Abstract
Endothelial-selective delivery of therapeutic agents, such as drugs or genes, would provide a useful tool for modifying vascular function in various disease states. A potential molecular target for such delivery is E-selectin, an endothelial-specific cell surface molecule expressed at sites of activation in vivo and inducible in cultured human umbilical vein endothelial cells (HUVEC) by treatment with cytokines such as recombinant human interleukin 1β (IL-1β). Liposomes of various types (classical, sterically stabilized, cationic, pH-sensitive), each conjugated with mAb H18/7, a murine monoclonal antibody that recognizes the extracellular domain of E-selectin, bound selectively and specifically to IL-1β-activated HUVEC at levels up to 275-fold higher than to unactivated HUVEC. E-selectin-targeted immunoliposomes appeared in acidic, perinuclear vesicles 2–4 hr after binding to the cell surface, consistent with internalization via the endosome/lysosome pathway. Activated HUVEC incubated with E-selectin-targeted immunoliposomes, loaded with the cytotoxic agent doxorubicin, exhibited significantly decreased cell survival, whereas unactivated HUVEC were unaffected by such treatment. These results demonstrate the feasibility of exploiting cell surface activation markers for the endothelial-selective delivery of biologically active agents via immunoliposomes. Application of this targeting approach in vivo may lead to novel therapeutic strategies in the treatment of cardiovascular disease.
Keywords: cytokine activation, drug delivery, E-selectin, immunoliposome, vascular endothelium
The endothelial lining of the cardiovascular system undergoes phenotypic modulation to an activated state in acute and chronic inflammation, injury and repair, angiogenesis, and atherogenesis (1). This localized process of endothelial activation is marked, in part, by increased cell surface expression of different types of adhesive glycoproteins, including E-selectin (ELAM-1), vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1) (2). Immunohistochemical studies in humans and animals have demonstrated characteristic spatial and temporal patterns of expression of these molecules in different pathophysiologic settings (3–5). Cultured human umbilical vein endothelial cells (HUVEC) activated by pro-inflammatory stimuli, such as the cytokine interleukin 1β (IL-1β), also show increased expression of ELAM-1, VCAM-1, and ICAM-1 in temporally well-characterized patterns, thus providing a useful in vitro model system for cell biological studies (6, 7).
Of these molecular markers, ELAM-1 exhibits the most distinct activation-dependent and endothelial-selective pattern of expression. In vivo ELAM-1 is not detectable in normal vessels, but in response to local inflammatory stimuli appears rapidly in endothelial cells (EC) in spatially circumscribed patterns (2, 3). Cultured HUVEC exhibit a similar pattern of inducible expression; whereas ELAM-1 is not detectable on unactivated cells, treatment with inflammatory cytokines results in abundant cell surface expression in 4–6 hr (7). Because of its highly activation-dependent pattern of induction, ELAM-1 may provide a useful molecular target for the site-specific delivery of agents to distinct regions within the cardiovascular system. Previous studies in this laboratory have demonstrated that hirudin, a potent thrombin inhibitor, can be selectively targeted to activated HUVEC by conjugation to the anti-ELAM-1 murine mAb H18/7, resulting in efficient antagonism of both cell surface and intracellular thrombin-mediated events (8).
Targeted liposomes [lipid vesicles bearing covalently conjugated mAb (immunoliposomes) or another targeting moiety (e.g., a specific peptide or lipid)] have several advantages over simple mAb–drug conjugates for specific drug delivery (9–11). Liposomes can contain a wide variety of both hydrophilic and hydrophobic diagnostic or therapeutic agents, provide a larger drug payload per particle, protect encapsulated agents from metabolic processes, and allow a high degree of cooperative binding to target cell antigens. In addition, the lipid composition of the bilayer can be modified to obtain other desirable properties, including prolonged circulatory half-life, the ability to complex with nucleic acids to mediate gene delivery or genetic regulation, and the capacity to deliver encapsulated contents to the cytosol through the endosome/lysosome pathway (12–14). Classical liposomes, having a lipid composition closely resembling the outer monolayer of the plasma membrane, do not destabilize under physiological conditions, but are rapidly cleared from the peripheral circulation by the reticuloendothelial system. Sterically stabilized liposomes contain lipids that provide a steric barrier to opsonization and eventual uptake by the reticuloendothelial system, and thus have a prolonged circulatory half-life. Cationic liposomes contain a lipid that is cationic under physiological conditions and are capable of condensing and carrying relatively large amounts of nucleic acid for delivery to target cells. Finally, pH-sensitive liposomes are composed of lipids that form a stable lipid bilayer at neutral or basic pH but destabilize at acidic pH. Liposome destabilization in an acidifying endosome may facilitate the delivery of liposomal contents to the target cell cytosol.
In this study, we explore the potential of using immunoliposomes of various chemical compositions, each targeted to ELAM-1, to deliver both fluorescent probes and toxic compounds to intracellular compartments of cultured HUVEC in an activation-dependent manner. Our results establish the feasibility of this strategy for EC-selective targeting, and suggest that this approach may be useful for the site-selective delivery of drugs, genes, and other agents within the cardiovascular system.
MATERIALS AND METHODS
Cell Culture.
HUVEC were isolated as described (15) and cultured in medium 199 (BioWhitaker) supplemented with 20% fetal calf serum (Summit Biotechnologies, Ft. Collins, CO), 2 mM l-glutamine, 100 mg/ml heparin (grade 1; Sigma), 50 mg/ml endothelial mitogen (Biomedical Technologies, Stoughton, MA), and penicillin/streptomycin. Passage 2 cells were plated on 6- and 96- well culture plates coated with 1.0% gelatin, for liposome binding and doxorubicin delivery studies, or on 0.1% gelatin-coated glass coverslips for microscopy, and used at 70–100% confluency unless stated otherwise.
Immunoassay of Cell Surface ELAM-1 Expression.
HUVEC cultured in 96-well plates were activated with recombinant human IL-1β (10 units/ml; Biogen), or sham-treated for 0–24 hr at 37°C, and cell surface ELAM-1 protein was measured in a two-step immunobinding assay, utilizing a saturating concentration of murine mAb H18/7 (20 μg/ml) and a fluorescein isothiocyanate-conjugated goat anti-murine antibody (15 μg/ml; Caltag, Burlingame, CA), in a fluorescent plate reader, as described (7).
Liposome Preparation.
Dioleoylphosphatidylcholine (DOPC), dioleoyl-phosphatidylethanolamine (DOPE), dioleoylphosphatidylethanolamine-N-[4-(maleimidophenyl) butyrate] (MPB-PE), disteroylphosphatidylethanolamine-polyethylene glycol-2000 (PEG), and cholesterol were purchased from Avanti Polar Lipids; cholesteryl hemisuccinate (CHEMS) from Sigma; cholesteryl 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoate (Chol-BODIPY), 8-hydroxypyrene-1,3,6-trisulfonate (HPTS), and p-xylene-bispyridinium bromide (DPX) from Molecular Probes; polycarbonate membranes from Poretics (Livermore, CA); and extruders from Avestin (Ottawa, Canada).
Liposomes were prepared essentially as described (16). Briefly, lipids in chloroform were combined at the following ratios: classical liposomes, 62.5:35:2.5 mol% DOPC/Chol/MPB-PE; sterically stabilized liposomes, 57.5:35:5:2.5 mol% DOPC/Chol/PEG/MPB-PE; cationic liposomes, 20:27.5:50:2.5 mol% DOTAP/DOPC/DOPE/MPB-PE; pH-sensitive liposomes, 65.4:32.1:2.5 mol% DOPE/CHEMS/MPB-PE. Liposomes used in binding studies were intrinsically labeled with 0.5–1.0 mol% Chol-BODIPY. Chloroform was removed under an argon stream and the lipids were lyophilized from spectral grade cyclohexane. The lipid mixtures were hydrated in 10 mM Hepes, 150 mM NaCl, and 0.1 mM EDTA (HBSE buffer) for binding studies, or in either 2 mM Hepes, 40 mM HPTS, 40 mM NaCl, and 0.1 mM EDTA or 2 mM Hepes, 40 mM HPTS, 40 mM DPX, and 0.1 mM EDTA for internalization studies. All hydrating solutions were prepared at pH 7.5 and ≈290 mmol/kg osmolality. Hydrated lipids were frozen and thawed four times, then extruded for 15 cycles through two stacked 0.08 μm polycarbonate membranes using a hand-held extruder. Liposome diameter was determined by quasi-elastic light scattering with an N4 Plus Submicron Particle Sizer (Coulter), and ranged from 80 to 180 nm.
Antibody Conjugation.
Murine mAb H18/7, an IgG2α antibody that recognizes the extracellular portion of human ELAM-1 and does not crossreact with other selectins (7), and murine mAb Rb1/9, an IgG1 antibody to rabbit VCAM-1 that does not crossreact with human VCAM-1 or other HUVEC antigens (4), were purified from hybridoma ascites. Purified murine pooled serum IgG2α was purchased from Chemicon).
Antibodies were chemically modified as described (17). Purified immunoglobulins were incubated with succinimidyl-S-acetylthioacetate (SATA) [solubilized in dimethylformamide at 1:10 mol and 100:1 volume ratios (antibody/SATA)] for 30 min at room temperature, dialyzed against 50–100 volumes of HBSE buffer (two changes), deacetylated with a 10% (vol/vol) addition of 50 mM sodium phosphate, 25 mM EDTA, and 0.5 M hydroxylamine (pH 7.5) for 2 hr, and then immediately added to liposomes. The liposome/antibody mixture was gently shaken at 4°C overnight, and immunoliposomes were separated from free antibody and deacetylation solution by chromatography (Sepharose CL-4B; 1 × 20 cm; HBSE buffer). Lipid mass was determined by phosphate assay (18, 19) or from intrinsic fluorescent label content; protein content was quantified by SDS/PAGE. The estimated antibody molecules per liposome ranged from 35 to 120, and were comparable among different immunoliposome types in a given experiment.
Preparation of Doxorubicin-Loaded Liposomes.
pH-sensitive liposomes were prepared as above, with the following modifications. Lipids were hydrated in 120 mM ammonium sulfate. After extrusion, liposomes were desalted in HBSE buffer by chromatography on Sepharose CL-4B, loaded with doxorubicin HCl (50–200 μg/ml, 98% pure; Sigma) as described (20, 21), and then conjugated with antibody. Doxorubicin concentration was determined spectrofluorometrically (ex. 470 nm; em. 590 nm), and lipid concentration by phosphate assay. Liposomes more than 1 week old were rechromatographed to rule out doxorubicin leakage prior to use in cell culture.
Liposome Binding Assay.
Activated (IL-1β, 10 units/ml, 4 hr, 37°C) or unactivated (sham-treated) HUVEC monolayers were incubated with fluorescently labeled liposomes (100 μM lipid) for 2 hr on ice, washed four times with fresh medium, detached with a trypsin/versene mixture (BioWhitaker), pelleted, and resuspended in fresh medium. Liposome binding was analyzed by flow cytometry (488 nm excitation, 520 nm bandpass/cutoff; Coulter EPICS Flow Cytometer). For competitive inhibition experiments, activated HUVEC were incubated with a saturating concentration of free mAb (50 mg/ml) for 30 min prior to incubation with liposomes. The mean fluorescence intensity (fluorescence per cell, in arbitrary units; MFI) of aliquots of 10 × 103 cells was determined for each incubation condition.
Intravital Microscopy of Immunoliposome Fate.
Activated and sham-treated HUVEC monolayers, on glass coverslips, were incubated for 1 hr on ice with classical or pH-sensitive liposomes (40 μM lipid) bearing mAb H18/7, mAb Rb1/9, or no mAb, and loaded with HPTS, a membrane-impermeant, pH-dependent fluorophore (22, 23); the coverslips were washed four times with fresh medium at 37°C and examined by phase contrast and fluorescence microscopy with a Zeiss inverted microscope, equipped with an image analysis system (Universal Imaging, West Chester, PA) (24). Liposomes loaded with both HPTS and DPX were used in some studies.
Incubation of HUVEC with Doxorubicin-Loaded Liposomes.
Comparable amounts of doxorubicin (6 nmol/well; 1.5 ml volume), either in free solution or encapsulated in pH-sensitive liposomes bearing mAb H18/7, mAb Rb1/9, or no mAb, were added to activated or sham-treated HUVEC plated at subconfluent (20–50%) or confluent densities. After incubation for 17–22 hr at 37°C, the supernatant was removed, the monolayers washed, and fresh culture medium added. The number of adherent (viable) cells per well was determined at various times after initial drug exposure by resuspension in trypsin-EDTA and direct microscopic counting in a hemocytometer and/or by flow cytometry.
RESULTS
ELAM-1-Targeted Immunoliposome Binding to HUVEC Is Activation-Dependent and Immunospecific.
Consistent with previously published data (6), unactivated HUVEC monolayers displayed essentially undetectable levels of ELAM-1 protein at the cell surface, as determined by mAb H18/7 binding. Upon stimulation with IL-1β, ELAM-1 expression was rapidly up-regulated, with maximal surface protein levels observed at 4–6 hr, followed by a decline toward baseline by 24 hr (Fig. 1).
Figure 1.
Fluorescence immunoassay of cell surface ELAM-1 expression on IL-1β-activated (□) and sham-treated (⧫) HUVEC monolayers. Each data point represents mean values from 8 microtiter wells. The SEM was <2.5% for all data points.
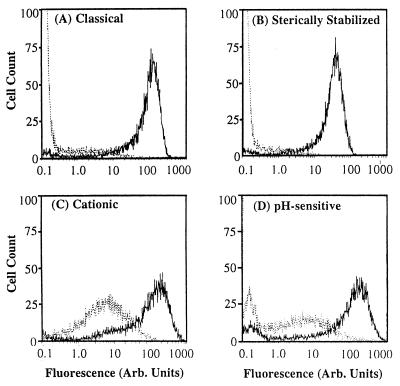
Fluorescently labeled classical, sterically stabilized, cationic, and pH-sensitive immunoliposomes, each bearing the mAb H18/7 (L-H18/7), bound to activated HUVEC at levels 13- to 275-fold higher than to unactivated HUVEC (Fig. 2; Table 1). Classical L-H18/7 displayed the highest ratio of activation-dependent binding (275:1, activated/unactivated), whereas cationic L-H18/7 showed the lowest ratio (13:1). Classical and sterically stabilized L-H18/7 both showed negligible binding (MFIs ≪ 1.0) to unactivated HUVEC (Fig. 2 A and B). In contrast, cationic and pH-sensitive L-H18/7 bound to unactivated HUVEC at low but detectable levels (MFIs of approximately 5.0 and 1.7, respectively) (Fig. 2 C and D).
Figure 2.
Flow cytometry data demonstrating activation-dependent targeting of fluorescently labeled (A) classical, (B) sterically stabilized, (C) cationic, and (D) pH-sensitive liposomes conjugated with H18/7, to 4 hr IL-1β activated (solid line) vs. unactivated (dotted line) HUVEC. Data are from a single experiment and are representative of at least four such experiments.
Table 1.
Immunoliposome binding to activated and unactivated HUVEC
| Immunoliposome | L-H18/7 binding
|
L-Rb1/9 binding
|
||||
|---|---|---|---|---|---|---|
| Activated | Unactivated | Ratio | Activated | Unactivated | Ratio | |
| Classical | 60.6 | 0.22 | 275 | 0.45 | 0.12 | 3.8 |
| Sterically stabilized | 24.7 | 0.17 | 145 | 0.25 | 0.12 | 2.1 |
| Cationic | 63.0 | 5.01 | 12.6 | 2.78 | 3.27 | 0.9 |
| pH-sensitive | 40.0 | 1.65 | 24.2 | 1.40 | 0.85 | 1.6 |
MFI values by flow cytometry are from one experiment and are representative of four such experiments.
To test the molecular target specificity of immunoliposome binding to activated HUVEC, parallel studies were conducted with classical, sterically stabilized, cationic and pH-sensitive immunoliposomes bearing mAb Rb1/9 (L-Rb1/9), a murine mAb that does not recognize antigens on unactivated or activated HUVEC. Each type of L-Rb1/9 bound comparably, at negligible to low levels, to both activated and unactivated HUVEC (Fig. 3; Table 1). Liposomes of each type bearing no mAb also bound at negligible levels (Fig. 3), as did liposomes bearing purified murine serum IgG2α, the same immunoglobulin isotype as anti-ELAM-1 mAb H18/7 (data not shown). To confirm that immunoliposome binding was target antigen-specific, activated HUVEC monolayers were pretreated for 30 min with a saturating concentration of free mAb H18/7. For classical, sterically stabilized, and pH-sensitive L-H18/7, this pretreatment abrogated activation-dependent binding to HUVEC (Fig. 3 A, B, and D). In the case of cationic L-H18/7, a significant degree of residual binding (≈33%) was observed, possibly reflecting the propensity of these charged particles for nonspecific interactions with proteins (such as free mAb) or components of the activated HUVEC surface.
Figure 3.
Flow cytometry data demonstrating molecular target specificity of binding of fluorescently labeled (A) classical, (B) sterically stabilized, (C) cationic, and (D) pH-sensitive liposomes conjugated with no mAb (Lp), mAb Rb1/9 (Lp-Rb1/9), mAb H18/7 (Lp-H18/7), or to 4-hr IL-1β-activated (filled bars) vs. unactivated (open bars) HUVEC. ∗∗, Preincubation with excess (50 μg/ml) mAb H18/7. SEM < 2.5% for all data bars. MFI values are plotted from a single experiment and are representative of four such experiments.
ELAM-1-Targeted Immunoliposomes Are Internalized via an Endocytic Pathway.
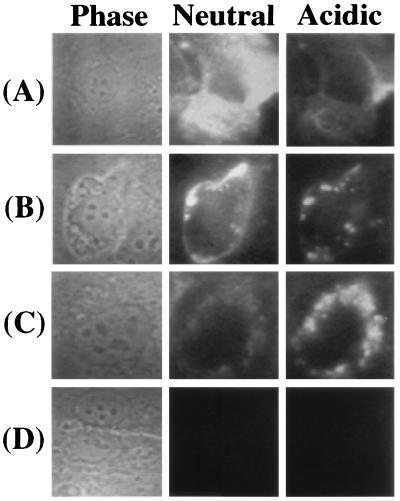
To trace the fate of immunoliposomes during their interaction with activated HUVEC, classical and pH-sensitive L-H18/7 were loaded with HPTS, a membrane-impermeant, pH-dependent fluorophore, that emits maximally (em = 510 nm) at excitation wavelengths of ≈405 nm at acidic pH and ≈440 nm at neutral pH. Comparing the relative emission intensities at these two excitation wavelengths thus reveals whether the dye is in an acidic or neutral environment (22, 23). After incubating classical, HPTS-loaded L-H18/7 with activated HUVEC for 1 hr on ice and then rinsing, a diffuse pattern of fluorescence was visible at ex = 440 nm (neutral pH maximum), presumably indicating a large number of cell surface-bound immuno-liposomes (Fig. 4A). Two hours after warming the cells to 37°C, a significant amount of HPTS dye appeared as punctate spots, which had significant fluorescence at ex = 405 nm, consistent with intracellular uptake into early and late endosomes (Fig. 4B). At the same time, some HPTS dye was still visualized in a diffuse pattern at neutral pH. Four hours after warming to 37°C, most of the HPTS dye appeared ex = 405 nm (low pH maximum) in punctate, perinuclear bodies, suggesting that immuno-liposomal entrapment and/or degradation was occurring in lysosomes (Fig. 4C). Little HPTS dye was detectable at ex = 440 nm (neutral pH) at this time.
Figure 4.
Digitized images of classical liposomes conjugated with mAb-H18/7, loaded with the pH-dependent fluorophore HPTS, interacting with HUVEC. Rows A, B, and C contain images of the same field of activated HUVEC, viewed by phase contrast microscopy (Phase) or fluorescence microscopy at either ex = 440 nm (Neutral) or ex = 405 nm (Acidic), recorded after 0, 2, or 4 hr of incubation at 37°C, respectively (following 1 hr liposome preincubation at 4°C and washing). Row D depicts HPTS-loaded Lp-H18/7 interacting with unactivated HUVEC after 2 hr. These results are representative of at least four such experiments.
When activated HUVEC were treated with NH4Cl, which elevates the pH of endocytic compartments, HPTS dye delivered by classical L-H18/7 appeared in large, round, neutral compartments up to 4 hr after incubation at 37°C (data not shown). HPTS-loaded, classical L-H18/7 incubated with unactivated HUVEC showed no detectable surface binding or dye delivery (Fig. 4D). Similar results were obtained for classical or pH-sensitive L-Rb1/9 and unconjugated control liposomes incubated with activated HUVEC, and for classical L-H18/7 incubated with activated HUVEC pretreated with excess mAb H18/7 (data not shown).
Recent reports suggest that pH-sensitive liposomes can enhance the delivery of encapsulated agents to the cytosol of certain cell types (13, 14). To determine whether this was the case in this cultured endothelium model system, we conducted studies similar to those above, using HPTS-loaded, pH-sensitive L-H18/7. As was the case with classical L-H18/7, HPTS dye delivered by pH-sensitive L-H18/7 was detectable primarily at an excitation wavelength of 405 nm (low pH maximum) in the perinuclear vesicles of targeted HUVEC 4–9 hr after warming to 37°C. In control experiments, free HPTS microinjected directly into the cytosol of activated HUVEC accumulated in the cell nucleus within 15 min and fluoresced brightly at 440 nm excitation (data not shown). In contrast, the HPTS dye delivered to activated HUVEC by either classical or pH-sensitive L-H18/7 showed no appreciable nuclear accumulation.
To determine whether classical or pH-sensitive L-H18/7 underwent significant degradation and/or leakage of their contents during the process of internalization, we conducted studies, utilizing L-H18/7 loaded with both HPTS and DPX (a concentration-dependent, diffusible fluorescence quenching agent) (22, 24). Neither classical nor pH-sensitive L-H18/7 showed evidence of detectable dye leakage when bound at the cell surface. Degradation of both types of liposomes was first evident at 2–4 hr and appeared to be occurring in slightly acidic intracellular compartments (as indicated by comparable HPTS fluorescence at ex = 405 nm and 440 nm). Maximal HPTS fluorescence was observed after 4–9 hr incubation at 37°C.
Doxorubicin-Loaded, ELAM-1-Targeted Immunoliposomes Reduce HUVEC Survival in an Activation-Dependent, Immunospecific Manner.
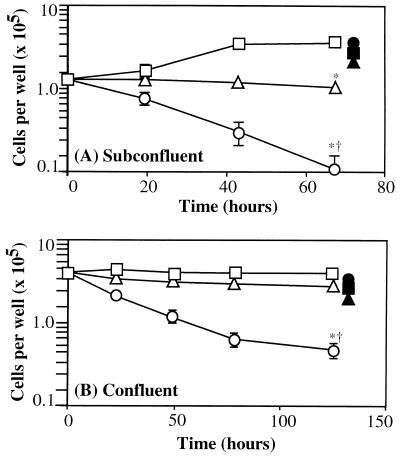
Doxorubicin is a membrane-perturbing, DNA-intercalating agent that can mediate a variety of cytotoxic actions on target cells (25, 26). We compared the effects of equivalent doses of doxorubicin (4 μM), added to subconfluent (actively growing) and confluent HUVEC monolayers, as either the free compound or encapsulated in pH-sensitive liposomes conjugated with mAb H18/7 (Fig. 5). Free doxorubicin significantly inhibited cell replication in actively growing, subconfluent, IL-1β-activated HUVEC cultures (Fig. 5A), essentially blocking viable cell number increase over a 72-hr interval (>3.3-fold fewer cells relative to control medium, P < 0.003). This cytostatic effect was independent of HUVEC activation state (data not shown). In contrast, pH-sensitive L-H18/7 loaded with a comparable amount of doxorubicin induced a marked decrease in cell viability over the same 72-hr interval (32-fold decrease relative to control medium, P < 0.002; 9.4-fold decrease relative to free doxorubicin, P < 0.001). However, the same immunoliposome preparation incubated with unactivated, subconfluent HUVEC resulted in neither cytostatic nor cytotoxic effects (94% cell survival, relative to control medium). Nontargeted, unconjugated, or conjugated with mAb Rb1/9 pH-sensitive liposomes loaded with doxorubicin, as well as ELAM-1-targeted liposomes not loaded with doxorubicin, failed to have any significant cytostatic or cytotoxic effect on activated or unactivated subconfluent HUVEC (Fig. 5A; data not shown).
Figure 5.
Cell survival in (A) subconfluent and (B) confluent, activated HUVEC monolayers following no treatment (□) or exposure to comparable doses (4 μM) of free doxorubicin (▵) or pH-sensitive liposome-encapsulated doxorubicin [ELAM-1-targeted = H18/7-conjugated (○), nontargeted = unconjugated liposomes (▴), or Rb1/9-conjugated liposomes (▪)]. •, Doxorubicin-loaded H18/7-conjugated liposomes interacting with unactivated HUVEC monolayers. Note markedly reduced cell survival resulting from ELAM-1-targeted drug delivery and the protective effect of nontargeted drug delivery. ∗, Statistical significance (P < 0.005) compared with untreated cultures; †, Statistical significance (P < 0.005) compared with free doxorubicin-treated HUVEC; P values determined by Student’s t test. Results are means ± SD of three determinations from one experiment and are representative of three subconfluent and two confluent experiments.
In HUVEC monolayers plated at confluency (Fig. 5B), free doxorubicin had a less dramatic effect on cell viability (70% cell survival relative to control medium). In contrast, ELAM-1 targeting of doxorubicin-loaded pH-sensitive liposomes resulted in a marked decrease in cell survival (8.25-fold decrease relative to control medium, P < 0.0005; 5.8-fold decrease relative to free doxorubicin, P < 0.0005). Unactivated confluent HUVEC monolayers incubated with doxorubicin-loaded liposomes conjugated with mAb H18/7 showed negligible loss of cell viability (86% cell survival; Fig. 5B). As was observed with subconfluent HUVEC, nontargeted doxorubicin-loaded, pH-sensitive liposomes (unconjugated or conjugated with mAb Rb1/9), as well as ELAM-1-targeted liposomes not loaded with doxorubicin, did not mediate any consistent cytostatic or cytotoxic effects on activated HUVEC (Fig. 5B; data not shown).
DISCUSSION
In this study, we demonstrate the feasibility of selectively targeting immunoliposomes and their contents to vascular EC based on a defined cell surface molecular marker of activation. Various types of immunoliposomes, each bearing the anti-ELAM-1 mAb H18/7, bound to activated cultured HUVEC at levels 13- to 275-fold higher than to unactivated HUVEC. In our in vitro experimental model system, this marked differential selectivity reflects the strictly activation-dependent pattern induced by a recombinant cytokine stimulus, IL-1β (7). In the in vivo setting, ELAM-1 expression also appears to be highly regulated, both temporally and spatially, and is associated primarily with inflammatory and immune processes (2, 3, 5) and also certain noninflammatory angiogenic states (27). In addition, ELAM-1 expression appears to be restricted to vascular endothelium, thus providing an endothelial-specific marker. In contrast, other cell surface molecules, such as ICAM-1, are constitutively expressed on both quiescent vascular endothelium and other cell types. A recent in vitro study demonstrated immunoliposome targeting to ICAM-1-expressing cells in culture, such as bronchial epithelium and endothelium, at significantly higher lipid concentrations than used in the present study (28). Given its endothelial specificity and marked inducibility, ELAM-1 may provide a more practical molecular target for in vivo applications in the cardiovascular system.
The variations in binding to activated and unactivated HUVEC observed with the different types of immunoliposomes most likely reflect their differing lipid compositions. Classical ELAM-1-targeted immunoliposomes bound to activated cells at relatively high levels and showed negligible nonspecific binding to unactivated cells. The sterically stabilized liposomes used in this study contain a polyethylene glycol-modified lipid intended to sterically hinder opsonization and phagocytosis by the reticuloendothelial system (29). Such liposomes exhibit a dramatically prolonged circulatory half-life in vivo (30). This modified lipid may also have sterically hindered antibody-mediated binding to HUVEC. ELAM-1-targeted, sterically stabilized liposome binding to activated HUVEC was consistently lower than classical immunoliposome binding. However, the extremely low levels of nonspecific binding of these sterically stabilized liposomes resulted in a 145-fold activation-dependent binding ratio. The cationic immunoliposomes had a relatively high level of nonspecific binding due to interactions with the anionic cell surface, as reported previously (14). Finally, ELAM-1-targeted pH-sensitive liposomes did not exhibit as high an activation-dependent binding ratio as classical or sterically stabilized liposomes. This primarily reflects their higher nonspecific binding to unactivated HUVEC. The reasons for this are unclear, but might reflect the propensity of pH-sensitive liposomes to coalesce, perhaps as a result of their relatively high phosphatidylethanolamine content. Despite these differences, each formulation of immunoliposome tested showed activation-dependent, antigen-specific targeting, and therefore may have potential application in vivo.
Previous studies have demonstrated that liposomes bound to the cell surface are often internalized, via an endocytic pathway, into lysosomes (31). Using a pH-dependent, membrane-impermeant fluorophore (HPTS) as a fluid phase marker of liposomal contents, we observed that both classical and pH-sensitive immuno-liposomes were internalized by activated HUVEC via an endocytic pathway, and that their contents were delivered primarily to acidifying endosomes and lysosomes. This is in agreement with previous studies in which very little liposome-encapsulated material was found in endocytic vesicles (13, 14, 22, 23, 31–33). However, these morphological studies do not preclude the possibility that some liposomal content was delivered into cytosolic or nuclear compartments. Interestingly, the kinetics of this uptake process in cultured EC appeared to be quite slow compared with other cell types (e.g., macrophages, tumor cell lines) in culture (22–24, 31–34).
To extend these studies to a biological end-point, we exposed both activated and unactivated HUVEC to comparable amounts of doxorubicin, a potent cytotoxic agent, either free in culture medium or encapsulated in immunoliposomes. Doxorubicin-loaded L-H18/7 efficiently targeted activated HUVEC, resulting in significant cytostatic and/or cytotoxic effects. Under conditions where free doxorubicin had a marked cytostatic action (in actively growing, subconfluent cultures), encapsulation of doxorubicin in nontargeted liposomes had a protective effect. Conversely, encapsulation of doxorubicin in targeted (H18/7-bearing) pH-sensitive liposomes increased cytotoxicity significantly. This increased efficacy presumably indicates immunoliposome-mediated drug uptake into HUVEC. Although we have not explored the details of this increased drug delivery, it is likely that uptake is occurring into various intracellular compartments, including the nucleus. These results with doxorubicin-loaded immunoliposomes illustrate the potential for simultaneously protecting nontargeted cells from a toxic drug while enhancing its action on a targeted cell population.
Inducible cell surface glycoproteins in addition to ELAM-1 can serve as markers of the activated and/or dysfunctional endothelial phenotype. For example, detailed studies in experimental animal models have revealed that the expression of VCAM-1 (ATHERO-ELAM, atherosclerosis associated-ELAM) is among the earliest detectable molecular changes in EC in the vicinity of a developing atheromatous plaque (4). Radiolabeled mAb directed against rabbit VCAM-1, infused into Watanabe (low density lipoprotein-receptor-deficient, atherosclerosis-prone) rabbits localize selectively and specifically (compared with isotypic matched, nonbinding control mAb) at sites of early lesion formation (M.I.C. and M.A.G., unpublished observations). Thus, VCAM-1, or an analogous human ATHERO-ELAM, would be a potential molecular target for immunoliposomal intervention in atherosclerosis. In addition to activation-dependent phenotypic markers, there is increasing evidence that EC in different regions of the vasculature can be distinguished by the constitutive expression of tissue-specific cell surface antigens (35). A previous study demonstrated selective immunoliposome binding to pulmonary EC and lung targeting in vivo (36). Recent reports have also described the selective expression of certain integrin receptors by the EC in tumor vessels and other neovascular disease settings (37, 38). Strategies to recognize and exploit such markers for selective liposomal targeting purposes need not be limited to monoclonal antibodies but might also involve cognate ligands (39), and conceivably could encompass gene delivery (40).
In conclusion, vascular endothelium serves as an extensive interface between circulating blood and various tissues and organs of the body. As such, it offers an accessible target for blood-borne pharmacological and genetic manipulations that can mediate both local and systemic effects. Thus, immunotargeting of liposomes to activated vascular EC may provide a strategy for site-selective delivery in the cardiovascular system with broad therapeutic applicability.
Acknowledgments
We thank W. Atkinson and K. Case for assistance in tissue culture, J. McGrath for cell microinjection, M. Stevenson for liposome preparation, and J.-M. Kiely, J. Topper, R. Padgett, and F. W. Luscinskas for helpful discussions. This research was supported in part by National Institutes of Health Grants P01-HL48743 and PO1-HL36028. D.D.S. was a recipient of a medical student research fellowship from The Stanley J. Sarnoff Endowment for Cardiovascular Science, Inc. M.A.G. is a recipient of an unrestricted research grant for cardiovascular research from the Bristol–Myers Squibb Research Institute. Monoclonal antibody H18/7 is deposited with the American Type Culture Collection (ATCC HB11684).
ABBREVIATIONS
- EC
endothelial cells
- ELAM-1
E-selectin
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- IL-1β
interleukin 1β
- L
liposome
- L-H18/7
mAb H18/7-conjugated immunoliposome
- L-Rb1/9
mAb Rb1/9-conjugated immunoliposome
- MFI
mean fluorescence intensity
- VCAM-1
vascular cell adhesion molecule 1
- HPTS
8-hydroxypyrene-1,3,6-trisulfonate
- DPX
p-xylene-bispyridinium bromide
References
- 1.Gimbrone M A., Jr . In: Molecular Cardiovascular Medicine. Haber E, editor. New York: Scientific American Medicine; 1995. pp. 49–61. [Google Scholar]
- 2.Pober J S, Cotran R S. Physiol Rev. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 3.Cotran R S, Gimbrone M A, Jr, Bevilacqua M P, Mendrick D L, Pober J S. J Exp Med. 1986;164:661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cybulsky M I, Gimbrone M A., Jr Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 5.Briscoe D M, Schoen F J, Rice G E, Bevilacqua M P, Ganz P, Pober J S. Transplantation. 1991;51:537–547. [PubMed] [Google Scholar]
- 6.Pober J S, Bevilacqua M P, Mendrick D L, Lapierre L A, Fiers W, Gimbrone M A., Jr J Immunol. 1986;136:1680–1687. [PubMed] [Google Scholar]
- 7.Bevilacqua M P, Pober J S, Mendrick D L, Cotran R S, Gimbrone M A., Jr Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiely J M, Cybulsky M I, Luscinskas F W, Gimbrone M A., Jr Arterioscler Thromb Vasc Biol. 1995;15:1211–1218. doi: 10.1161/01.atv.15.8.1211. [DOI] [PubMed] [Google Scholar]
- 9.Heath T D, Fraley R T, Papahadjopoulos D. Science. 1980;210:539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- 10.Soriano P, Dijkstra J, Legrand A, Spanjer H, Londos-Gagliardi D, Roerdink R, Scherphof G, Nicolau C. Proc Natl Acad Sci USA. 1983;80:7128–7131. doi: 10.1073/pnas.80.23.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moradpour D, Compagnon B, Wilson B E, Nicolau C, Wands J R. Hepatology. 1995;22:1527–1537. [PubMed] [Google Scholar]
- 12.Lasic D D, Papahadjopoulos D. Science. 1995;267:1275–1276. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- 13.Straubinger R M. Methods Enzymol. 1993;221:361–376. doi: 10.1016/0076-6879(93)21030-c. [DOI] [PubMed] [Google Scholar]
- 14.Bentz J, Alford D, Ellens H. In: The Structure of Biological Membranes. Yeagle P, editor. Boca Raton, FL: CRC; 1992. pp. 915–947. [Google Scholar]
- 15.Gimbrone M A., Jr Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- 16.Compagnon B, Moradpour D, Alford D R, Larsen C E, Stevenson M J, Mohr L, Wands J R, Nicolau C. J Liposome Res. 1997;7:127–141. [Google Scholar]
- 17.Weston P D, Devries J A, Wrigglesworth R. Biochim Biophys Acta. 1980;612:40–49. doi: 10.1016/0005-2744(80)90276-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen P S, Jr, Toribara T Y, Warner H. Anal Chem. 1956;28:1756–8. [Google Scholar]
- 19.Morrison W R. Anal Biochem. 1964;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- 20.Haran G, Cohen R, Bar L K, Barenholz Y. Biochim Biophys Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz A T, Barenholz Y, Gabizon A A. Biochim Biophys Acta. 1992;1109:203–209. doi: 10.1016/0005-2736(92)90084-y. [DOI] [PubMed] [Google Scholar]
- 22.Daleke D L, Hong K, Papahadjopoulos D. Biochim Biophys Acta. 1990;1024:352–366. doi: 10.1016/0005-2736(90)90365-u. [DOI] [PubMed] [Google Scholar]
- 23.Straubinger R M, Papahadjopoulos D, Hong K L. Biochemistry. 1990;29:4929–4939. doi: 10.1021/bi00472a025. [DOI] [PubMed] [Google Scholar]
- 24.Lee K-D, Oh Y K, Portnoy D A, Swanson J A. J Biol Chem. 1996;271:7249–7252. [PubMed] [Google Scholar]
- 25.Tritton T R. In: Apoptosis: The Molecular Basis of Cell Death. Tomei L D, Cope F O, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1991. pp. 121–137. [Google Scholar]
- 26.Tritton T R. Pharmacol Ther. 1991;49:293–309. doi: 10.1016/0163-7258(91)90060-y. [DOI] [PubMed] [Google Scholar]
- 27.Kraling B M, Razon M J, Boon L M, Zurakowski D, Seachord C, Darveau R P, Mulliken J B, Corless C L, Bischoff J. Am J Pathol. 1996;148:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- 28.Bloemen P G, Henricks P A, van Bloois L, van den Tweel M C, Bloem A C, Nijkamp F P, Crommelin D J, Storm G. FEBS Lett. 1995;357:140–144. doi: 10.1016/0014-5793(94)01350-a. [DOI] [PubMed] [Google Scholar]
- 29.Lasic D D, Martin F J, Gabizon A, Huang S K, Papahadjopoulos D. Biochim Biophys Acta. 1991;1070:187–192. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- 30.Woodle M C, Matthay K K, Newman M S, Hidayat J E, Collins L R, Redemann C, Martin F J, Papahadjopoulos D. Biochim Biophys Acta. 1992;1105:193–200. doi: 10.1016/0005-2736(92)90194-q. [DOI] [PubMed] [Google Scholar]
- 31.Straubinger R M, Hong K, Friend D S, Papahadjopoulos D. Cell. 1983;32:1069–1079. doi: 10.1016/0092-8674(83)90291-x. [DOI] [PubMed] [Google Scholar]
- 32.Cudd A, Nicolau C. Biochim Biophys Acta. 1985;845:477–491. doi: 10.1016/0167-4889(85)90214-9. [DOI] [PubMed] [Google Scholar]
- 33.Cudd A, Nicolau C. Biochim Biophys Acta. 1986;860:201–214. doi: 10.1016/0005-2736(86)90516-x. [DOI] [PubMed] [Google Scholar]
- 34.Lee K-D, Nir S, Papahadjopoulos D. Biochemistry. 1993;32:889–899. doi: 10.1021/bi00054a021. [DOI] [PubMed] [Google Scholar]
- 35.Tan X-Y, Schnitzer J E. Int J Microcirc Clin Exp. 1996;16:239. [Google Scholar]
- 36.Maruyama K, Kennel S J, Huang L. Proc Natl Acad Sci USA. 1990;87:5744–5748. doi: 10.1073/pnas.87.15.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedlander M F, Brooks P C, Schaffer R W, Kincaid K M, Varner J A, Cheresh D A. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 38.Friedlander M F, Theesfeld C L, Sugita M, Fruttiger M, Thomas M, Chang S, Cheresh D A. Proc Natl Acad Sci USA. 1996;93:9764–9. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kansas G S. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 40.Isner J M, Walsh K, Symes J, Pieczek A, Takeshita S, Lowry J, Rosenfield K, Weir L, Brogi E, Jurayj D. Hum Gene Ther. 1996;7:959–988. doi: 10.1089/hum.1996.7.8-959. [DOI] [PubMed] [Google Scholar]