Abstract
Previously, it was shown that the lack of a functional estrogen receptor (ER) α gene (ERα) greatly affects reproduction-related behaviors in both female and male mice. However, widespread expression of a novel second ER gene, ERβ, demanded that we examine the possible participation of ERβ in regulation of these behaviors. In dramatic contrast to our results with ERα knockout (αERKO) males, βERKO males performed at least as well as wild-type controls in sexual behavior tests. Moreover, not only did βERKO males exhibit normal male-typical aggressive behavior, including offensive attacks, but they also showed higher levels of aggression than wild-type mice under certain conditions of social experience. These data revealed a significant interaction between genotype and social experience with respect to aggressive behavior. Finally, females lacking a functional β isoform of the ER gene showed normal lordosis and courtship behaviors, extending in some cases beyond the day of behavioral estrus. These results highlight the importance of ERα for the normal expression of natural reproductive behaviors in both sexes and also provide a background for future studies evaluating ERβ gene contributions to other, nonreproductive behaviors.
Keywords: testosterone, progesterone, lordosis, sexual behavior, aggression
One of the most reliable phenomena in neuroendocrinology is the facilitation of the female reproductive behavior, lordosis, by estrogens (1). The neural circuitry for this behavior has been well defined (2). Estrogen binding to neurons in the ventromedial nucleus of the hypothalamus (VMH) is the first neuroendocrine step activating neural circuitry for the behavior (3). In particular, antiestrogens delivered directly to the VMH block the behavior, thus revealing the essential nature of this particular hormone action (4). The neurobiological effects of estrogen binding in brain were long conceived to depend exclusively on the classical estrogen receptor (ER) (now renamed as ERα). Indeed, we have demonstrated that the classical ERα gene is required for normal expression of a number of reproduction-related social behaviors in female mice regardless of their hormonal status (5, 6). ERα-deficient knockout (αERKO) female mice showed no sign of lordosis behavior, greatly reduced pup-caring behavior, and elevated levels of infanticide and aggression. These results suggest that the presence of ERβ, a novel ER, by itself may not be sufficient to compensate for behavioral changes caused by the lack of the ERα gene. However, widespread expression of ERβ in the central nervous system (7–9) led us to hypothesize that the presence of ERβ also may be important for normal performance of female reproductive behaviors. In the present study, we tested this possibility by the use of ERβ gene-specific KO (βERKO) mice (10, 11).
In the male, brain mechanisms underlying the regulation of reproductive behaviors by gonadal androgenic hormones have received much attention (reviewed in ref. 12). Notably, it is known that conversion of testosterone to estradiol, by a process referred to as aromatization, and eventual activation of brain ER plays an important role not only in male sexual behavior but also in male-typical aggressive behavior. Our previous studies demonstrated that the presence of the ERα gene is critically involved in the regulation of testosterone-dependent male reproductive behaviors. We have shown that αERKO males are infertile (13) and do not exhibit a normal profile of masculine sex behaviors (14, 15), and that these effects of ERα gene disruption are not simply caused by abnormal levels of circulating hormones (16). Particularly, αERKO showed reduced levels of intromission and virtually no ejaculation whereas they showed normal levels of mounts. Furthermore, testosterone-inducible aggressive behavior of αERKO was greatly reduced (14, 15). These results suggest that ERα activation is required for normal expression of male reproductive behavior, and the lack of ERα gene cannot be completely compensated for by the activation of androgen receptor or ERβ. As discussed above, however, possible contributions of ERβ gene expression in supporting the normal occurrence of these behaviors have not been explored. ERα and ERβ are known to form heterodimers in vitro (17), and ERα and ERβ colocalize in neurons (18) in the preoptic area and amygdala, brain areas known to be responsible for the regulation of male sexual and aggressive behaviors. Therefore, it is conceivable that the presence of ERβ gene is necessary for normal induction of these behaviors. In the present study, we also tested open-field activity of male βERKO mice because we found previously that αERKO male mice had significantly higher levels of locomotor activity than wild-type (WT) control mice (15).
Materials and Methods
Male Mice.
Gonadally intact male βERKO mice (n = 9) and their WT (n = 6) littermates from a mixed background of C57BL/6J and 129 (11) were used. They were obtained from the breeding colony maintained at the National Institute of Environmental Health Sciences. Upon arrival at the Rockefeller University (βERKO, 32.9 ± 1.1 weeks old; WT, 32.3 ± 1.5 weeks old), all mice were individually housed in plastic cages (30 × 20 × 13 cm) throughout the extent of the studies and maintained on a 12/12-h light/dark cycle (light off at noon) at constant temperature (22°C). Food and water were available ad libitum. They were tested for sexual and aggressive behaviors as well as open-field activity as shown in Fig. 1A. All tests were done during the dark phase of the light/dark cycle starting at 2 h after lights off. Sexual and aggressive behavioral tests were performed under red light and videotaped for further analysis. Open-field tests were done under white light.
Figure 1.
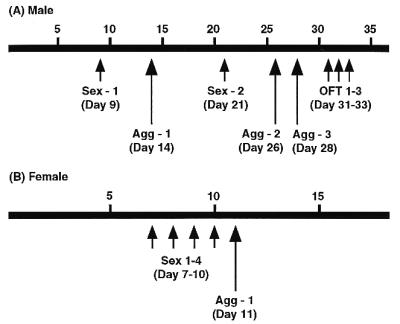
Schematic diagrams showing the experimental design. Time scale indicates the days after mice were singly housed. Sex, sexual behavior tests. Agg, aggressive behavioral test. OFT, open-field test.
Female Mice.
Gonadally intact βERKO (n = 7) and WT (n = 7) littermates from the same breeding colony as males were used. Starting 7 days before the first behavioral tests, they (βERKO, 16.1 ± 1.4 weeks old; WT, 15.4 ± 1.2 weeks old) were individually housed in plastic cages and maintained on a 12/12-h light/dark cycle (light off at 10 a.m.) at constant temperature (22°C). Food and water were available ad libitum. Mice were tested for female sexual and aggressive behavior as shown in Fig. 1B at the National Institute of Environmental Health Sciences. All tests were done under red light during the dark phase of the light/dark cycle starting at 2 h after lights off and videotaped for further analysis.
Male Sexual Behavior Test.
Male mice were tested twice (with an interval of 12 days; see Fig. 1A) for sexual behavior during a 30-min behavioral test with a Swiss–Webster (SW) female mouse [(SW)fBR purchased from Taconic Farms] in the male’s home cage. All females were ovariectomized and s.c. injected with 10 μg estradiol benzoate (48 h before the tests) and 500 μg progesterone (4–7 h before the tests) to ensure high sexual receptivity. For each male, the number of attempted mounts (including head mounts), the number and latency (1,800 sec was used for mice that did not show the behavior) of mounts, intromissions, and ejaculations were recorded. Numbers of thrusts per intromission and ejaculation duration also were recorded for mice that showed these behaviors. Male sexual behaviors were defined as described (19).
Male Aggressive Behavior Test.
Aggressive behaviors were tested three times (see Fig. 1A) in a resident-intruder paradigm. Each male was tested in his home cage (as a resident) against a group-housed (4–5 mice/cage) olfactory bulbectomized (OBX) male SW intruder mouse for 15 min. Expression of aggression in mice is regulated mainly by olfactory cues, and therefore OBX intruders rarely show aggression. However, because their gonads are intact, they can elicit aggressive behaviors from resident mice (20, 21). By testing against OBX intruder mice, the aggressive behaviors of resident animals, which were not influenced by any experience of defeat, were measured. An aggressive bout was defined as a continuous series of behavioral interactions including at least one aggressive behavioral act (see below). Three seconds was the maximum amount of time that could elapse between aggressive behavioral acts to be considered part of the same aggressive bout: if intervals between the occurrences of two aggressive behavioral acts exceeded 3 sec, the two behavioral acts were scored as two separate aggressive bouts. Chasing, boxing, tail rattling, biting, and offensive attack (often accompanied by biting and wrestling), previously shown to be typical for intermale (male vs. male) aggression (20, 22), were defined as aggressive behavior acts. For each experimental male, cumulative duration of aggressive bouts, number of aggressive bouts with offensive attacks, and cumulative duration of sexual behavior by resident mice toward intruder mice (chasing with attempted mounts), as well as latency to the first aggressive act, offensive attacks, and sexual behavior (900 sec was given to mice that did not show the behavior), were recorded.
Open-Field Behavior Tests.
Mice were tested for 5 min on 3 consecutive days in an open-field apparatus (40.5 × 40.5 cm, 30-cm high wall), which was illuminated with white light from the top at the center of the apparatus. At the beginning of the test, a mouse was placed gently in a corner square with his head facing the corner. Horizontal activity was monitored automatically with IR beams, and the data were analyzed and stored by the Digiscan Analyzer and Digiscan software (Digiscan model RXYZCM, Accuscan Instruments, Columbus, OH). The total moving distance, moving time, moving distance in the center area, and time spent in the center area were recorded for each mouse.
Female Sexual Behavior Test.
Sexual behaviors were tested in the males’ home cages, using single-housed stud WT male mice (14–17 weeks old) obtained from the breeding colony of βERKO mice maintained at the National Institute of Environmental Health Sciences. Each female was tested on 4 consecutive days while its estrus cycle was monitored by taking vaginal smears. One βERKO mouse and one WT mouse were tested at the same time against the same two male mice to eliminate confounds caused by variability of males’ behavior. After the females were tested with the first male (e.g., βERKO female with male 1 and WT female with male 2), they then were switched and tested with a second male (e.g., βERKO female with male 2 and WT female with male 1). Each test lasted either 12 min or until both females received 10 mounts or intromissions. Male intromissions were terminated by the experimenter after females’ responses were scored (after about 10 thrusts) to avoid unnecessary insemination. Female responses to male mounts or intromissions were scored as either (a) totally unreceptive with kicking, rearing, or fleeing (score 0), (b) proceptive/still posture without any extension of legs (score 0.1), (c) proceptive/still posture with extension of legs (score 0.5), or (d) receptive lordosis posture with dorsiflexion of the vertebral column (scores 1–3 with 0.5 intervals, depending on the degree of dorsiflexion). Female responses with the score of 1 or higher were considered as lordosis response for the calculation of lordosis quotient (number of lordosis/number of mounts and intromissions). The percent of proceptive/still postures (score 0.1 and 0.5) among total numbers of mounts and intromissions was calculated separately for each female mouse. In addition, receptivity scores were calculated as the average of 10 lordosis scores (0–3) for each mouse.
Female Aggressive Behavior Test.
Aggressive behaviors were tested in a resident-intruder paradigm for 10 min against group-housed ovariectomized female CD-1 mice (Charles River Breeding Laboratories) as intruders.
Statistics.
Data were analyzed by a two-way ANOVA for repeated measurements for the main effects of genotype and test day and their interaction, followed by post-hoc one-way ANOVAs on each test day if necessary. Data of nonrepeated measurements were analyzed by one-way ANOVAs. Differences in the percent of animals showing certain behavior were tested with Fisher’s exact probability or χ2 test.
Results
Male Sexual Behavior.
βERKO male mice were not different from WT male mice in any aspects of male sexual behavior (Table 1; Fig. 2 A and B). We found that βERKO male mice were not deficient in either frequency or latency of mounts or intromissions. The behavioral pattern of intromission (see Fig. 2A) and mean number of thrusts per intromission (Table 1) of βERKO male mice were not different from those of WT male mice. In both tests, βERKO male mice tended to show slightly higher numbers of attempted mounts, mounts, and intromissions with slightly shorter latencies. Only one mouse of each genotype ejaculated during the second 30-min behavioral test. Ejaculation latency and duration were similar between these two mice (Table 1, test 2). We confirmed that some βERKO male mice (at least six of nine) successfully inseminated C57BL/6J female mice within 20 days of continuous cohabitation. Female mice delivered 4–9 pups (7.1 ± 0.5, mean ± SEM) after 22–38 days (27.5 ± 1.6) of cohabitation.
Table 1.
Results of male sexual behavior tests
| Test | Behavior | WT (N = 6) | βERKO (N = 9) |
|---|---|---|---|
| 1 | Attempted mounts | ||
| No. of mice | 5/6* | 8/9 | |
| Frequency | 2.1 ± 1.2† | 6.2 ± 2.5 | |
| Mounts | |||
| No. of mice | 4/6 | 8/9 | |
| Latency | 1,245 ± 211 | 1,005 ± 154 | |
| Frequency | 2.3 ± 1.4 | 3.6 ± 1.0 | |
| Intromissions | |||
| No. of mice | 1/6 | 5/9 | |
| Latency | 1,624 ± 176 | 1,434 ± 153 | |
| Frequency | 0.5 ± 0.5 | 2.6 ± 1.4 | |
| No. thrusts/I per intromission | (2.7)‡ | 7.9 ± 3.8§ | |
| Ejaculation | |||
| No. of mice | 0/6 | 0/9 | |
| 2 | Attempted mounts | ||
| No. of mice | 4/6 | 7/9 | |
| Frequency | 1.8 ± 0.7 | 5.8 ± 3.0 | |
| Mounts | |||
| No. of mice | 4/6 | 7/9 | |
| Latency | 1,215 ± 204 | 960 ± 193 | |
| Frequency | 1.2 ± 0.6 | 4.6 ± 2.2 | |
| Intromissions | |||
| No. of mice | 4/6 | 5/9 | |
| Latency | 1,380 ± 188 | 1,142 ± 213 | |
| Frequency | 3.8 ± 1.8 | 6.0 ± 2.5 | |
| No. thrusts/I per intromission | 18.3 ± 4.5§ | 16.7 ± 5.5§ | |
| Ejaculation | |||
| No. of mice | 1/6 | 1/9 | |
| Latency | (1,238)‡ | (1,148)‡ | |
| Ejaculation duration | (34)‡ | (44)‡ |
Number of mice that showed behavior/number of mice tested.
Mean ± SEM.
Data of the mice that showed the behavior.
Mean ± SEM including only the mice that showed intromissions.
Figure 2.
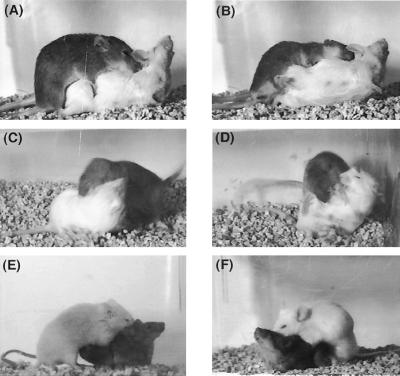
Photographs (digitized images captured from video recordings) showing the representative behavioral patterns of male sexual, male aggressive, and female sexual behaviors exhibited by βERKO mice. (A) A βERKO male mouse (agouti, top) exhibited an intromission to a SW female mouse (albino), which showed lordosis behavior. (B) A βERKO male mouse ejaculated. (C and D) Offensive attack exhibited by a βERKO male mouse (agouti) toward OBX SW male intruder (albino). (E and F) Lordosis responses of a βERKO female mouse (agouti, bottom) induced by male mounting behavior.
Male Aggressive Behavior.
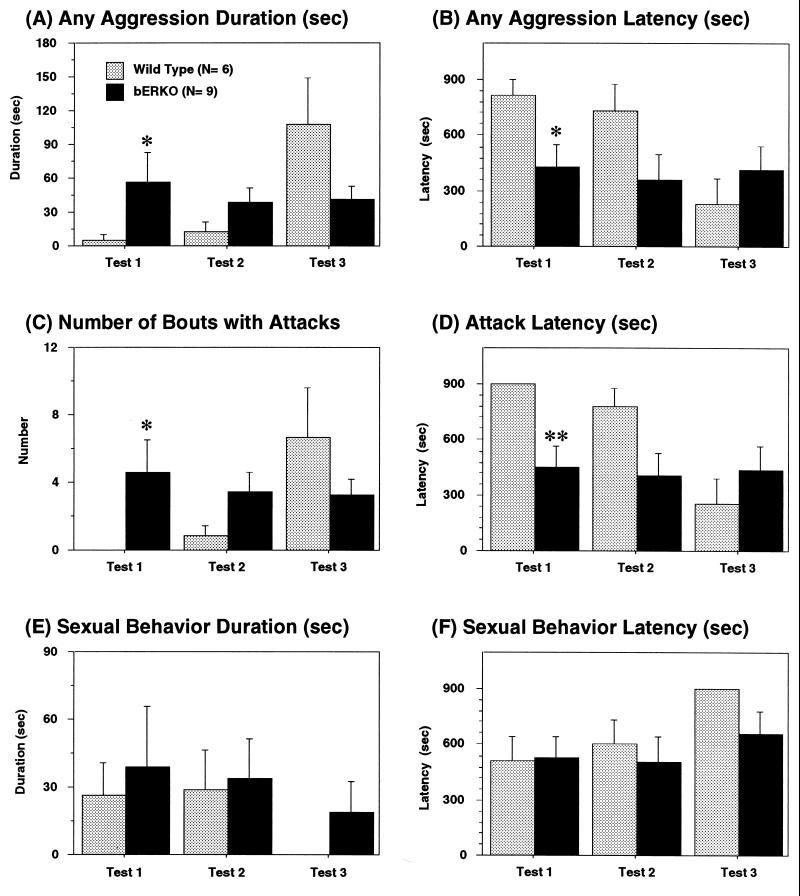
ERβ gene disruption did not abolish aggressive behavior in male mice. βERKO male mice exhibited male-typical offensive attacks toward OBX male intruder mice (see Fig. 2 C and D). Detailed analyses of male aggressive behavior revealed that there were no overall genotype differences in cumulative duration of aggressive behavior (Fig. 3A) or number of aggressive bouts with offensive attacks (Fig. 3C) and in latencies to the first aggressive acts (Fig. 3B) or the first offensive attack (Fig. 3D). However, interactions between genotype and test days were significant in all four measurements [cumulative duration of aggression, F(2,26) = 5.05, P < 0.05; latency to the first aggressive act, F(2,26) = 14.29, P < 0.001; number of attacks, F(2,26) = 4.00, P < 0.05; latency to the first attack, F(2,26) = 15.98, P < 0.001]. βERKO male mice showed similar levels of aggression with similar latencies throughout the three aggression tests whereas WT male mice became more aggressive with repetition of aggression tests. Post-hoc ANOVAs revealed that βERKO male mice were more aggressive than WT male mice in the first test, but comparable to WT in the second and third tests. Duration and latency of sexual behavior toward male intruder mice were not different between genotypes (Fig. 3 E and F).
Figure 3.
Effects of ERβ gene disruption on (A) cumulative duration of aggression, (B) latency to the first aggressive act, (C) number of attacks, and (D) latency to the first attack during resident-intruder tests. βERKO male mice showed higher levels of aggression in the first tests compared with WT male mice, whereas there were no genotype differences in the levels of aggression in the second and third tests (see text for details of statistical analyses). Resident βERKO and WT male mice also exhibited attempted sexual behaviors toward male intruder mice as demonstrated in E and F. ∗∗, P < 0.01; ∗, P < 0.05 vs. WT.
Male Open-Field Behavior.
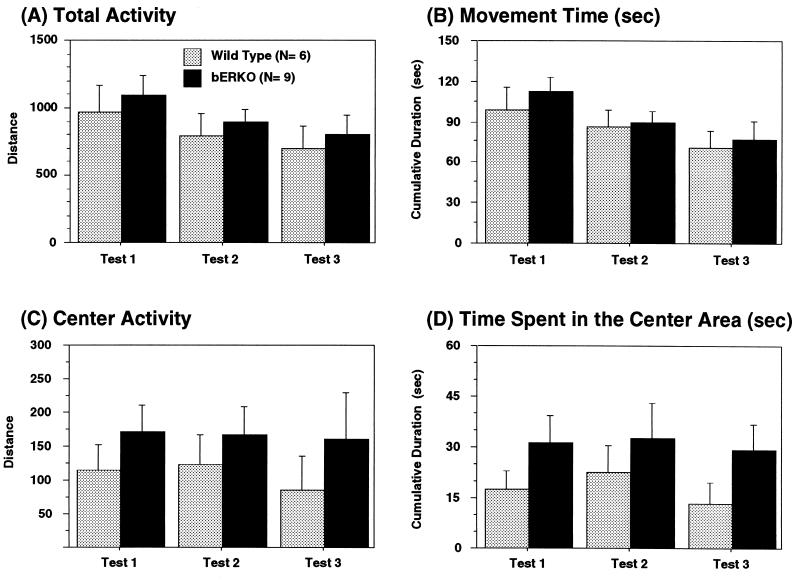
Total locomotor activity measured as total moving distance (Fig. 4A) and cumulative duration of moving time (Fig. 4B) was not different between genotypes. Activity in the center area (Fig. 4C) and time spent in the center area (Fig. 4D), indications of fearfulness and emotionality, were not different between βERKO and WT male mice.
Figure 4.
Effects of ERβ gene disruption on locomotor activity in the open-field tests in male mice. Locomotor activity in the open-field apparatus was measured 3 consecutive days. There were no overall genotype differences in total activity (A), cumulative moving time (B), center activity (C), and time spent in the center area (D).
Female Sexual Behavior.
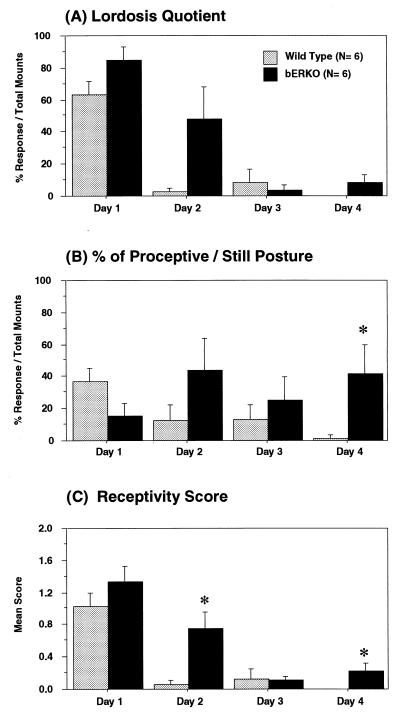
One βERKO and one WT female mouse was inseminated during behavioral tests on the second day and excluded from the study. Females’ responses to two males on each test day were highly correlated (lordosis quotient, r = 0.78, P < 0.0001; receptivity score, r = 0.88, P < 0.0001). Therefore, we analyzed female responses to the first 10 mounts in each day regardless of which stimulus male mouse was used. Data were sorted according to the days of the estrous cycle (the day of behavioral estrus was designated as day 1), regardless of the calendar day of the tests. Lordosis quotient (Fig. 5A), the percent of proceptive/still posture (Fig. 5B), and receptivity score (Fig. 5C) then were averaged for each genotype. There were certain significant genotype differences in the lordosis quotient [F(1,36) = 7.79, P < 0.01] and the receptivity score [F(1,36) = 9.05, P < 0.01] and marginal differences in the percent of proceptive/still posture (P = 0.079). βERKO showed slightly higher levels of female sexual behavior compared with WT female mice. Their receptivity was not significantly different from WT female mice on the day of estrus. However, βERKO females showed high sexual receptivity even on the day after estrus and exhibited a proceptive/still posture throughout the cycle, whereas WT females were receptive only on the day of estrus (interaction between genotype and estrous day of lordosis quotient, P = 0.056; percent of proceptive/still posture, P = 0.060).
Figure 5.
Genotype differences in female sexual behaviors. Female mice were tested 4 consecutive days, and the data were sorted based on the days of estrous cycle. Day 1 represents the day of behavioral estrus. Overall, βERKO female mice showed higher levels of receptivity compared with WT female mice as demonstrated in the lordosis quotient (A), the percent of proceptive/still posture (B), and the receptivity score (C). They especially showed higher levels of sexual behavior on the day after behavioral estrus (day 2). *, P < 0.05 vs. WT.
Female Aggressive Behavior.
Neither βERKO nor WT female mice, regardless of the day of estrous cycle, showed aggressive behavior toward female intruder mice.
Discussion
In sexual and aggressive behavior tests in the present study, βERKO male and female mice performed at least as well as their respective WT controls. These results contrast markedly with our previous findings in αERKO male and female mice and highlight the importance of the classical ERα gene for the facilitation of reproductive behaviors. Moreover, the present findings in βERKO mice suggest that simultaneous activation of ERβ may not be essential for the regulation of reproductive behaviors by estrogen through ERα-mediated mechanisms.
Unlike αERKO males, which are infertile (13, 16) with reduced levels of intromissions and absence of ejaculation (15), βERKO male mice are known to be fertile (10, 11). The present study provides quantitative behavioral evidence to support the normal fertility of male βERKO mice. In comparison to WT male mice, βERKO male mice not only showed no sign of deficiency in any aspects of male sexual behavioral profile, but they also tended to show slightly higher levels of intromissions, particularly in the first test (Table 1). We could not completely exclude the possibility that βERKO male mice might actually be more sexually active or aggressive (see below) than WT mice, through unopposed ERα action. We assume that these behavioral characteristics of βERKO male mice are not simply caused by compensating mechanisms mediated by androgen receptor although at present we do not have reportable circulating testosterone levels from βERKO males. Whether βERKO male mice actually may show different behavioral sensitivity to androgen, however, needs to be determined in further studies using gonadectomized and androgen-treated mice.
Male aggressive behavior was not abolished by the lack of the ERβ gene. βERKO male mice exhibited a variety of aggressive behavioral acts, including offensive attacks (Fig. 2 C and D). These findings differ dramatically from the almost complete suppression of male typical offensive attacks in gonadally intact αERKO male mice (14, 15). In addition, it should be noted that during the very first tests of aggressive behaviors, βERKO males had rather higher frequencies and lower latencies of aggression than WT controls (Fig. 3). With increased fighting experience (tests 2 and 3), however, these genotype differences disappeared and were almost reversed in the third test. These data thus reveal an important interaction between genotype and social experience with respect to aggressive behavior. Such dependencies defy simplistic thinking about the relations between genes and mammalian behaviors and add to the list of complex dependencies in the emerging field of studies on gene/behavior relationships (reviewed in ref. 23).
In the genetic male, estrogen actions in the brain during development are important for the masculinization of certain behaviors, even as hormone actions in adulthood more directly activate these behaviors. The present results suggest that a functional ERβ gene is not essential for such masculinization, at least as far as mating and aggressive behaviors are concerned. In addition, there were no effects of ERβ gene disruption in any measurements of open-field activity, which is known to be influenced by the presence or absence of neonatal brain ER stimulation and found to be significantly elevated in male αERKO mice compared with WT control mice.
We found that female βERKO mice showed no reduction of sexual receptivity in terms of either the lordosis quotient or the degree of lordosis responses, which was quite the opposite from the complete abolition of lordosis behavior in gonadally intact (6) as well as steroid-primed gonadectomized αERKO female mice (5). In this respect, mating behavior results in the present study are similar to those in the uterus, where hyperplasia after estrogen administration is present in βERKO females as in WT females (11). The normal performance of lordosis behavior in βERKO females is, likewise, consonant with the neuroanatomical distribution of ERβ, in which very little expression, if any, has been found in the ventromedial nucleus of the hypothalamus (24, 25) where estrogen binding plays a critical role for the neural circuit for this behavior (2).
The neuroanatomical distribution of ERβ gene expression in the telencephalon (7–9) favors the notion that this gene could be involved in hormonal modulation of cognitive processes. It is noted that the normal performances of a variety of natural behaviors, including the normal levels of general locomotor activity observed in the present study, provide a sort of control to be compared with potential changes in learning or memory by ERβ gene disruption.
Acknowledgments
We thank Dr. C. Pavlides for critical reading of the manuscript and Ms. Lucy Frank for her editorial assistance. We also are indebted to Mr. Todd Washburn and Ms. Vickie Walker at the National Institute on Environmental Health Sciences for their administration and maintenance of the βERKO animal colony. Support for this work was provided by National Science Foundation Grant IBN-9728579 (S.O.) and National Institutes of Health Grant HD-05751 (D.W.P.).
Abbreviations
- ER
estrogen receptor
- KO
knockout
- WT
wild type
- SW
Swiss–Webster
- OBX
olfactory bulbectomized
References
- 1.Beach F. Hormones and Behavior. New York: Hoeber Harper; 1948. [Google Scholar]
- 2.Pfaff D W. Estrogen and Brain Function: Neural Analysis of a Hormone-Controlled Mammalian Reproductive Behavior. New York: Springer; 1980. [Google Scholar]
- 3.Pfaff D W. Endocrinology. 1968;82:1149–1155. doi: 10.1210/endo-82-6-1149. [DOI] [PubMed] [Google Scholar]
- 4.Meisel R L, Dohanich G P, McEwen B S, Pfaff D W. Neuroendocrinology. 1987;45:201–207. doi: 10.1159/000124726. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa S, Eng V, Taylor J A, Lubahn D B, Korach K S, Pfaff D W. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa S, Taylor J A, Lubahn D B, Korach K S, Pfaff D W. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 7.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper G G J M, Enmark E, Peltohuikko M, Nilsson S. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuiper G G J M, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-A. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 10.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 11.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meisel R L, Sachs B D. In: Physiology of Reproduction. 2nd Ed. Knobil E, Neill J D, editors. 1 and 2. New York: Raven; 1994. pp. 3–105. [Google Scholar]
- 13.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa S, Washburn T F, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddy E M, Washburn T F, Bunch D O, Goulding E H, Gladen B C, Lubahn D B, Korach K S. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 17.Pettersson K, Grandien K, Kuiper G G J M, Gustafsson J-A. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 18.Shughrue P J, Scrimo P J, Merchenthaler I. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 19.McGill T E. In: Contributions to Behavior-Genetic Analysis: The Mouse as a Prototype. Lindzey G, Thiessen D D, editors. New York: Appleton Century Crofts; 1970. pp. 57–88. [Google Scholar]
- 20.Ogawa S, Robbins A, Kumar N, Pfaff D W, Sundaram K, Bardin C W. Horm Behav. 1996;30:74–84. doi: 10.1006/hbeh.1996.0011. [DOI] [PubMed] [Google Scholar]
- 21.Denenberg V H, Gaulin-Kremer E, Gandelman R, Zarrow M X. Anim Behav. 1973;21:590–598. doi: 10.1016/s0003-3472(73)80021-1. [DOI] [PubMed] [Google Scholar]
- 22.Maxson S C, Didier-Erickson A, Ogawa S. Behav Neural Biol. 1989;52:251–259. doi: 10.1016/s0163-1047(89)90369-5. [DOI] [PubMed] [Google Scholar]
- 23.Pfaff D W. Drive: Neural and Molecular Mechanisms for Sexual Motivation. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 24.Shughrue P J, Lane M V, Scrimo P J, Merchenthaler I. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper G G, Shughrue P J, Merchenthaler I, Gustafsson J A. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]