.
Cytoplasmic HBx Sensitizes Cells to TNFα Killing by Inducing MEKK1/JNK and N-Myc.
Studies performed with HBx variants containing a functional nuclear localization signal (HBx-NLS) that targets HBx exclusively to the nucleus or a mutated signal that does not (HBx-SLN) previously showed that HBx functions in the cytoplasm to activate the Ras-Raf-mitogen-activated protein kinase cascade (14–16). Here it was found that targeting HBx exclusively to the nucleus (HBx-NLS) blocked sensitization of cells to TNFα killing, suggesting activation of cytoplasmic signal transduction in induction of apoptosis by HBx. Cotransfection of HBx with dominant-interfering forms of MEKK1 or Myc also impaired HBx sensitization of Chang cells to TNFα-mediated killing (Fig. 2A). Cotransfection of the Ad E1B-19k gene, a strong inhibitor of apoptosis, with HBx blocked TNFα-induced cell killing (Fig. 2A), further confirming that cell death occurred apoptotically. Collectively, these data and those of Fig. 1 indicate that HBx sensitizes cells to induction of apoptosis by TNFα through a pathway that involves activation of MEKK1/JNK and Myc proteins.
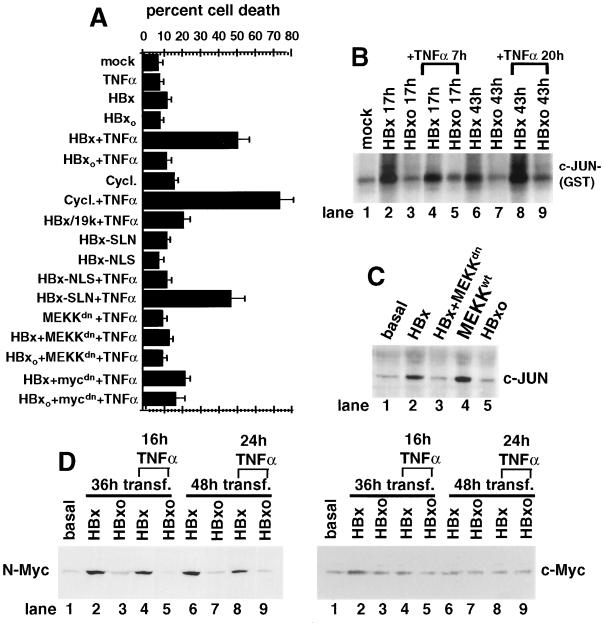
Figure 2.
Characterization of HBx protein-induced apoptosis. (A) HBx sensitization of cells to TNFα-mediated cell killing. Chang cells were transfected with HBx plasmids with or without Ad E1B-19k protein or transfected with dominant-interfering forms of MEKK1dn or Myc for 48 h followed by TNFα treatment at 1.0 ng/ml for 16 h. HBx-NLS contains an N-terminal SV40 nuclear localization sequence, which is mutated and nonfunctional in the HBx-SLN control (14). (B) Time course for HBx activation of JNK. Chang cells were transfected with HBx plasmids, extracts were prepared, JNK activity was measured by in vitro kinase assay using γ-32P-ATP, and an N-terminal c-Jun–glutathione S-transferase protein was resolved by electrophoresis (15). Cells were treated with 1.0 ng/ml TNFα at 10 h posttransfection for the remaining 7 h or at 23 h after transfection for an additional 20 h as indicated. (C) HBx induces prolonged activation of MEKK1/JNK. Chang cells were transfected with wild-type MEKK-1 or a dominant-interfering mutant (MEKKdn) and HBx plasmids, and then endogenous c-Jun levels were determined by gel electrophoresis and immunoblot analysis (15). (D) HBx induces prolonged activation of endogenous N-Myc. Chang cells were transfected with HBx plasmids for 36 or 48 h with or without treatment with TNFα for the last 16 or 24 h as indicated and resolved by SDS/gel electrophoresis, and proteins were immunoblotted with an antisera to N-Myc or c-Myc.
It was previously demonstrated that HBx induces activation of MEKK1/JNK and Myc in different cell lines including Chang and HepG2 cells (15, 26, 28). These components also contribute to induction of apoptosis in a variety of systems, particularly sustained activation of the MEKK1/JNK signaling pathway (30) and activation of Myc proto-oncogenes that sensitizes cells to TNFα-mediated cell killing (29, 35). Studies therefore were conducted to further examine the contribution of MEKK1 signaling and Myc activities in sensitization of cells to apoptosis by HBx. The activity and duration of JNK activity were assayed as a measure of stress-induced signaling in cells transfected with HBx or HBxo expression plasmids and were treated for various times with a subapoptotic dose (1.0 ng/ml) of TNFα (Fig. 2B). Activation of JNK was measured by binding and phosphorylation of the N terminus of c-Jun–glutathione S-transferase protein. In cells treated with TNFα, HBx but not HBxo induced prolonged activation of JNK. HBx but not HBxo also induced the sustained accumulation of endogenous c-Jun, further demonstrating that HBx mediated physiologically significant activation of JNK, which could be blocked by cotransfection with a dominant-negative MEKK1 (Fig. 2C). HBx therefore induced strong and prolonged activation of MEKK1/JNK that was not observed by TNFα treatment of control HBxo-expressing cells. HBx was shown previously to induce sustained activation of MEKK1/JNK in HepG2 cells (15). These results show that HBx sensitized cells to killing by TNFα, in part by activating the MEKK1/JNK signaling pathway. Induction and nuclear accumulation of Myc also promotes TNFα-mediated apoptosis (36), and HBx induces c-Myc or N-Myc genes (26–27, this report). The nuclear accumulation of Myc proteins therefore was examined in cells transfected with HBx plasmids and treated with TNFα (Fig. 2D). HBx induced strong (≈5–10 fold) and persistent accumulation of the endogenous N-Myc protein. Expression of the HBxo gene during TNFα treatment did not induce N-Myc. HBx only weakly and briefly induced the endogenous c-Myc gene in Chang cells (Fig. 2D). The significant increase by HBx of N-Myc levels and MEKK1 signaling is consistent with sensitization of cells to TNFα-mediated killing. It is not known why HBx strongly induced N-Myc and not c-Myc in Chang cells although reports indicate that expression of the different Myc family members is probably related to stages of cell differentiation and lineage (37).
Cell Killing by HBx Is Suppressed by Mitogenic Growth Factors.
Mitogenic growth factors can suppress induction of apoptosis in cells that receive conflicting proliferative signals or sustain an unregulated loss of cell cycle checkpoint controls (31). The majority of hepatocellular carcinomas derived from mammalian hepadnavirus infection express growth factors, particularly insulin-like growth factor II (IGF-II) in WHV and some HBV-derived tumors. IGF-II suppresses apoptotic killing by overexpression of N-myc during WHV infection of cultured cells (28, 38). To determine whether mitogens could suppress apoptotic killing induced by HBx, Chang cells transfected with HBx or HBxo expression plasmids were treated with TNFα alone or in combination with human IGF-II at 20 h after transfection (Fig. 3A). Condensed, Hoechst-stained nuclei indicative of apoptosis were evident only in TNFα-treated cells expressing wild-type HBx. Cotreatment of cells with IGF-II blocked cell killing by HBx and TNFα by ≈90% (Fig. 3A). These findings indicate that a mitogenic growth factor strongly associated with WHV tumors can impair the induction of apoptosis by HBx.
HBx Sensitizes Cells to TNFα Killing During Virus Replication in Chang Cells.
The effect of HBx on cell killing during WHV replication of cultured cells was studied. We have found that WHV and HBV replicate well in Chang cells in a manner dependent in HBx protein, in contrast to poor replication in HepG2 cells, which are only slightly stimulated by HBx (ref. 7; N.K. and R.J.S., unpublished work). Chang cells were transfected with a replication-competent cDNA of WHV that expresses the wild-type HBx gene (wtWHV) or a replication-impaired cDNA that expresses a C-terminally deleted (inactive) HBx mutant (CWHV). Treatment of 3-day, wtWHV-transfected cells with 1.0 ng/ml TNFα for 16 h induced significant apoptotic cell killing, evident by Hoechst nuclei staining (Fig. 3 B and C). Killing was not observed in the CWHV(HBx)-transfected cultures after TNFα treatment. The extent of apoptotic cell death (≈45%) correlated with the fraction of cells containing replicating wtWHV, as determined by indirect immunofluorescence staining of the WHV envelope proteins (HBsAg) (Fig. 3B). Important to note, only HBsAg-stained cells that contained wtWHV and expressed HBx showed evidence of apoptotic killing, determined by Hoechst staining of condensed fragmented nuclei (Fig. 3B, filled arrows). HBsAg antibodies did not stain cells in the absence of WHV transfection or when apoptosis was induced in control cells by TNFα and cycloheximide (data not shown). Addition of IGF-II to TNFα-treated cells containing replicating wtWHV blocked cell killing by ≈60% (Fig. 3C). The inability to fully suppress apoptosis with IGF-II might indicate that HBx is more toxic when expressed during wtWHV replication or that the high level of WHV replication promoted by HBx is itself somewhat toxic. Nevertheless, it is clear that HBx sensitizes cells to TNFα-mediated killing in the context of WHV replication in cultured cells, which is partially blocked by a mitogenic growth factor expressed by virus-induced tumors.
CONCLUSIONS
Previous work has demonstrated that the apoptotic death of hepatocytes in HBV and WHV infection, which is driven by cell-mediated immune responses, underlies liver damage and viral pathogenesis that occurs during viral infection (1). The results presented here demonstrate that the viral HBx protein might be a significant cofactor in HBV pathogenesis. HBx might participate in destruction of liver tissue during HBV infection in mammals by inducing Myc protein accumulation and by stimulating MEKK1/JNK signaling pathways. Four features of the study presented here validate the likelihood that HBx might participate in hepadnavirus pathogenesis during authentic infection of animals and humans: (i) Expression of low levels of HBx in Chang and HepG2 cells sensitized them to killing by low levels of TNFα. (ii) Production of TNFα is often part of the repertoire of immune responses directed against HBV- and WHV-infected liver (1, 3). (iii) HBx sensitized cells to TNFα killing by an established mechanism, activation of Myc, and the stress-mediated MEKK1 signaling pathway (29–31, 35), which also are previously reported properties of HBx protein (15, 26). (iv) HBx sensitized cells to TNFα-mediated killing when expressed during WHV replication in cultured cells. Thus, the sensitization of cells to killing by TNFα occurs at levels of HBx protein that may be physiologically relevant. The molecular contribution by TNFα to cell killing was not directly examined in this study.
The ability of HBx to promote apoptotic cell killing by TNFα must be considered within the context of authentic infection of animals and humans by the mammalian hepadnaviruses. In particular, elegant studies by Guidotti et al. (39), using a transgenic mouse model, have shown that HBV replication can be inhibited by the combined exposure of cells to interferon-γ and TNFα secreted by cytotoxic T lymphocytes without destruction of infected hepatocytes. Destruction of virus-infected liver may therefore be a pathogenic response unrelated to viral clearance by the immune system, particularly because most hepatocytes can be infected although only a fraction are normally killed (39). The results of Guidotti et al. are not inconsistent with the findings presented in this report, for two reasons. First, in the transgenic mouse model, HBx protein either is not expressed or is only expressed at extremely low levels (39). On the other hand, our studies indicate that, in the transfected cells examined here, HBx protein may be expressed at physiologically relevant levels. Second, although HBV can be cleared from infected liver without the requirement of tissue destruction, there is still a significant pathogenic killing of hepatocytes that varies significantly in magnitude in different individuals (1). It is plausible that the balance and levels of INFγ and TNFα presented to infected hepatocytes, as well as the level of HBx protein expressed, are important determinants of viral clearance or pathogenesis.
The potential role of HBx in HBV pathogenesis is in seeming contradiction to its established role in replication of the virus and a possible role in carcinogenesis. However, these activities may not represent a paradox. We note that myc proteins can participate in both cell transformation and apoptosis (40). In addition, transgenic mice that coexpressed HBx and c-myc displayed both enhanced hepatocyte apoptosis and progression to carcinoma compared with expression of either gene alone (41). Finally, liver cancer in humans and rats display excessive hepatocyte apoptotic death rates as well as replication rates at all stages of transformation, indicative of a consistent species-independent mechanism in hepatocellular carcinoma (42). Furthermore, increased cell death rates can select for survival growth factors that in turn lead to enhanced malignancy. A key question that needs to be addressed is the possible role that enhanced hepatocyte turnover by HBx might play during natural infection. It is possible that, by increasing hepatocyte death rates, induction of liver cell growth factors may be enhanced. This in turn could enhance liver regeneration, creating a larger reservoir of new uninfected hepatocytes to propagate the viral infection.
Acknowledgments
We thank T. Lee, E. Ziff, E. White, and B. Su for dominant-interfering plasmids and C. Seeger for WHV genomic clones. Thanks to N. Klein for comments on the paper. This work was supported by National Institutes of Health Grant CA54525 to R.J.S.
Footnotes
Abbreviations: HBV, hepatitis B virus; WHV, woodchuck hepatitis B virus; TNFα, tumor necrosis factorα; MEKK1, mitogen-activated-protein kinase kinase 1; JNK, JUN kinase; Ad, adenovirus; IGF-II, insulin-like growth factor II; wtWHV, wild-type WHV; CWHV, WHV with C-terminally deleted HBX protein.
References
- 1.Chisari F V. Curr Topics Microbiol Immunol. 1996;206:149–173. doi: 10.1007/978-3-642-85208-4_9. [DOI] [PubMed] [Google Scholar]
- 2.Ganem D, Varmus H E. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 3.Galle P R, Hagelstein J, Kommerell B, Volkmann M, Schranz P, Zentgraf H. Gastroenterology. 1994;106:664–673. doi: 10.1016/0016-5085(94)90700-5. [DOI] [PubMed] [Google Scholar]
- 4.Rogler C E. In: Cellular and Molecular Mechanisms in Hepatocarcinogenesis Associated with Hepadnavirus Infection. Mason W S, Seeger C, editors. Vol. 168. Berlin: Springer; 1991. pp. 103–140. [DOI] [PubMed] [Google Scholar]
- 5.Yen T S B. J Biomed Sci. 1996;3:20–30. doi: 10.1007/BF02253575. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Kaneko S, Girones R, Anderson R W, Hornbuckle W E, Tennant B C, Cote P J, Gerin J L, Purcell R H, Miller R H. J Virol. 1993;67:1218–1226. doi: 10.1128/jvi.67.3.1218-1226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoulim F, Saputelli J, Seeger C. J Virol. 1994;68:2026–2030. doi: 10.1128/jvi.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C-M, Koike K, Saito I, Miyamura T, Jay G. Nature (London) 1991;353:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 9.Benn J, Schneider R J. Proc Natl Acad Sci USA. 1995;92:11215–11219. doi: 10.1073/pnas.92.24.11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross J C, Wen P, Rutter W J. Proc Natl Acad Sci USA. 1993;90:8078–8082. doi: 10.1073/pnas.90.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kekule A S, Lauer U, Weiss L, Luber B, Hofschneider P H. Nature (London) 1993;361:742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- 12.Benn J, Schneider R J. Proc Natl Acad Sci USA. 1994;91:10350–10354. doi: 10.1073/pnas.91.22.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natoli G, Avantaggiati M L, Chirillo P, Puri P L, Ianni A, Balsano C, Levrero M. Oncogene. 1994;9:2837–2843. [PubMed] [Google Scholar]
- 14.Doria M, Klein N, Lucito R, Schneider R J. EMBO J. 1995;14:4747–4757. doi: 10.1002/j.1460-2075.1995.tb00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benn J, Su F, Doria M, Schneider R J. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su F, Schneider R J. J Virol. 1996;70:4558–4566. doi: 10.1128/jvi.70.7.4558-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aufiero B, Schneider R J. EMBO J. 1990;9:497–504. doi: 10.1002/j.1460-2075.1990.tb08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwee L, Lucito R, Aufiero B, Schneider R J. J Virol. 1992;66:4382–4389. doi: 10.1128/jvi.66.7.4382-4389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H-D, Yuh C-H, Dang C V, Johnson D L. Mol Cell Biol. 1995;15:6720–6728. doi: 10.1128/mcb.15.12.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguire H F, Hoeffler J P, Siddiqui A. Science. 1991;252:842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- 21.Williams J S, Andrisani O M. Proc Natl Acad Sci USA. 1995;92:3819–3823. doi: 10.1073/pnas.92.9.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haviv I, Vaizel D, Shaul Y. EMBO J. 1996;15:3413–3420. [PMC free article] [PubMed] [Google Scholar]
- 23.Cheong J-H, Yi M-K, Lin Y, Murakami S. EMBO J. 1995;14:143–150. doi: 10.1002/j.1460-2075.1995.tb06984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X W, Forrester K, Yeh H, Feitelson M A, Gu J, Harris C C. Proc Natl Acad Sci USA. 1994;91:2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee T-H, Elledge S J, Butel J S. J Virol. 1995;69:1107–1114. doi: 10.1128/jvi.69.2.1107-1114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balsano C, Avantaggiati M L, Natoli G, DeMarzio E, Will H, Perricaudet M, Levrero M. Biochem Biophys Res Commun. 1991;176:985–992. doi: 10.1016/0006-291x(91)90379-l. [DOI] [PubMed] [Google Scholar]
- 27.Avantaggiati M L, Balsano C, Natoli G, DeMarzio E, Will H, Elfassi E, Levrero M. Arch Virol. 1992;4:57–61. doi: 10.1007/978-3-7091-5633-9_12. [DOI] [PubMed] [Google Scholar]
- 28.Ueda K, Ganem D. J Virol. 1996;70:1375–1383. doi: 10.1128/jvi.70.3.1375-1383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evan G, Wyllie A, Gilbert C, Littlewood T, Land H, Brooks M, Waters C, Penn L, Hancock D. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 30.Xia Z, Dickens M, Raingeaud J, Davis R, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 31.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 32.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 33.Dandri M, Schirmacher P, Rogler C E. J Virol. 1996;70:5246–5254. doi: 10.1128/jvi.70.8.5246-5254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L, Moreno-Otero R, Alonso J L, Yague E, Pivel J P, Lopez-Cabrera M, Fernandez-Ruiz E, Sanchez-Madrid F. J Exp Med. 1994;179:841–848. doi: 10.1084/jem.179.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klefstrom J, Vastrik I, Saksela E, Valle J, Eilers M, Alitalo K. EMBO J. 1994;13:5442–5450. doi: 10.1002/j.1460-2075.1994.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L, Nishioka W K, Th’ng J, Bradbury M, Litchfield D W, Greenberg A H. Science. 1994;263:1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- 37.Mugrauer G, Alt F W, Ekblom P. J Cell Biol. 1988;107:1325–1335. doi: 10.1083/jcb.107.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Faris R, Hixson D, Affigne S, Rogler C E. J Virol. 1996;70:6260–6288. doi: 10.1128/jvi.70.9.6260-6268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 40.Harrington E A, Bennett M R, Fanidi A, Evan G I. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terradillos O, Billet O, Rnard C-A, Levy R, Molina T, Briand P, Buendia M A. Oncogene. 1997;14:395–404. doi: 10.1038/sj.onc.1200850. [DOI] [PubMed] [Google Scholar]
- 42.Grasl-Kraupp B, Ruttkay-Nedecky B, Mullhauser L, Taper H, Huber W, Bursch W, Schulte-Hermann R. Hepatology. 1997;25:906–912. doi: 10.1002/hep.510250420. [DOI] [PubMed] [Google Scholar]