Abstract
Ethanol and glucocorticoids are risk factors associated with osteonecrosis. Previous reports suggest ethanol and glucocorticoids induce adipogenesis, decrease osteogenesis in bone marrow stroma cells, and produce intracellular lipid deposits resulting in death of osteocytes. The Wnt/β-catenin signal pathway is involved in the regulation of homeostasis of bone and we presume glucocorticoids and ethanol may induce osteonecrosis in humans through a similar mechanism as in rodents. We hypothesized (1) ethanol, like glucocorticoids, decreases osteogenesis and increases adipogenesis through the Wnt/β-catenin signaling pathway in human bone marrow stromal cells; and (2) ethanol decreases intranuclear translocation of β-catenin. We found both dexamethasone and ethanol decrease the gene and protein expression of osteogenesis and increase that of adipogenesis through Wnt signaling-related genes by semiquantitative and quantitative polymerase chain reaction and Western blot. Ethanol hampered intranuclear translocation of β-catenin by immunofluorescence analysis. The data suggest the Wnt/β-catenin signaling pathway may be associated with ethanol-induced osteonecrosis.
Introduction
Osteonecrosis (ON) is a pathologic process resulting from direct and indirect injury to the osteoblasts [23]. Numerous risk factors associated with nontraumatic ON include corticosteroid treatment, alcoholism, smoking, hyperlipidemia, and hyperviscosity [1, 11, 23, 28]. However, the pathogenesis of nontraumatic ON remains controversial and no clear connection between adipogenesis and ON has been established as yet. In our previous studies, we demonstrated chickens treated with steroids developed fat cell hypertrophy and eventual ON in the femoral head [7, 8]. Ethanol, on the other hand, induces adipogenesis and also produces intracellular lipid deposits resulting in the death of osteocytes, which may be associated with the development of ON, especially in patients with long-term and excessive consumption of alcohol [28].
Wnt signaling pathway and the various Wnt family members are involved in morphogenesis, organogenesis, oncogenesis, cell fate determination, regulation of cell proliferation, and differentiation during embryogenesis [14, 22]. Recent studies suggest the Wnt signaling pathway is involved in the regulation of homeostasis of bone mass. The Wnt proteins activate two types of signaling pathways: canonic and noncanonic. The canonic Wnt proteins bind to a member of the Frizzled family receptor and its coreceptors, LRP5/LRP6, at the cell membrane leading to glycogen synthase kinase-3β (GSK-3β) inactivation and the nuclear accumulation of β-catenin by inhibiting phosphorylation of β-catenin [20]. Nuclear β-catenin acts as a transcriptional coactivator by interacting with transcription factors of the T-cell factor (Tcf)/lymphoid enhancer factor (Lef) family to regulate gene expression [20]. Low-density-lipoprotein receptor-related proteins 5 and 6 (LRP5/LRP6) are indispensable transmembrane proteins for Wnt/β-catenin signaling and are likely to act as Wnt coreceptors [14]. Knockout of LRP5 in mice leads to osteopenia [29]. Overexpression of LRP5 increases bone mass and reduces osteoblast apoptosis [2]. Therefore, the Wnt signaling pathway likely plays important roles in the development of bone.
Glucocorticoids may affect the Wnt signaling pathway and reduce bone formation by inhibiting the activity of β-catenin and regulating the expression of Wnt signal-related molecules in osteoblasts [27]. Because of the similar pathologic changes between steroid-induced and alcohol-induced ON [28], we proposed the mechanism between these two situations might be similar. In previous experiments, we demonstrated ethanol decreased the mRNA expression of osteogenic genes and increased the mRNA expression of adipogenic genes [28] similar to the effects of dexamethasone in human bone marrow stroma cells from the patients with ON [4]. Based on these previous studies, we inferred glucocorticoids and ethanol may induce ON in humans through a similar mechanism.
We therefore hypothesized (1) ethanol, similar to glucocorticoids, decreases osteogenesis and increases adipogenesis through the regulation of the Wnt signal pathway on human marrow cells by mRNA and protein expression; and (2) ethanol decreases intranuclear translocation of β-catenin on human marrow cells.
Materials and Methods
We monitored the responses of multipotent human marrow cells treated with dexamethasone and ethanol to evaluate the pathologic change of ON by monitoring gene expressions related to osteogenesis and adipogenesis and that of Wnt signaling-related genes. To confirm the posttranscriptional effects of ethanol on adipogenesis and the Wnt signaling pathway, we analyzed the protein expression of β-catenin and PPARγ by Western blot analysis. β-catenin, the pivotal protein in the Wnt signaling pathway, was also observed by confocal microscopy for intranuclear translocation.
We enrolled 13 patients with ethanol-induced ON of the femoral head and nine patients without ON. Patients with impaired renal or liver function, patients receiving hormone therapy, and those with malignancy or diabetes mellitus were excluded. Age, gender, and body mass index were similar between groups. The non-ON group included three patients who had THA for dysplastic arthritis of the hip and six patients who had internal fixation for a fresh fracture at the acetabulum, pelvis, or femoral shaft within 2 days after injury. Frequent alcohol consumption of more than 400 mL ethanol per week was reported for all patients with alcohol-induced ON.
We previously reported the detailed procedures of HMC culture [4]. Cells from 22 patients were cultured and tested separately. The cells were separated by a Percoll™ gradient (Amersham Pharmacia, Piscataway, NJ); the nucleated stroma cells were then collected for primary cell culture [13]. The third passage of culture was used for experiments. Donor cells (104 cells/cm2) were seeded on a plate and when they reached 80% confluence were treated with either 10 or 30 mmol/L ethanol (Sigma, St Louis, MO) or 100 nmol/L dexamethasone. For immunohistochemistry assay, the cells were pretreated with 25 mmol/L LiCl (Sigma) as provocative treatment and then further treated with and without ethanol. Messenger RNA expression of all target genes was evaluated by semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) and quantitative real-time PCR after treatment for 3 days. Total protein was isolated for Western blotting after treatment for 3 days. All independent experiments containing at least three tests were repeated at least twice (Table 1).
Table 1.
List of tests performed
| Case | ON | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ON#1 | ON#2 | ON#3 | ON#4 | ON#5 | ON#6 | ON#7 | ON#8 | ON#9 | ON#10 | ON#11 | ON#12 | ON#13 | |
| PCR | N = 3 | N = 3 | N = 3 | N = 3 | |||||||||
| n = 3 | n = 3 | n = 3 | n = 3 | ||||||||||
| Real-time PCR | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 |
| n = 3 | n = 2 | n = 2 | n = 3 | n = 2 | n = 2 | n = 2 | n = 2 | n = 3 | n = 2 | n = 2 | n = 3 | n = 2 | |
| Western blotting | N = 3 | N = 3 | N = 3 | N = 3 | |||||||||
| n = 3 | n = 3 | n = 3 | n = 3 | ||||||||||
| β-catenin intranuclear translocation | N = 3 | N = 3 | N = 3 | ||||||||||
| n = 3 | n = 3 | n = 3 | |||||||||||
| Case | Non-ON | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TA#1 | TA#2 | TA#3 | TA#4 | TA#5 | TA#6 | OA#1 | OA#2 | OA#3 | |||||
| PCR | N = 3 | N = 3 | N = 3 | ||||||||||
| n = 3 | n = 3 | n = 3 | |||||||||||
| Real-time PCR | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | ||||
| n = 3 | n = 3 | n = 2 | n = 2 | n = 2 | n = 3 | n = 3 | n = 3 | n = 2 | |||||
| Western blotting | N = 3 | N = 3 | N = 3 | N = 3 | |||||||||
| n = 3 | n = 3 | n = 3 | n = 3 | ||||||||||
| β-catenin intranuclear translocation | N = 3 | N = 3 | N = 3 | ||||||||||
| n = 3 | n = 3 | n = 3 | |||||||||||
HMCs were isolated from 22 patients (13 ON and nine non-ON cases; non-ON cases include six trauma and three osteoarthritis cases); we have RT-PCR data from seven patients, real-time PCR data from 22 patients, Western blotting data from eight patients, and β-catenin intranuclear translocation data from six patients; all independent experiments contain at least three repeat tests (n); each individual experiment was tested at least twice (N); ON = osteonecrosis; RT-PCR = reverse transcriptase–polymerase chain reaction; TA = Trauma; HMC = Human marrow cell.
All 22 cell lines were tested for the surface markers and the ability of osteogenic, adipogenic, and chondrogenic differentiation. The surface markers in all cell lines are compatible with those of mesenchymal stem cells. All cell lines showed good osteogenic, chondrogenic, and adipogenic differentiation after proper induction as previously reported [19, 30].
We examined the mRNA expression of the Wnt signaling ligand, Wnt 3a; the Wnt protein antagonist, SFRP2; membrane coreceptor, LRP5; and osteogenic-related genes, including BMP2, Runx2, and osteocalcin. The adipogenic-related genes, including PPARγ and adipsin, were examined using RT-PCR and quantitative real-time PCR. For each gene, the quantitative RT-PCR experiments were performed with at least three independent batches of cDNAs. Changes (x-fold) in gene expression level were calculated by the 2-ΔΔct method [21]. Analysis of variance was performed using Excel software (Microsoft Corp, Cupertino, CA) as in previous studies [4, 5]. HMCs were isolated from 22 patients (13 ON and nine non-ON cases; non-ON cases included six trauma and three osteoarthritis cases). In total, we have RT-PCR data from seven patients, real-time PCR data from 22 patients, Western blot data from eight patients, and β-catenin intranuclear translocation data from six patients. All independent experiments containing at least three tests were repeated at least twice (Table 1).
We performed Western blots on cell extracts in eight different cell lines, including four patients with ON and four patients without ON, separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and blotted onto Hybond-C membrane (Amersham Pharmacia). The membranes were blocked by 5% nonfat milk and probed with β-catenin, PPARγ, and β-actin. Blots were incubated with a horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by the enhanced chemiluminescence system (Amersham Biosciences). The optical densities of the resolved bands were then semiquantified using Image-Pro Plus® analysis software (Media Cybernetics, Bethesda, MD). All independent experiments containing at least three tests were repeated at least twice (Table 1).
To further understand whether ethanol suppresses the canonic Wnt signaling pathway, human marrow cells from six different cell lines, three from patients with ON and three from patients without ON, were treated with LiCl, an inhibitor of GSK-3β, to activate β-catenin. LiCl treatment was used as a provocative treatment in the intranuclear translocation of β-catenin by immunofluorescence analysis. Human marrow cells were cultured on glass coverslips. After drug treatments, the cells were fixed and permeated with 0.2% Triton X-100 (Sigma) and then blocked. Cells were incubated with anti-β catenin antibody for 1 hour and goat anti-mouse IgG coupled to FITC for 40 minutes. Meanwhile, the cells were counterstained with DAPI (Sigma) to highlight the nuclei. All images were observed with a Fluoview FV 500 (Olympus, Tokyo, Japan) confocal microscope and processed by a Fluoview FV 500 analysis system (Olympus). All independent experiments containing at least three tests were repeated at least twice (Table 1).
In the first hypothesis, data from RT-PCR, real-time PCR, and Western blotting were presented as mean ± standard deviation and evaluated by one-way analysis of variance (ANOVA) and Post Hoc test by Scheffe’s method. The independent variables in the test of RT-PCR, real-time PCR, and Western blotting are dexamethasone and ethanol. A p value less than 0.05 was considered significant. In the second hypothesis, the intranuclear translocation of β-catenin was not evaluated statistically because of the difficulty in the quantitation of β-catenin on the image. The variables in the test of intranuclear translocation of β-catenin were LiCl and ethanol.
Results
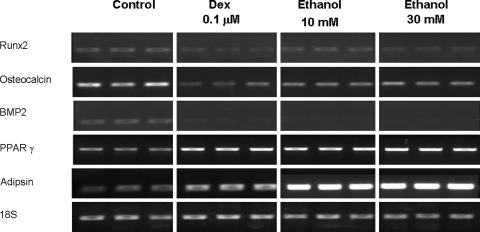
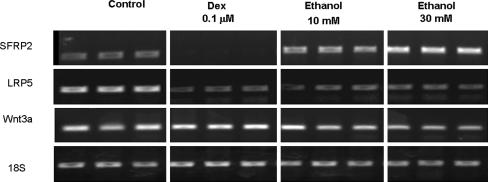
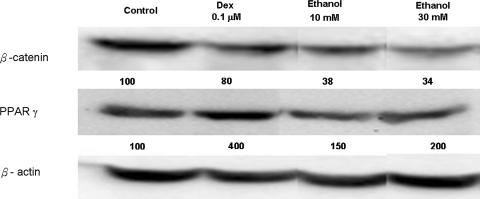
Ethanol suppressed the mRNA expression of osteogenic genes, including BMP2 (p = 0.005 and 0.009), Runx2 (p = 0.01 and 0.002), and osteocalcin (p = 0.003 and 0.002) (Fig. 1). On the other hand, mRNA expression of PPARγ (p = 0.005 and 0.03) and adipsin (p = 0.03 and 0.02) was upregulated after treatment with ethanol (Fig. 1). Ethanol suppressed osteogenic gene expression and induced adipogenic gene expression, similar to dexamethasone-treated cells. Like with dexamethasone treatment, ethanol, 10 and 30 mmol/L, decreased mRNA expression of LRP5 (p = 0.002 and < 0.001) and Wnt3a (p < 0.001 and < 0.001) genes. On the other hand, the expression of Wnt3a was suppressed (p < 0.001) in a dose-dependent manner by ethanol treatment (Fig. 2). The expression of SFRP2 was increased by ethanol treatment, 10 and 30 mmol/L, (p = 0.01 and 0.003) but suppressed (p = 0.02) by dexamethasone treatment (Fig. 2). The trends of gene expression were the same in RT-PCR and real-time PCR. Both dexamethasone and ethanol increased the mRNA expression of PPARγ and decreased that of BMP2, osteocalcin, Runx2, Wnt 3a, and LRP5 (Table 2). The ethanol, 10 and 30 mmol/L, suppressed (p = 0.002 and 0.006) β-catenin expression and increased PPARγ (p < 0.001 and 0.01) expression in a dose-dependent manner similar to mRNA expression by Western blot analysis (Fig. 3).
Fig. 1.
The human bone marrow cells were treated with dexamethasone and ethanol for 3 days and the expression of osteogenic and adipogenic genes were evaluated by reverse transcriptase–polymerase chain reaction. Ethanol, 10 and 30 mmol/L, and dexamethasone inhibited the mRNA expression of osteogenic genes, including Runx2, osteocalcin, and BMP2, in a dose-dependent manner and both increased mRNA expression of adipogenic genes, including PPARγ and adipsin, in a dose-dependent manner. The data support our first hypothesis.
Fig. 2.
Dexamethasone decreased SFRP2 expression, but ethanol, 10 and 30 mmol/L, increased SFRP2 gene expression in a dose-dependent manner by reverse transcriptase–polymerase chain reaction. Dexamethasone and ethanol, 10 and 30 mmol/L, decreased LRP5 and Wnt 3a gene expression. The data are consistent with our first hypothesis.
Table 2.
Result of osteogenic, adipogenic, and Wnts signal pathway-specific mRNA expression after drug treatment by real-time polymerase chain reaction measurement in 22 samples of human bone marrow stroma cells
| Gene | Control | Dexamethasone 0.1 μM | Ethanol 10 mM | Ethanol 30 mM | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P | Mean ± SD | P | Mean ± SD | P | |
| BMP2 | 100 ± 10 | 22 ± 9 | 0.018 | 24 ± 12 | 0.012 | 22 ± 10 | 0.021 |
| Osteocalcin | 100 ± 10 | 27 ± 8 | 0.013 | 27 ± 6 | 0.036 | 17 ± 6 | 0.016 |
| Runx2 | 100 ± 8 | 42 ± 13 | 0.022 | 34 ± 12 | 0.011 | 22 ± 11 | 0.0041 |
| PPARγ | 100 ± 7 | 150 ± 6 | 0.049 | 186 ± 3 | 0.020 | 272 ± 5 | 0.031 |
| SFRP2 | 100 ± 11 | 47 ± 9 | 0.022 | 134 ± 12 | 0.041 | 174 ± 12 | 0.021 |
| Wnt 3a | 100 ± 14 | 42 ± 12 | 0.035 | 59 ± 12 | 0.036 | 44 ± 13 | 0.045 |
| LRP5 | 100 ± 6 | 60 ± 10 | 0.038 | 64.7 ± 8 | 0.006 | 59 ± 7 | 0.014 |
p < 0.05 was considered significant; SD = standard deviation.
Fig. 3.
Cell extracts were subjected to immunoblotting using antibodies against β-catenin and PPARγ The amounts of loading control were determined by β-actin. Dexamethasone and ethanol, 10 and 30 mmol/L, decreased β-catenin protein expression but increased PPARγ protein expression in a dose-dependent pattern. The results support our first hypothesis.
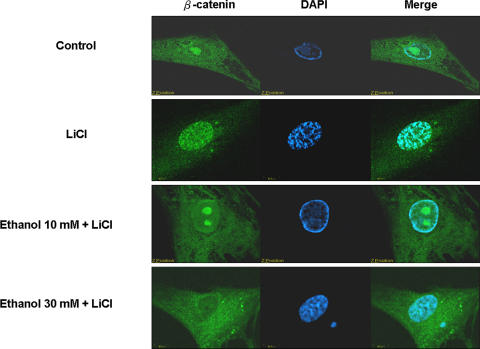
Addition of the GSK-3β inhibitor, LiCl, at 25 mmol/L intensified the green light in the nucleus, which meant the increase of β-catenin–FITC complexes in the nucleus. After ethanol treatment, immunofluorescence staining showed β-catenin–FITC complexes were reduced in the nucleus (Fig. 4). Decrease of β-catenin in the nucleus may hamper the transcriptional activity.
Fig. 4.
Human bone marrow cells were stained with β-catenin antibody (green) and the cells were costained with DAPI to make the cell nuclei (blue). Immunofluorescent images were observed with a confocal microscope. After the treatments with ethanol, immunofluorescence staining showed β-catenin–FITC complexes decreased in the nucleus. The results support our second hypothesis.
Discussion
Previous reports indicate an association of prolonged ethanol intake and ON [1, 9, 15–18, 24, 28]. Ethanol-induced ON is associated with marrow cell changes on histologic sections similar to those from prolonged glucocorticoid administration [28]. Because of the similar pathologic changes between glucocorticoid-induced and ethanol-induced ON, we presumed the mechanism between these two situations might also be similar. Thus, we presumed glucocorticoids and ethanol induce ON in humans through a similar mechanism such as that in the murine cells. We proposed two hypotheses: (1) ethanol, like glucocorticoids, decreases osteogenesis and increases adipogenesis through the regulation of the Wnt signal pathway on human marrow cells; (2) ethanol diminishes intranuclear translocation of β-catenin.
There are some limitations to this study. First, the number of patients enrolled in this study is not large enough to provide a conclusive answer, but rather a preliminary one. Second, although we demonstrated ethanol suppresses Wnt/β-catenin signaling in mRNA expression, protein expression, and intranuclear translocation of β-catenin, we cannot clearly identify the protein expression of GSK3β, especially the phosphorylated form. Third, the activity of GSK3β is not confirmed in this study, although we clearly observed the changes of intranuclear translocation of β-catenin. The important role of GSK3β is not clearly linked in this study. Fourth, the control osteoarthritis subjects in this study were not exposed to a substantial amount of ethanol; we do not know whether they will develop ON if they drink excessively. Furthermore, patients with osteoarthritis may have abnormal bone remodeling, and therefore we cannot presume their data are normal.
Gaur et al. [12] reported canonic Wnt signaling promoted osteogenesis by directly stimulating Runx2 gene expression. We found ethanol suppressed the mRNA expression of BMP2, Runx2, and osteocalcin, whereas it activated the mRNA expression of adipogenic genes, including PPARγ and adipsin. Therefore, ethanol causes human marrow cells toward adipogenic differentiation rather than osteogenic differentiation. This may be one of the possible mechanisms of ethanol-induced ON.
Wang et al. reported death of osteocytes in alcohol-induced ON [28]. Calder et al. [3] reported steroid-induced and alcohol-induced ON was accompanied by widespread apoptosis of osteoblasts and osteocytes. β-catenin was reported to modulate cell proliferation and survival [29] We found ethanol decreased the quantity of β-catenin and hampered the intranuclear translocation of β-catenin in human marrow cells. It is worthwhile to delineate the relationship between β-catenin and alcohol-induced apoptosis.
Wang et al. [27] suggested dexamethasone increases the mRNA expression of SFRP1 in primary mesenchymal cells from male Sprague-Dawley rats. Our data suggest dexamethasone decreases the mRNA expression of SFRP2, whereas ethanol increases that of SFPR2. Ethanol and glucocorticoids may act on different SFRPs to modulate Wnt signaling.
Bone morphogenetic proteins (BMPs) have emerged as key regulators of stem cell fate commitment [26]. Wnts and TGF-β superfamily members interact to regulate the transcription of a number of genes [6]. BMPs and Wnt are important signals determining the fate of immature cells into cells of the osteoblastic lineage [10]. However, Nakashima et al. [25] reported BMP-2 did not induce canonic Wnt expression and Tcf/Lef1-dependent transcriptional activation in C2C12 cells. Our data suggest ethanol decreases the gene expression of BMP2 and osteocalcin. Meanwhile, it also decreased gene expression of LRP5 and Wnt 3a and enhanced the gene expression of SFRP2 and diminished β-catenin protein level. Crosstalk between BMP and the Wnt signal pathway may coregulate osteogenic gene expression, but the relation between Wnt and the BMP signal pathway requires further investigation.
Acknowledgments
We thank Yi-Jen Chen for helping in the experimental process and Dr Chung-Hwan Chen for the preparation of the manuscript. We also thank Chihuei Wang, PhD, for help with the experiment and discussion.
Footnotes
Ching-Hua Yeh and Je-Ken Chang contributed equally to this manuscript.
One or more of the authors (GJW, JKC, MLH) have received funding from the National Health Research Institute of Taiwan (NHRI-EX94-9316EP and NHRI-EX96-9615EP), the Hip Society, Technology Development Program for Academia in Taiwan (96-EC-17-A-17-S1-041), and Zimmer, Inc.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed]
- 2.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974. [DOI] [PubMed]
- 3.Calder JD, Buttery L, Revell PA, Pearse M, Polak JM. Apoptosis—a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2004;86:1209–1213. [DOI] [PubMed]
- 4.Chang JK, Ho ML, Yeh CH, Chen CH, Wang GJ. Osteogenic gene expression decreases in stromal cells of patients with osteonecrosis. Clin Orthop Relat Res. 2006;453:286–292. [DOI] [PubMed]
- 5.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16:2039–2045. [DOI] [PubMed]
- 6.Crease DJ, Dyson S, Gurdon JB. Cooperation between the activin and Wnt pathways in the spatial control of organizer gene expression. Proc Natl Acad Sci USA. 1998;95:4398–4403. [DOI] [PMC free article] [PubMed]
- 7.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. [DOI] [PubMed]
- 8.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [DOI] [PubMed]
- 9.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006;88(Suppl 3):148–154. [DOI] [PubMed]
- 10.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. [DOI] [PubMed]
- 11.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;1:902–906. [DOI] [PubMed]
- 12.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. [DOI] [PubMed]
- 13.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. [DOI] [PubMed]
- 14.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. [DOI] [PubMed]
- 15.Hirota Y, Hirohata T, Fukuda K, Mori M, Yanagawa H, Ohno Y, Sugioka Y. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137:530–538. [DOI] [PubMed]
- 16.Hungerford DS, Lennox DW. The importance of increased intraosseous pressure in the development of osteonecrosis of the femoral head: implications for treatment. Orthop Clin North Am. 1985;16:635–654. [PubMed]
- 17.Hungerford DS, Zizic TM. Alcoholism associated ischemic necrosis of the femoral head. Early diagnosis and treatment. Clin Orthop Relat Res. 1978;130:144–153. [PubMed]
- 18.Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res. 1978;130:51–67. [PubMed]
- 19.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [DOI] [PubMed]
- 20.Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. [DOI] [PubMed]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C[T]) method. Methods. 2001;25:402–408. [DOI] [PubMed]
- 22.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. [DOI] [PubMed]
- 23.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992;326:1473–1479. [DOI] [PubMed]
- 24.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. [DOI] [PubMed]
- 25.Nakashima A, Katagiri T, Tamura M. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J Biol Chem. 2005;280:37660–37668. [DOI] [PubMed]
- 26.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–5721. [DOI] [PubMed]
- 27.Wang FS, Lin CL, Chen YJ, Wang CJ, Yang KD, Huang YT, Sun YC, Huang HC. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146:2415–2423. [DOI] [PubMed]
- 28.Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–224. [DOI] [PubMed]
- 29.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. [DOI] [PubMed]
- 30.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. [DOI] [PubMed]