Abstract
Incubation of Na/K-ATPase with ascorbate plus H2O2 produces specific cleavage of the α subunit. Five fragments with intact C termini and complementary fragments with intact N termini were observed. The β subunit is not cleaved. Cleavages depend on the presence of contaminant or added Fe2+ ions, as inferred by suppression of cleavages with nonspecific metal complexants (histidine, EDTA, phenanthroline) or the Fe3+-specific complexant desferrioxamine, or acceleration of cleavages by addition of low concentrations of Fe2+ but not of other heavy metal ions. Na/K-ATPase is inactivated in addition to cleavage, and both effects are insensitive to OH⋅ radical scavengers. Cleavages are sensitive to conformation. In low ionic strength media (E2) or media containing Rb ions [E2(Rb)], cleavage is much faster than in high ionic strength media (E1) or media containing Na ions (E1Na). N-terminal fragments and two C-terminal fragments (N-terminals E214 and V712) have been identified by amino acid sequencing. Approximate positions of other cleavages were determined with specific antibodies. The results suggest that Fe2+ (or Fe3+) ions bind with high affinity at the cytoplasmic surface and catalyze cleavages of peptide bonds close to the Fe2+ (or Fe3+) ion. Thus, cleavage patterns can provide information on spatial organization of the polypeptide chain. We propose that highly conserved regions of the α subunit, within the minor and major cytoplasmic loops, interact in the E2 or E2(Rb) conformations but move apart in the E1 or E1Na conformations. We discuss implications of domain interactions for the energy transduction mechanism. Fe-catalyzed cleavages may be applicable to other P-type pumps or membrane proteins.
Keywords: Fe ions, energy transduction mechanism, Na, K-pump
Recent studies of structure-function relations of P-type cation pumps, such as Na/K-, H/K-, and Ca-ATPases, have focused on identification of residues of the catalytic subunits involved in cation or ATP binding or on their topological organization, comprising 10 transmembrane segments (M1-M10) (1–3). Cation occlusion sites are located within trans-membrane segments, and site-directed mutagenesis results suggest that side chains within M4, M5, M6, and M8 ligate cations (2, 4–6). The ATP binding site is located within the major cytoplasmic loop, and probable ligating residues are being identified by chemical modification or mutagenesis (1). By comparison with progress in these areas, our understanding of the energy transduction mechanism lags behind. Earlier studies provided a wealth of information on kinetics of cation transport, phosphorylation-dephosphorylation, transport-linked E1-E2 conformational transitions, and cation occlusion (7, 8). Transmission of force between ATP and cation binding sites and their structural reorganization in E1 and E2 conformations must underlie the energy transduction process. However, the structural basis of these events is poorly understood. Some evidence has been obtained using proteolysis or site-directed mutations. Specific proteolytic cleavages of Na/K- or Ca-ATPase in the first cytoplasmic loop between trans-membrane segments M2 and M3, characteristically inhibit ATPase activity and stabilize E1 conformations (9–11). In addition, there are several highly conserved sequences in the cytoplasmic domains of all P-type pumps, including TGES in the loop between M2 and M3 and MVTGD and TGDGVNDSPALKK in the major cytoplasmic loop before M5. Mutations in these sequences, in Ca- or H-ATPase, inhibit activity and stabilize E1 forms (and in some cases also inhibit phosphorylation) (2, 12–14). Thus, the conserved sequences serve an important function, perhaps mediating conformational interactions between ATP and cation sites. However, their specific roles are unknown.
This paper describes metal-catalyzed cleavage for study of structure-function relations and conformational transitions of Na/K-ATPase. The use of complexed transition metals, such as EDTA-Fe or phenanthroline-Cu, for selective oxidative cleavage of nucleic acids is well established (15). Although specific metal-catalyzed cleavage of proteins is less characterized, there are several examples of applications to soluble enzymes using complexed or free Fe2+ and Cu2+ ions, directed or naturally bound to specific sites (16–20). The term “affinity cleavage reagent” describes substrates or other molecules that complex the heavy metals and direct them to sites where cleavage occurs (16). In principle, analysis of the cleavage pattern should provide spatial information on peptide bonds in proximity to the metal. Applications to membrane proteins include identification of proximal trans-membrane segments of lac permease detected by cleavages dependent on Cu-phenanthroline targeted chemically to engineered cysteines (21) and proximal residues on neighboring subunits of cytochrome bd quinol oxidase using Fe-chelate-dependent cleavages (22).
We describe here a chance discovery that incubation of renal Na/K-ATPase with ascorbate and H2O2 causes selective cleavages of the α subunit. We demonstrate that cleavages depend on the presence of contaminating Fe2+ ions in the media and describe properties of the Fe-catalyzed cleavages.
MATERIALS AND METHODS
Materials.
For SDS/PAGE, all reagents were electrophoresis-grade from Bio-Rad. Tris (ultra pure) was from Bio-Lab, Jerusalem. l(+)-ascorbic acid and 30% H2O2 were from Merck. All other reagents were of analytical grade.
Enzyme Preparation.
Na/K-ATPase (13–18 units/mg protein) was prepared from pig kidneys, assayed, and stored at −20°C in a solution of 250 mM sucrose, 25 mM histidine (pH 7.4), and 1 mM EDTA [as described by Jørgensen (23)]. Before use, membranes were washed twice and suspended in a solution containing 10 mM Tris⋅HCl, pH 7.4.
Cleavage Reaction.
Membrane suspensions (0.1–1 mg/ml), with or without added 30 mM RbCl or NaCl, were incubated at 20°C with freshly prepared solutions of 4 mM ascorbate (Tris) plus 4 mM H2O2, without or with added FeSO4 or other metals, in a total volume of 30–40 μl. To arrest the reaction, 10 μl of 5 mM EDTA or 5-fold concentrated gel sample buffer with 5 mM EDTA was added, and samples were assayed for Na/K-ATPase activity or applied to gels, respectively.
Gel Electrophoresis, Blotting to Polyvinylidene Difluoride, Immunoblots, Sequencing.
Procedures for running of 10% Tricine (N-[tris(hydroxymethyl]glycine) SDS/PAGE, including precautions before sequencing, electroblotting to polyvinylidene difluoride paper, immunoblots, and microsequencing of fragments, have been described in detail (3, 24). In some cases (see Fig. 5), the enzyme (1 mg/ml) was first mixed with 3 mg/ml of the nonionic detergent C12E10 (polyoxyethylene 10 lauryl ether), the undissolved protein was removed (50,000 rpm in a Beckman TLX-100 centrifuge), and soluble protein was precipitated with 4 vol of methanol/ether 2:1 and overnight storage at −20°C. This procedure selectively extracts the pump subunits and their fragments (unpublished work). For quantification of the α subunit, the band was cut out of the gel, the Coomassie stain was extracted by homogenization in 1% SDS solution, acrylamide was removed by centrifugation, and OD was measured at 595 nm.
Figure 5.
Fragments of the α subunit produced in E2(Rb) and E1Na conformations. The enzyme, 1 mg/ml, with added 30 mM RbCl or NaCl, was incubated at 20°C for 20 or 60 min in the standard conditions, without added Fe2+ ions. The figure presents a Coomassie-stained gel.
Antibodies.
Rabbit antisera, prepared as described by Grossman et al. (25), were raised against fragments of trypsinized Na/K-ATPase, “19-kDa membranes” (5, 24). Antisera included (i) “anti-M1/M2,” prepared from a 11.7-kDa fragment D68-R168, containing transmembrane segments M1 and M2 and (ii) “anti-β,” prepared from a 16-kDa fragment A5-R142 of the β subunit. Bands were cut out of stained gels, extracted, and precipitated (26), and aliquots of ≈200 μg were used per injection. Anti-peptide antibodies also were raised against the synthetic peptides L337-N348 and I263- P276, coupled to keyhole limpet hemocyanin. The epitope of the latter antibody is known to be I263-L266 (A. Shainskaya and S.J.D.K., unpublished work). In addition, the following anti-peptide antibodies, supplied by colleagues, were used to characterize α subunit fragments: (i) anti-K1012-Y1016, referred to as “anti-KETYY,” (ii) anti-K347-E358, (iii) anti-I366-N377, (iv) anti-D636-R651, (v) anti-L815-Q828, and (vi) anti-N889-Q903.
Heavy Metals.
Induced coupled plasma atomic emission spectroscopy was performed to determine heavy metal composition of the media (Spectrolabs, Rehovot, Israel). Concentrated solutions of ascorbic acid, Tris⋅HCl (pH 7.4), and H2O2 were analyzed, and the contents of the reaction media were then calculated.
Calculations.
Nonlinear curve-fitting was performed using Enzfitter (Elsevier-Biosoft, Cambridge, U.K.).
RESULTS
Fig. 1 presents the basic finding that incubation of renal Na/K-ATPase with ascorbate plus H2O2 produces selective cleavage of the α subunit but does not cleave the β subunit. Intact α subunit and its fragments were visualized in immunoblots to detect intact C-terminal residues (anti-KETYY) or the N-terminal region (anti-M1/M2) (see Materials and Methods). Five principal fragments with intact C-terminals and five fragments with intact N-terminal fragments were observed. Fragments are numbered as in Table 1, which summarizes apparent molecular mass values (range 22.6–81.8 kDa), antibody binding, and amino-acid sequences. Based on their apparent lengths, the following pairs of fragments appear to be complementary:10 and 1, 8 and 3, 6 and 5, 4 and 7, and 2 and 9 (for supporting evidence see Table 1).
Figure 1.
Selective cleavage of the α subunit induced by incubation with ascorbate and H2O2. Enzyme, 1 mg/ml, was incubated for 60 min in the standard conditions (without added Rb or FeSO4, see Materials and Methods). C, control; A, ascorbate; and HP, H2O2. The figure presents immunoblots using anti-KETYY, anti-M1/M2, or anti-β (see Materials and Methods).
Table 1.
Fragments produced by Fe-catalyzed cleavage of the α subunit
| Fragment approximate molecular mass, kDa | Antibody binding | Sequence found | Position of cleavages |
|---|---|---|---|
| 1. 81.8 ± 2.5 (n = 5) | Anti-KETYY | ESEPQTR | E214-Y1016 |
| 2. 79.8 ± 2 (n = 4) | Anti-M1/M2 | GRDKYEP | G1-G711 |
| 3. 76 ± 2.2 (n = 4) | Anti-KETYY | Blocked N terminus | ≥(I262-L266)-Y1016 |
| 4. 69.1 (n = 1) | Anti-M1/M2 | Not sequenced | G1-≤(D636-R651) |
| 5. 68.8 (n = 2) | Anti-KETYY | Not sequenced | ≥(I366-N377)-Y1016 |
| 6. 41.5 ± 0.3 (n = 3) | Anti-M1/M2 | GRDKYEP | G1-≥(I366-N377) |
| 7. 39.1 ± 1 (n = 5) | Anti-KETYY | Blocked N terminus | ≤(D636-R651)-Y1016 |
| 8. 33.9 ± 1.5 (n = 3) | Anti-M1/M2 | GRDKY | G1-≥(I262-L266) |
| 9. 27.4 ± 0.7 (n = 5) | Anti-KETYY | VNDSPALKK | V712-Y1016 |
| 10. 22.6 ± 0.4 (n = 4) | Anti-M1/M2 | GRDKYEPAAV | G1-G213 |
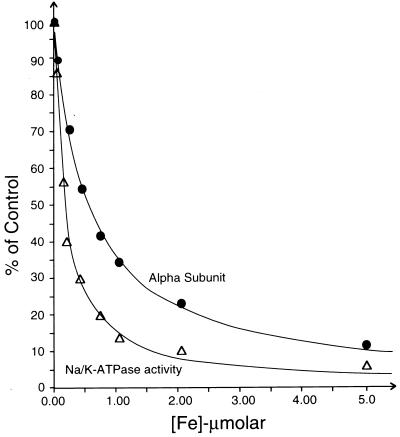
Both ascorbate and H2O2 were required for efficient specific cleavage. Ascorbate alone was slightly effective whereas H2O2 alone was ineffective. This finding hinted at possible involvement of transition metal ions as catalysts of oxidation/reduction reactions although no metal ions were added. The experiments in Fig. 2 tested this hypothesis. In Fig. 2A various heavy metal chelators were added to the medium; 25 mM histidine (Hist) partially protected the α subunit whereas 2 mM EDTA or 1 mM phenanthroline (Phen) completely prevented cleavage. The result indicated the requirement for a heavy metal, which is either tightly bound to the enzyme or is present as a contaminant in the medium. The following experiment showed that the first possibility is unlikely. We incubated the enzyme in a medium containing 4 mM ascorbate/H2O2 and 30 mM EDTA(Tris) so as to release and chelate any bound ions, washed the enzyme twice in 10 mM Tris⋅HCl to remove EDTA, and then reincubated with ascorbate/H2O2. The enzyme was cleaved to the same extent as control enzyme (not shown). Analysis of heavy metal content of other components showed that a medium consisting of 4 mM ascorbic acid, 4 mM H2O2 and 10 mM Tris⋅HCl contains: Fe, 47.5 nM; Ni, 14.4 nM; Cu, 8 nM; Mn, 3.3 nM; Cr, 1.12 nM; Mo, 1.1 nM; Zn, 0.9 nM; and Sn, 0.8 nM. One approach to identify the relevant contaminant metal ion has been to use metal-specific complexants. The Fe3+-selective complexant desferrioxamine suppresses cleavage at 10–100 μM (Fig. 2B) whereas neither a Cu-selective complexant, bicinchoninic acid, nor a Ni-specific complexant, dimethyl glyoxime, prevented cleavage at 100-1000 μM (not shown). The result points to Fe2+ or Fe3+ ions, but it might be misleading because the complexant is in large molar excess over all contaminants and, because selectivity is not absolute, other metal ions might also be complexed. More conclusive evidence was obtained by looking at effects of added Fe2+ and other metals. The experiments used short incubation times (2 minutes) to minimize cleavage due to the contaminant Fe2+ ions. Fig. 2C shows that addition of 0.25–10 μM Fe2+ ions greatly amplifies the extent of cleavage. Note that the same five cleavages appear as in the absence of added Fe2+ ions and that the cleavages display a similar Fe2+ concentration dependence. Fig. 2D shows that, of the heavy metals ions Fe2+, Cu2+, Ni2+, or Mn2+ added at 5 μM, only Fe2+ ions were effective. Cr3+, Ru3+, Sn2+, Ag+, Pb2+, Zn2+, Cd2+, and Ti3+ ions also were ineffective (not shown). Fig. 3 presents the Fe2+-concentration dependence of cleavage of the α subunit and, for comparison, inactivation of Na/K-ATPase. Inactivation of the enzyme is attributable to the combined action of Fe2+/ascorbate/H2O2 because elimination of any of these components prevented effective inactivation. Both inactivation of Na/K-ATPase and cleavage show hyperbolic dependence on added Fe2+ ions with fitted K0.5 values of 180 ± 12 and 560 ± 23 nM, respectively. The presence of OH⋅ radical scavengers such as mannitol, formate, or tert-butanol (20 mM) did not affect inactivation of Na/K-ATPase or cleavage (not shown).
Figure 2.
Demonstration that selective cleavages are dependent on presence of contaminant or added Fe2+ (or Fe3+) ions. (A) Enzyme, 1 mg/ml, was incubated for 30 min in the standard conditions (without added Rb or FeSO4) without or with 25 mM histidine or 2 mM EDTA or 1 mM phenanthroline. (B) As in A but incubation with indicated concentrations of desferrioxamine. (C) Enzyme, 0.1 mg/ml, was incubated for 2 min in standard condition with indicated concentrations of added FeSO4. (D) Incubation as in C without or with 5 μM added FeSO4, CuSO4, NiCl2, or MnCl2. The figure presents immunoblots using anti-KETYY.
Figure 3.
Fe2+ concentration dependence of cleavage and inactivation of Na/K-ATPase activity. Incubation was as in Fig. 2C. The curves represent best-fits to experimental points assuming a single site inhibition model.
In the course of optimizing conditions, the rate of cleavage was found to be greatly slowed down in media containing 150 mM choline chloride or at pH 8. These conditions are known to favor E1 conformations (27), so it was of interest to compare cleavages in Na- or K(Rb)-containing media, conditions that stabilize the E2(Rb) or E1Na conformations, respectively. Fig. 4 shows the time course of appearance of fragments visualized with either anti-KETYY (A and B) or the anti-M1/M2 antibody (C and D) and numbered as in Table 1. In the presence of Rb ions (Fig. 4 A and C), we observed four major fragments. The intensity of staining of all fragments rises in parallel, i.e., smaller fragments are not products of secondary cleavages. Fragments 1, 3, 7, and 9 recognize anti-KETYY, and complementary fragments 2, 4, 8, and 10 recognize anti-M1/M2. By comparison with the low ionic strength medium (Figs. 1 and 2), which also stabilizes primarily an E2 form, fragments 5 and 6 were less pronounced in the presence of Rb ions. A minor fragment of ≈100 kDa that binds anti-KETYY also was seen in these experiments. In the Na-medium (Fig. 4 B and D), the rate of cleavage was slow, and only one major cleavage was observed, producing fragment 3, which recognizes anti-KETYY, and fragment 8, which recognizes anti-M1/M2. Fig. 5 shows Coomassie-stained gels of fragments produced in Rb- or Na-containing media and digested for 20 or 60 min, without added Fe2+ ions. To clearly distinguish pump fragments from contaminant proteins, intact and cleaved Na/K-ATPase were selectively solubilized with C12E10 (see Materials and Methods). In the Rb medium, six fragments marked 1/2, 3, 7, 8, 9, and 10 were observed. Fragment 4 is concealed by the β subunit. In the Na-medium, the α subunit is much less cleaved, and again, only two fragments, 3 and 8, were observed.
Figure 4.
Time course of cleavage in E2(Rb) or E1Na conformational states. The enzyme, 1 mg/ml, with added 30 mM RbCl or NaCl, was incubated for the indicated times in the standard conditions without added Fe2+ ions. The figure presents immunoblots using anti-KETYY or anti-M1/M2. Anti-M1/M2 stains much less intensely than anti-KETYY, thus precluding quantitative comparisons of amounts of fragments binding the two antibodies.
Fragments from gels, as in Fig. 5, were transferred to polyvinylidene difluoride paper and sequenced (Table 1). Fragments 4 and 5 could not be isolated because they are overlapped by the β subunit. Note that fragments that bind anti-M1/M2 begin at the N-terminal G1 of the α subunit. Of the fragments that bind anti-KETYY, sequence was obtained from fragments 1 and 9 whereas fragments 3 and 7 appear to have blocked N termini. The band marked 1/2 in Fig. 5 consists of a mixture of two fragments with sequences ESEPQTR (N-terminal E214) and GRDKEY (N-terminal G1). Fragment 9 gave a single sequence VNDSPALKK (N-terminal V712). The C termini of the complementary fragments 2 and 10, beginning at G1, can be assigned as G711 and G213. For fragments that could not be sequenced, limits on the positions of cleavages were determined using anti-peptide antibodies. Salient findings are: (i) Fragment 8 binds anti-I263-P276 whereas its complementary fragment 3 does not, (ii) fragment 6 binds anti-K347-E358 and anti-I366-N377 whereas its complementary fragment 5 does not, and (iii) fragment 7 binds D636-R651 whereas its complementary fragment 4 does not (see Table1). Finally, use of anti-L815-Q828, between M6 and M7, or N889-Q903, between M7 and M8, produced the same cleavage pattern as observed with anti-KETYY. The latter finding demonstrates that only fragments binding either anti-KETYY or anti-M1/M2 are produced. The data in Table 1 indicate that the splits are cytoplasmic (see also Fig. 6). Using right side-out renal microsomal vesicles to define sidedness, the Fe2+-dependent cleavages also have been shown directly to occur at the cytoplasmic surface, i.e., vesicles must first be opened by addition of a detergent (M. Bar Shimon and S.J.D.K., unpublished).
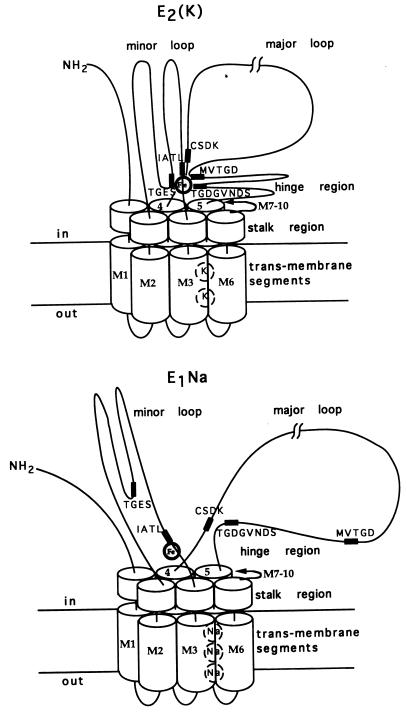
Figure 6.
Schematic models depicting formation [E2(K)] or disruption (E1Na) of interactions between cytoplasmic domains.
DISCUSSION
Mechanism of Cleavage of the α Subunit.
The following properties of Fe-dependent cleavages suggest that Fe2+ (or Fe3+) ions bind with high affinity at the cytoplasmic surface of the α subunit and catalyze cleavages of peptide bonds close to or in contact with the bound Fe2+ ion. (i) Cleavages were specific. We detected five cleavages and only fragments containing either intact C or N termini. (ii) Cleavage and inactivation of Na/K-ATPase displayed hyperbolic dependence on low Fe2+ concentrations (K0.5, 560 and 180 nM, respectively) (Fig. 3). (iii) All cleavages showed a similar Fe2+ concentration dependence (Fig. 2C). (iv) Cleavages showed a similar time course of appearance. (Fig. 4A). (v) Four cleavages were virtually absent in Na compared with the Rb medium (see Figs. 4 and 5). One cleavage producing fragments 3 and 8 occurred slowly. This is discussed below. (vi) Cleavages were insensitive to OH⋅ scavenging reagents in the medium. These properties suggest further that (a) cleavages at the different positions occur with a probability depending on the spatial arrangement of the polypeptide chain and proximity of the different peptide bonds to the bound Fe2+ ion and that (b) once the chain is cleaved at a particular position further cleavages do not occur to a significant extent. Selective Fe-catalyzed cleavages may be applicable to other pumps or membrane proteins (28).
The chemistry of selective Fe-mediated cleavage of peptide bonds is uncertain. Both oxidative and hydrolytic mechanisms have been proposed (16–18, 29). In the presence of ascorbate, H2O2, and Fe2+ ions, OH⋅ radicals are generated by the Fenton reaction, but OH⋅ radicals in the medium are not involved in cleavage of Na/K-ATPase. One hypothesis, for Fe-dependent, specific oxidative cleavages, is that OH⋅ radicals generated within sites are responsible (16). Resulting C- or N-terminal fragments are the amide and ketoacyl derivatives, respectively (16, 29). The blocked N termini preclude sequencing of C-terminal fragments by Edman degradation. Another oxidative mechanism (18) produces an isocyanate derivative at the N terminus of the cleaved fragment, which can be sequenced because the NCO derivative is rapidly hydrolyzed. In the hydrolytic mechanism (17), a bound Fe-peroxo intermediate acts as a nucleophile to attack the carbonyl carbon of the peptide bond, producing an intermediate that rearranges to generate free carboxyl and amino groups. This reaction produces fragments that can be sequenced. Our results are compatible with any of these mechanisms and suggest that different reactions can occur simultaneously because two C-terminal fragments could be sequenced (1 and 9) while two fragments (3 and 7) were blocked. Both E214 and V712 in the sequenced fragments lie adjacent to glycine residues, hinting that the same reaction mechanism operates in these cases. Another observation compatible with different types of oxidative reactions is the higher sensitivity to Fe2+ of inactivation of Na/K-ATPase compared with cleavage (Fig. 3). In time course experiments, the rate of enzyme inactivation is also faster than cleavage. A reasonable explanation is that enzyme inactivation precedes chain cleavage, but not all inactivating events lead to cleavage, e.g., oxidation of side chains (29). Similar phenomena have been described for Fe2+- and Cu2+-dependent cleavage of enzymes (19, 20).
Interactions Between Cytoplasmic Domains: Implications for Energy Transduction.
Fig. 6 presents schematic models of peptide segments close to the bound Fe2+ ion in the K(Rb)-bound, E2(K), or Na-bound, E1Na state. Sites for 2 K or 3 Na ions are depicted within transmembrane segments in a domain comprising M1-M6 (2, 4–6). The C-terminal region may also be involved in cation binding, but for simplicity trans-membrane segments M7-M10 and the β and γ subunits are omitted. Approximate positions of cleavages that could not be determined exactly are assigned on the basis of antibody binding, apparent molecular mass values, and topological considerations. Cleavages in the minor loop between M2 and M3 include (i) E214 in TGES (fragments 1 and 10) and (ii) that producing fragments 3 and 8 that lies beyond IATL but before the entrance to M3, i.e., close to IATL. The cleavage producing fragments 5 and 6 is located after M4 at or beyond I366-N377, near the phosphorylation site CSDK. The two other cleavages in the major loop are (i) that producing fragments 7 and 4 that lies before D636-R651 and may be near the segment MVTGD and (ii) V712 in TGDGVNDSPALKK (fragments 9 and 2), within the so-called “hinge region” that leads into M5.
A priori, it is likely that segments close to bound Fe2+ also interact with each other. The sequences CSDK, TGES, and VAVTGDGVNDSPALKK, and MVTDG, are the most highly conserved in P-type pumps. A strong implication is that major and minor loop domains do interact (in E2 states), and the sequences are highly conserved because the interactions serve an important function. In E1 states, major and minor loops may move apart and change configuration so that the conserved segments no longer make contact with the Fe2+ ion (see Fig. 6). The bond near IATL is cleaved (slowly), even in the E1Na state. A possible explanation is that Fe2+ binding residues are near IATL in the primary sequence and IATL cannot move away. The sequence HFIH, near the entrance of M3, might form part of a Fe coordination site, especially if the imidazole nitrogens of the two histidines are oriented in an α-helical arrangement. In the E2(K) state (Fig. 6), of the five cleavage points, CSDK is placed furthest away from the Fe2+ ion and is perhaps barely in contact with it because the split producing fragments 5 and 6 is a minor one (Fig. 4). In an E2 conformation (absence of Rb and low ionic strength), this split is more pronounced (Figs. 1 and 2) and CSDK might lie closer to the Fe2+ ion.
Are the models in Fig. 6 compatible with known characteristics of E1/E2 conformational transitions? The notion that the major and minor loops move apart in the E2/E1 transition fits well with studies on proteolytic digestion of Na/K-ATPase. In the E1Na state, the α subunit is cleaved at R262 by trypsin (9) or at L266 by chymotrypsin (10), consistent with exposure of the IATL sequence to proteases in the medium. By contrast, in the E2(Rb) state, the IATL sequence is inaccessible to proteases, and the α subunit is cleaved in the major loop. The model predicts that structural modifications, such as mutations or proteolytic splits, could interfere with the domain interactions and stabilize E1 states in which such interactions are already suppressed. Stabilization of E1 states of Ca- Na/K- or H-ATPase is indeed observed when these proteins are mutated at or close to the positions of the Fe-catalyzed cleavage sites described here (2, 12–14, 30, 31). Specific proteolytic cleavages of Na/K- and Ca-ATPase in the minor loop also stabilize E1 forms (9–11). Conversely, modifications that favor interactions could stabilize E2 states. As a recent example, mutations of D369 to neutral residues N and A have been found to stabilize E2 forms (32).
What are the implications for the energy coupling mechanism? One important issue concerns catalytic effects of bound cations and ATP-cation interactions. Binding of Na ions at cytoplasmic sites stabilizes the E1Na state, in which ATP is bound with high affinity (Kd ≈ 0.2 μM) and catalyzes phosphorylation of D369. Conversely, binding of K(Rb) at cytoplasmic sites stabilizes E2(K) and antagonizes ATP binding (Kd ≈ 200–300 μM). A simple hypothesis to explain K-ATP antagonism could assume that interactions of TGDVNDSPALKK or the MVTDG sequence with the minor loop [in the E2(K) state] distort the major loop and interfere with high affinity ATP binding. Conversely, these interactions are suppressed in the E1Na state, allowing reorganization of the major loop for high affinity ATP binding and phosphorylation of D369. Consistent with the hypothesis, chemical modification of D710 and K719, using an ATP analog, ClRATP [γ-(4-N-2-chloroethyl-N-methylamino)benzylamide-adenosine triphosphate; ref. 33], with the reactive group attached to the γ phosphate or the adenosine derivative FSBA [p-(fluorosulfonyl)benzoyladenosine; ref. 34] demonstrates proximity of D710 or K719 with D369 and the γ phosphate of ATP in the E1 state. The mechanism could also explain the finding (10) that chymotryptic cleavage of Na/K-ATPase at L266 stabilizes E1 with high affinity ATP binding and phosphorylation but also leaves intact Rb occlusion, a characteristic of E2. Cleavage may hinder domain interactions and so uncouple normal K-ATP antagonism. The second major issue concerns coupling of conformational transitions to active cation transport. One can envisage that the interactions between the minor and major cytoplasmic loops might cause movement of trans-membrane segments M4-M6, which are considered to play a major role in cation binding and transport (1). However, formulation of more specific hypotheses will require comprehensive information on cleavages in the different phosphorylated and nonphosphorylated states of the catalytic cycle. Experiments to obtain such information are in progress.
A final question of interest concerns the possibility that binding of Fe2+ ions to the Na/K-pump in vivo and subsequent cleavage reactions have physiological significance or a pathological role in oxidative stress (35). Alternatively, the Fe-catalyzed cleavages may have no significance in vivo and serve only as a useful tool for in vitro experiments.
Acknowledgments
We are greatly indebted to Drs. J. Kyte, University of California, La Jolla; J. V. Møller, Aarhus University; T. Pressley, Texas Technical University; J. W. Ball, Cincinnati University; and Y. C. Ng, Pennsylvania State University for providing antibodies and to Dr. R. Tarrab-Hazdai for help in preparing antibodies. We thank also Prof. A. Shanzer for a gift of desferrioxamine. This research was supported by the Israel Science Foundation (founded by the Israel Academy of Sciences and Humanities) and by the Weizmann Institute Renal Research Fund.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Møller J V, Juul B, Le Maire M. Biochim Biophys Acta. 1996;1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.Andersen J P, Vilsen B. FEBS Lett. 1995;359:101–106. doi: 10.1016/0014-5793(95)00019-6. [DOI] [PubMed] [Google Scholar]
- 3.Goldshleger R, Tal D M, Karlish S J D. Biochemistry. 1995;34:8668–8679. doi: 10.1021/bi00027a016. [DOI] [PubMed] [Google Scholar]
- 4.Clarke D M, Loo T W, Inesi G, MacLennan D H. Nature (London) 1989;339:476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- 5.Karlish S J D, Goldshleger R, Stein W D. Proc Natl Acad Sci USA. 1990;87:4566–4570. doi: 10.1073/pnas.87.12.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingrel J G, Kuntzweiler T. J Biol Chem. 1994;269:19659–19662. [PubMed] [Google Scholar]
- 7.Jørgensen P L, Andersen J P. J Membr Biol. 1988;103:95–120. doi: 10.1007/BF01870942. [DOI] [PubMed] [Google Scholar]
- 8.Glynn I M, Karlish S J D. Annu Rev Biochem. 1990;59:171–205. doi: 10.1146/annurev.bi.59.070190.001131. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen P L. Biochim Biophys Acta. 1975;401:399–415. doi: 10.1016/0005-2736(75)90239-4. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen P L, Petersen J. Biochim Biophys Acta. 1985;821:319–333. doi: 10.1016/0005-2736(85)90102-6. [DOI] [PubMed] [Google Scholar]
- 11.Le Maire M, Lund S, Viel A, Champeil P, Møller J V. J Biol Chem. 1990;265:1111–1123. [PubMed] [Google Scholar]
- 12.Andersen J P, Vilsen B, Leberer E, Maclennan D H. J Biol Chem. 1989;264:21108–21023. [PubMed] [Google Scholar]
- 13.Clarke D M, Loo T W, MacLennan D H. J Biol Chem. 1990;265:14088–14092. [PubMed] [Google Scholar]
- 14.Goffeau A, de Meis L. J Biol Chem. 1990;265:15503–15505. [PubMed] [Google Scholar]
- 15.Sigman D S, Chen C B. Annu Rev Biochem. 1990;59:207–236. doi: 10.1146/annurev.bi.59.070190.001231. [DOI] [PubMed] [Google Scholar]
- 16.Hoyer D, Cho J, Schultz P G. J Am Chem Soc. 1990;112:3249–3250. [Google Scholar]
- 17.Rana T M, Meares C F. Proc Natl Acad Sci USA. 1991;88:10578–10582. doi: 10.1073/pnas.88.23.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platis I E, Ermácora M R, Fox R A. Biochemistry. 1993;32:12761–12767. doi: 10.1021/bi00210a027. [DOI] [PubMed] [Google Scholar]
- 19.Chou W-Y, Tsai W-P, Lin C-C, Chang G-C. J Biol Chem. 1995;270:25935–25941. doi: 10.1074/jbc.270.43.25935. [DOI] [PubMed] [Google Scholar]
- 20.Soundar S, Colman R F. J Biol Chem. 1993;268:5264–5271. [PubMed] [Google Scholar]
- 21.Wu J, Perrin D M, Sigman D, Kaback H R. Proc Natl Acad Sci USA. 1995;92:9186–9190. doi: 10.1073/pnas.92.20.9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaim J B, Greiner D P, Meares C F, Gennis R B. Biochemistry. 1995;34:11311–11315. doi: 10.1021/bi00036a002. [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen P L. Methods Enzymol. 1988;156:29–43. doi: 10.1016/0076-6879(88)56005-6. [DOI] [PubMed] [Google Scholar]
- 24.Capasso J M, Hoving S, Tal D M, Goldshleger R, Karlish S J D. J Biol Chem. 1992;267:1150–1158. [PubMed] [Google Scholar]
- 25.Grossman Z, Ram D, Markovics A, Tarrab-Hazdai R, Lantner F, Ziv E, Schechter I. Exp Parasitol. 1990;70:62–71. doi: 10.1016/0014-4894(90)90086-r. [DOI] [PubMed] [Google Scholar]
- 26.Or E, Goldshleger R, Tal D M, Karlish S J D. Biochemistry. 1996;35:6853–6864. doi: 10.1021/bi960093q. [DOI] [PubMed] [Google Scholar]
- 27.Skou J C, Esmann M. Biochim Biophys Acta. 1980;601:386–402. doi: 10.1016/0005-2736(80)90543-x. [DOI] [PubMed] [Google Scholar]
- 28.Castilho R F, Carvalho-Alves P C, Vercesi A E, Ferreira S T. Mol Cell Biochem. 1996;159:105–114. doi: 10.1007/BF00420912. [DOI] [PubMed] [Google Scholar]
- 29.Stadtman E R. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 30.Daly S E, Blostein R, Lane L K. J Biol Chem. 1997;272:6341–6347. doi: 10.1074/jbc.272.10.6341. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Tamas M J, Hall M J, Pascual-Ahuir A, Perlin D S. J Biol Chem. 1996;271:25438–25445. doi: 10.1074/jbc.271.41.25438. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen P A, Rasmussen J H, Jørgensen P L. Biochemistry. 1996;35:16085–16093. doi: 10.1021/bi961614c. [DOI] [PubMed] [Google Scholar]
- 33.Ovchinikov Y A, Dzhandzugazyan K N, Lutsenko S V, Mustayev A A, Modyanov N N. FEBS Lett. 1987;217:111–116. doi: 10.1016/0014-5793(87)81253-x. [DOI] [PubMed] [Google Scholar]
- 34.Ohta T, Nagano K, Yoshida M. Proc Natl Acad Sci USA. 1986;83:2071–2075. doi: 10.1073/pnas.83.7.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadrazeh S M, Eaton J W. J Clin Invest. 1988;82:1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]