Abstract
An osteotropic alendronate-β-cyclodextrin conjugate (ALN-β-CD) was developed as a bone-targeting delivery system for improved treatment of skeletal diseases. The conjugate shows very strong binding to hydroxyapatite (HA, main component of the skeleton). Its ability in forming molecular inclusion complex with prostaglandins E1 (PGE1, a potent bone anabolic agent) was confirmed by phase-solubility experiments and differential scanning calorimetry (DSC). In a bilateral rat mandible model, ALN-β-CD/PGE1 molecular complex was shown to stimulate strong local bone anabolic reaction. In the control study, ALN-β-CD itself was also found to be bone anabolic. To investigate this finding, other control groups were studied. The histomorphometry data suggests that ALN-β-CD itself could generate more new bone at the injection site than its complex with PGE1. Alendronate (ALN) injection could also cause new bone formation, which locates peripheral to the site of injection. PGE1, saline or ethanol injections do not have anabolic effect. These findings were also confirmed by micro-CT evaluation of mandibular bones. It is clear that the bone anabolic effect of ALN-β-CD is independent of mechanical stimuli of the periosteum or ALN injection alone. Further studies are warranted to understand the working mechanism of ALN-β-CD as a bone anabolic agent.
Keywords: Bone regeneration, osteoporosis, hydroxyapatite, drug delivery
1. Introduction
Repair of bone defects due to trauma, arthritis, cancer treatment or other skeletal diseases is expensive and invasive. Substantial research efforts in this area have been focused on effective incorporation of synergistic growth factors, such as bone morphogenetic proteins (BMPs), into the bone grafts [1–3]. Compared to these biologics, the clinical applications of low molecular weight bone anabolic agents such as prostaglandins (E1 and E2) [4,5], statins [6] and prostaglandin EP4 receptor agonists [7,8] have not yet been developed. Prostaglandins are locally secreted, rapidly metabolized, biologically active fatty acids first identified in the prostate [9]. The potency of PGEs as authentic bone anabolic agents was demonstrated convincingly in vivo by both systemic and local delivery [10]. However, the clinical applications of these agents have been limited by their profound side effects on the soft tissues. Yet their outstanding anabolic potency, low cost and relatively stable chemical structures are intriguing for clinical bone anabolic therapy development. With the applications of appropriate delivery systems, one may anticipate widespread clinical applications of the PGEs to accelerate bone defect repair.
Cyclodextrins (CDs) are water-soluble cyclic oligosaccharides, composed of α-D-glucopyranoside units linked 1 to 4. The naturally occurring CDs are α, β and γ CDs having 6, 7 and 8 glucopyranose units, respectively. The annulus interior of CD is hydrophobic, which enables the formation of inclusion complexes with many lipophilic compounds. Being considered biological inert, CDs have been approved by US FDA as pharmaceutical excipients for numerous drug formulations [11]. Recently, we have designed and synthesized an alendronate-β-cyclodextrin (ALN-β-CD) conjugate for local delivery of therapeutic agents to the bone and teeth [12]. In this manuscript, this conjugate was studied as a delivery system for prostaglandin E1 for treatment of bone defects. Surprisingly, when evaluated in vivo, the new β-CD derivative showed a robust bone anabolic effect.
2. Materials and methods
2.1. Abbreviations
ALN, alendronate; ALN-β-CD, alendronate-β-cyclodextrin conjugate; β-CD, β-cyclodextrin; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; prostaglandins E1 (PGE1); HA, hydroxyapatite; HP-β-CD, 2-hydroxylpropyl-β-cyclodextrin; NHS, N-hydroxysuccinimide; RB, rhodamine B; RB-β-CD, RB-labeled β-CD; RB-ALN-β-CD, RB-labeled ALN-β-CD; differential scanning calorimetry (DSC).
2.2. Materials
β-CD was purchased from TCI America (Portland, OR, USA). p-Toluenesulfonyl chloride, 4-pentynoic acid, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), sodium azide, CuSO4.5H2O, sodium ascorbate, dimethylformamide and dichloromethane were purchased from Acros Organics (Morris Plains, NJ, USA). Alendronate (ALN) was purchased from Ultratech India Ltd. (New Mumbai, India). Hydroxyapatite (HA, DNA grade Bio-Gel HTP gel. Surface area ≈ 50 m2/g) was purchased from Bio-Rad (Hercules, CA, USA). Prostaglandins E1 (PGE1) was purchased from Hawkins Inc. (Minneapolis, MN, USA). The Sprague-Dawley rats (retired breeder) were purchased from Charles River Laboratories, Inc. (Wilmington, MA). All other reagents and solvents if not specified were purchased from Fisher Scientifics (Pittsburgh, PA, USA).
2.3. Synthesis of ALN-β-CD
ALN-β-CD (see Figure 1) was synthesized according to the method we reported previously [12]. Briefly, β-cyclodextrin was first toslated with p-toluenesulfonyl chloride and then converted to mono-6-(azido)-β-cyclodextrin (N3-β-CD). Active ester of 4-pentynoic acid was synthesized by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) coupling 4-pentynoic acid with N-hydroxysuccinimide (NHS), then it reacted with alendronate in water under controlled pH to obtain 1-hydroxy-4-pent-4-ynamidobutane-1,1-diyldiphosphonic acid (alkyene-ALN). Finally, alkyene-ALN was conjugated to N3-β-CD via Huisgen 1,3-dipolar cycloaddition (a “click” reaction) with CuSO4/sodium ascorbate as catalysts to obtain ALN-β-CD.
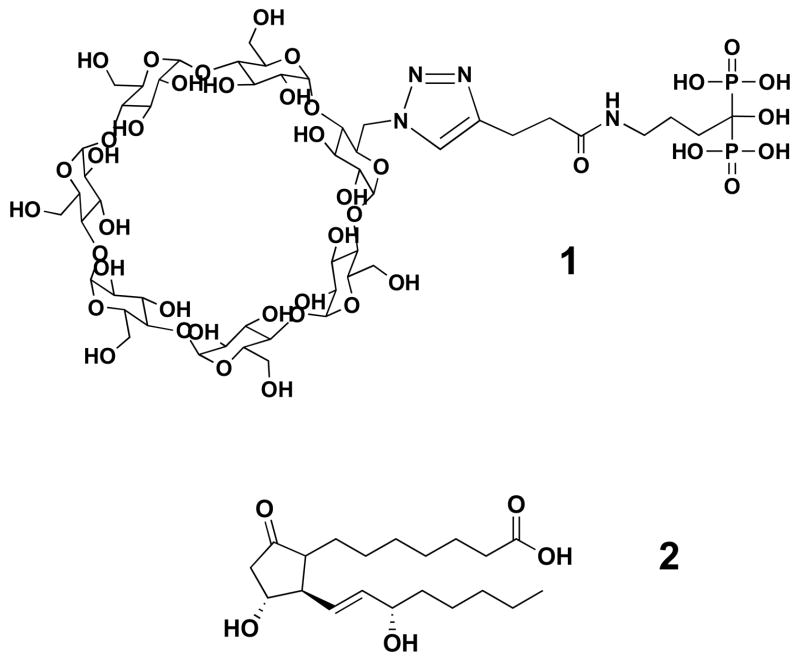
Figure 1.
The chemical structures of ALN-β-CD (1) and PGE1 (2).
2.4. Biomineral-binding ability of ALN-β-CD
ALN-β-CD was first labeled with rhodamine B (RB) by EDC coupling. RB-ALN-β-CD (20 mg) was then dissolved in 0.5 mL H2O and filtered through 0.22 μm filters. It was then agitated with HA (100 mg) for 10 min at 21 °C, HA was removed by centrifugation (10,000 rpm, 2 min), and then washed extensively with H2O and acetone. The recovered HA was dried under vacuum. RB-labeled β-CD (RB-β-CD) and RB were used as controls for non-specific binding.
2.5. Saturated binding of ALN-β-CD on HA
ALN-β-CD (100 mg) and RB-ALN-β-CD (0.2 mg) were dissolved together in PBS (5 mL, pH=7.4, 10 mM) and filtered through 0.22 μm filters. HA powder (50 mg) was added into 1 mL of this solution. After 10 min of agitation at 21 °C, HA was removed by centrifugation (10,000 rpm, 2 min). The absorbance of the supernatant at 560 nm was determined using an UV/Vis spectrophotometer (UV-1601PC, Shimadzu, Kyoto, Japan) and compared with that of the initial RB-ALN-β-CD solution. The analysis was performed in triplicate.
2.6. Phase solubility study of PGE1 in the presence of ALN-β-CD
The solubility study was performed according to Higuchi and Connors [13]. An excess amount of PGE1 (2 mg) was added to aqueous solutions (1.0 mL, pH 5) containing various concentrations of ALN-β-CD (from 0 to 10 mM). The mixtures were agitated at 21 °C for 2 days and then passed through a 0.22 μm filter. The concentration of PGE1 in the filtrate was determined by HPLC (1100 Series, Agilent Technologies, Santa Clara, CA). A reverse phase C18 column (Agilent, 4.6 × 250 mm, 5 μm) was used. Mobile phase: acetonitrile/0.01M KH2PO4 = 42/58 (v/v); UV detection wavelength = 205 nm; Flow rate = 1 mL/min. The analysis was performed in triplicate. The mean value and standard deviation were obtained with Excel.
2.7. Preparation of ALN-β-CD/PGE1 complex formulation
PGE1 (8 mg) was added to 1 mL of aqueous solution of ALN-β-CD (100 mg/mL). Tubes containing the solutions were sealed and shaken at 21 °C for 2 days. Suspensions were then filtered through 0.22 μm filters. Similarly, HP-β-CD/PGE1 complex formulation was also prepared.
2.8. Differential scanning calorimetry (DSC) analyses of ALN-β-CD/PGE1 complex
DSC of PGE1, ALN-β-CD, their mixture and their inclusion complex were performed on a DSC Thermal Analyzer (DSC-50, Shimadzu, Kyoto, Japan) with a temperature range of 30 to 180 °C. The calorimeter was calibrated with various standards covering a range of temperatures exceeding those over which the studied were performed. Samples were sealed in an aluminum pan for analysis with an empty pan as reference. Thermograms were recorded at a scanning speed of 5 °C/min under a nitrogen stream.
2.9. In vitro PGE1 release from ALN-β-CD/PGE1 complex immobilized on HA surface
PGE1 (7.5 mg) and ALN-β-CD (100 mg) was mixed to form a complex in 4 mL H2O according to procedures described in section 2.7. The resulting ALN-β-CD/PGE1 complex solution was added to HA (500 mg), agitated for 10 min, followed by filtration and lyophilization to give the HA powder loaded with ALN-β-CD/PGE1 complex. To determine the total PGE1 content in this HA powder, it was washed with methanol/water (1:1, v/v) 4 times to extract PGE1. After centrifugation (10,000 rpm, 2 min), the combined solution was analyzed with HPLC. The total content of PGE1 in HA loaded with ALN-β-CD/PGE1 was determined as 156 μg/100 mg of HA. HP-β-CD/PGE1 complex was prepared similarly and used as a control for this study. The content of PGE1 in HA incubated with HP-β-CD/PGE1 was only 28 μg/100 mg of HA, which might be attributed to non-specific binding of the complex to HA or the residual solution of the complex on HA surface after filtration. For the in vitro PGE1 release study, ALN-β-CD/PGE1 loaded HA (100 mg) and the control were mixed with PBS (1 mL, pH 7.4, 10 mM) and agitated for 10 min at 21 °C. After centrifugation (10,000 rpm, 2 min), the supernatant was removed and analyzed by HPLC. This extraction and analysis cycle repeated until over 90 % of the initial PGE1 loading was removed. This experiment was performed in triplicate. The mean value and standard deviation were obtained with Excel. The HPLC conditions for all the above PGE1 analyses were as follows: chromatographic column, Agilent C18 reverse-phase (4.6×250 mm, 5 μm); mobile phase, acetonitrile-0.01M KH2PO4 (42:58, v/v) at a flow rate of 1 ml/min; UV detection at 205 nm.
2.10. Animal procedures
A rat bilateral mandible model was used for this study, where the treatment formulation was injected supraperiosteally over the facial aspect of the one randomly-selected side of the mandible, and the control formulation was similarly applied on the contralateral side. Six to eight retired breeders were used in each pairing (Table 1). After one week of acclimation, the skin around the mandible was shaved and swabbed with disinfectant. Immediately before local drug application, animals were sedated with an intraperitoneal injection of 40–87 mg/kg ketamine and 5–13 mg/kg xylazine (Phoenix Pharmaceutical Co. St. Joseph, MO). Injections were done using a 27 gauge × 1 cm needle attached to a tuberculin syringe. The needle was inserted at a marked mandible angle 6 mm deep into muscle just supraperiosteally near the lateral aspect of the mandibular angle and 50 μL of formulation solution was slowly deposited. After 24 days of healing, animals were euthanized by CO2 asphyxiation, and mandibular bone with overlying soft tissue was harvested and placed in 10% buffered formalin immediately. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center.
Table 1.
Histomorphometrical analyses of new bone formation 24 days post-injection of different formulation pairs with treatments randomized to left and right sides of the same animal. “New bone area” and “New bone width-1” were measured with cross sections of decalcified mandibles at the injection sites. “New bone width-2” was measured at approximately 2 mm away from the injection sites. The same treatment was not comparable among different animal groups, since rat retired breeders have significant variation in bone metabolism due to differences in breeding background.
| Groups | New bone area (mm2±SEM) | New bone width-1 (mm±SEM) | New bone width-2 (mm±SEM) |
|---|---|---|---|
| ALN-β-CD/PGE1
HP-β-CD/PGE1 p |
0.97±0.23
0.18±0.09 <0.0001 |
0.50±0.14
0.14±0.06 <0.0001 |
0.17±0.06
0.16±0.06 NS |
| ALN-β-CD
HP-β-CD p |
0.78±0.10
0.25±0.08 <0.003 |
0.36±0.07
0.05±0.02 <0.0002 |
0.18±0.03
0.19±0.11 NS |
| ALN-β-CD/PGE1
ALN-β-CD p |
0.66±0.15
1.11±0.25 <0.02 |
0.23±0.05
0.47±0.14 <0.008 |
0.26±0.13
0.37±0.14 NS |
| ALN
Saline p |
0.61±0.12
0.008±0.008 <0.0004 |
0.14±0.05
0 NS |
0.24±0.11
0.02±0.02 <0.005 |
2.11. Histological preparation and analysis
After at least 24 hours fixation, mandibles were decalcified in 5% formic acid for one week at 4 °C, and gross cut in cross-section at the angle of the mandible to include both sides of the mandibular bone and overlying facial soft tissues at the injection sites. Specimens were dehydrated and embedded in paraffin, then three 5 μm-thick cross-sections were cut at 200 μm intervals to represent an average response, keyed for right and left sides, and stained with hematoxylin and eosin.
Histomorphometric analysis was performed as described previously [14] to include new bone width and area at one mm-intervals from the base of the mandible. Measurements were performed on digitized images using Sigma Scan Pro 5 software (SPSS Inc., Chicago, IL) by two masked evaluators.
2.12. Statistic Analysis
Histomorphometric data from treatment and control sides were compared using analysis of variance (ANOVA).
2.13. Micro-CT evaluation of the mandible bone
The mandibles were scanned at 20 micron resolution with an integration time of 250 milli-seconds using micro-CT (micro-CT-40, Scanco Medical AG, Bassersdorf, Switzerland). An average of 280 slices were used for both cortical and trabecular microarchitectural analyses. A standard convolution-back projection procedure with a Shepp and Logan filter was used to reconstruct the CT images in 1024 × 1024 pixel matrices. A customized thresholding technique (Scanco) was used to provide the best segmentation of the bone tissue. An optimal threshold of 275 was used because it is most appropriate for the trabecular bone tissue in this longitudinal study.
3. Results and discussion
3.1. Biomineral-binding ability of ALN-β-CD
Bisphosphonates are known to have very strong osteotropicity, and the phosphonate groups are vital for all bisphosphonates’ bone-binding ability and the hydroxyl group in between can strengthen the binding [15–17]. Since it is the –NH2 of alendronate that was used for conjugation of ALN to β-CD (Figure 1), we believe that ALN-β-CD should retain the bone affinity of ALN and bind strongly to HA (main bone component). As shown in Figure 2, incubation (10 min) of rhodamine B (RB), RB-labeled ALN-β-CD and β-CD with HA and subsequent repeated washing with water and acetone left the HA treated with RB-labeled ALN-β-CD deep pink, while the HA incubated with RB and RB-labeled β-CD returns to their original color (Figure 2). This observation supports the strong bone-binding ability of ALN-β-CD. Analysis of supernatants at different time points after incubation with HA reveals that the binding of the RB-ALN-β-CD to HA was very fast and could reach a plateau in 2-3 min. Prolonged incubation of the conjugate with HA would not increase its binding ratio. The saturated binding amount of RB-ALN-β-CD on the particular HA powder we used (Bio-Rad, DNA grade Bio-Gel HTP gel) is 256 mg/g.
Figure 2.
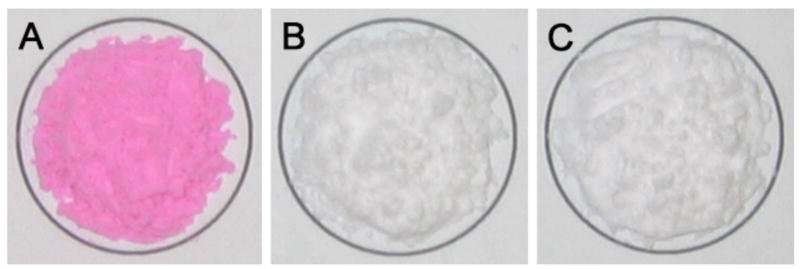
The appearance of hydroxyapatite (HA) powder after incubation with rhodamine B-labeled ALN-β-CD (A), rhodamine B-labeled β-CD (B) and rhodamine B (C). After incubation, the powder was extensive washed with water.
3.2. Formation and characterization of inclusion complex between ALN-β-CD and PGE1
It is known that β-CD can form molecular complexes with numerous compounds, including PGEs [18,19]. The CD ring of ALN-β-CD would act as the drug-loading structure to accommodate low molecular weight drugs. We hypothesize that when ALN-β-CD complexes with PGE1, it becomes water-soluble and adequate for injectable formulation.
In a classical phase solubility study, PGE1 was introduced in the presence of ALN-β-CD aqueous solution with different concentrations. It can be seen in Figure 3 that as the concentration of ALN-β-CD increases, the solubility of PGE1 increases linearly. The solubility diagrams can be classified as AL type according to Higuchi and Connors [13]. The result clearly indicates that ALN-β-CD forms a molecular complex with PGE1 with a stoichiometry of 1:1. The apparent complex stability constant Kc calculated using Equation 1 gave a value of 4.78×103 M−1 for ALN-β-CD/PGE1 molecular complex. This result is very close to the data reported previously with HP-β-CD/PGE1, which suggests that, the conjugation of ALN to β-CD does not impact the complexation ability of β-CD with PGE1[20].
Figure 3.
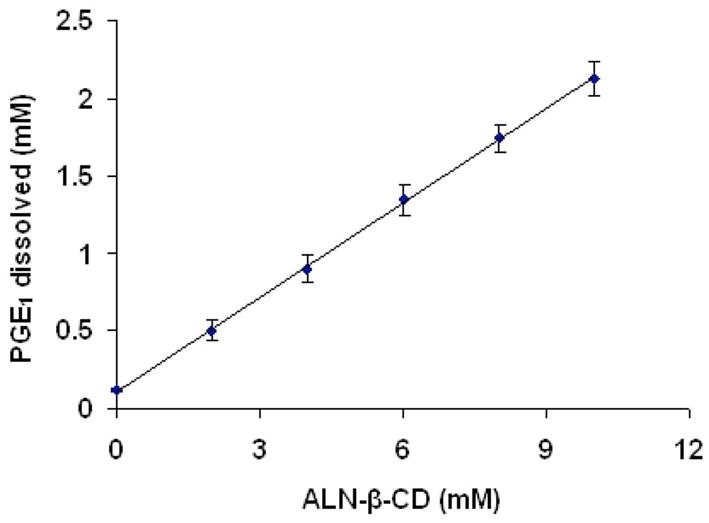
Phase solubility profile of prostaglandin E1 (PGE1) in the presence of ALN-β-CD.
| Eq. 1 |
The differential scanning calorimeter (DSC) analysis of ALN-β-CD, PGE1, their physical mixture and complex obtained by lyophilization are shown in Figure 4. PGE1 shows a characteristic endothermic fusion peak at approximately 116 °C. The thermograms for ALN-β-CD exhibit a dehydration process that takes place at about 80 °C. The DSC thermograms for the physical mixtures ALN-β-CD and PGE1 show peaks corresponding to the pure ALN-β-CD and PGE1, indicate the absence of interaction between the compounds. In the case of the complex obtained by lyophilization, the endothermic peak around 116 °C disappears, suggesting the formation of the molecular inclusion complex of the PGE1 and ALN-β-CD.
Figure 4.
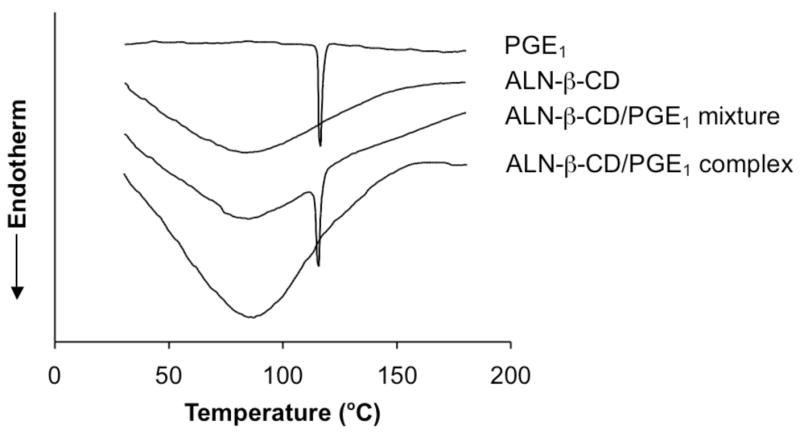
Differential scanning calorimeter (DSC) analysis of PGE1, ALN-β-CD, ALN-β-CD/PGE1 mixture and ALN-β-CD/PGE1 complex.
Clearly, both the phase solubility study and the DSC study support the formation of molecular inclusion complex of the PGE1 and ALN-β-CD. We hypothesize that when the ALN-β-CD/PGE1 formulation is injected subcutaneously onto the mandible bone surface, the bisphosphonate will immediately bind to bone apatite and anchor the entire drug complex at the injection site. Upon interstitial fluid exchange, the complexed PGE1 will be gradually released from the cyclodextrin annuli according to the dilution mechanism [21]. Due to the gradual drug release and local retention of the delivery system, the potential systemic toxicity of PGE1 may be reduced. In addition, the aqueous injectable formulation makes the bony defect repair a minimally invasive procedure. To explore the feasibility of this hypothesis, we performed an in vitro drug release study. ALN-β-CD/PGE1 was first bound to HA powder. It was then subjected to repeated extraction with PBS. As can be seen in Figure 5, ALN-β-CD/PGE1 loaded HA apparently release PGE1 much slower than HP-β-CD/PGE1 loaded HA. However, the release rate seems to be faster than ALN-β-CD/dexamethasone (Dex) complex in our previous report, which may be due to the better water-solubility of PGE1 than Dex [12].
Figure 5.
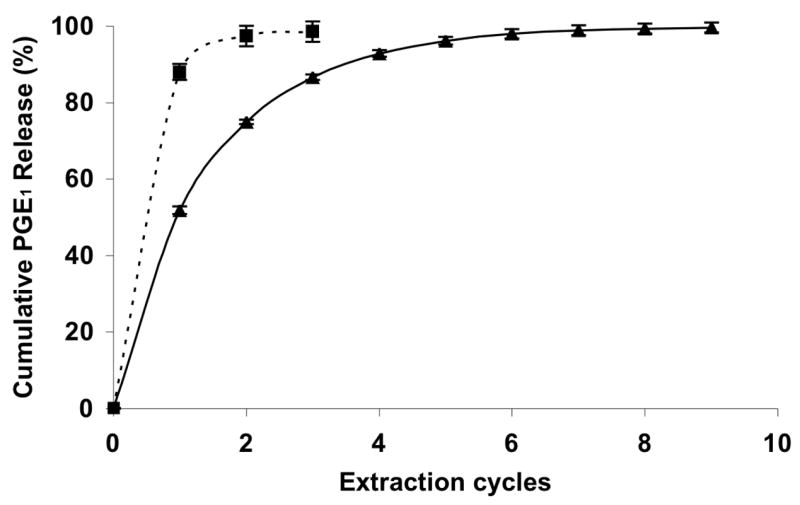
In vitro PGE1 release from ALN-β-CD after ALN-β-CD/PGE1 complex bound to hydroxyapatite (HA) (▲); and PGE1 release from HP-β-CD after HP-β-CD/PGE1 complex non-specifically bound to HA (■).
3.3. In vivo animal study
Modeling the repair of craniofacial bony defects, the anabolic efficacy of ALN-β-CD/PGE1 complex was tested in a rat bilateral mandible model [14], in which one facial side of the mandible is used for treatment and the other facial side for administration of the control. In the initial study, the ALN-β-CD/PGE1 complex (PGE1 = 0.75 mg, ALN-β-CD = 16 mg) was administered to one side of the mandible with HP-β-CD (2-hydroxylpropyl-β-cyclodextrin, with no osteotropicity)/PGE1 complex (PGE1 = 0.63 mg, HP-β-CD = 16.8 mg) as control. After euthanasia (24 days after treatment), mandibles were isolated and processed. The histology and histomorphometry data (Figure 6–A, Table 1) both suggest that ALN-β-CD/PGE1 can induce a very strong localized bone anabolic reaction, but the non-targeted HP-β-CD/PGE1 could not.
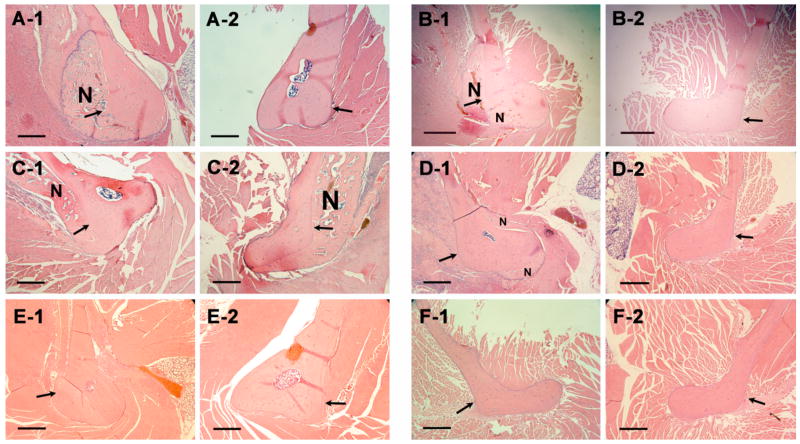
Figure 6.
Histology of decalcified rat mandible pairs 24 days after treatments with different compounds. ALN-β-CD/PGE1 (A-1) vs. HP-β-CD/PGE1 (A-2); ALN-β-CD (B-1) vs. HP-β-CD (B-2); ALN-β-CD/PGE1 (C-1) vs. ALN-β-CD (C-2); ALN (D-1) vs. saline (D-2); PGE1 (E-1) vs. ethanol (E-2); saline (F-1) vs. no treatment (F-2); N = new bone. Bar = 0.5 mm. Arrow indicates the approximate site of injection.
Anticipating no bone anabolic effect, ALN-β-CD (32 mg) and HP-β-CD (33.6 mg) were tested in the control group. Surprisingly, the histological and histomorphometric analysis indicate a strong bone anabolic reaction at the site of ALN-β-CD injection (Figure 6, B–1; Table 1). Conversely, HP-β-CD caused very little bone formation. Histological analyses of areas ~ 2 mm away from the injection site (Table 1, “New bone width-2”) suggest that neither of the two compounds showed much bone formation there. To further confirm this interesting observation, ALN-β-CD/PGE1 complex (PGE1 = 0.75 mg, ALN-β-CD = 20 mg) vs. ALN-β-CD (20 mg) was tested. It was found that ALN-β-CD caused significantly more bone formation than the ALN-β-CD/PGE1 complex (p<0.02). Another observation was that animals receiving the ALN-β-CD/PGE1 complex had a more negative postoperative experience that those with ALN-β-CD alone. PGE1 rats routinely had porphyrin staining about the eyes (sign of stress) and were lethargic for several days’ post-injections, while the ALN-β-CD rats were not. Two PGE1 rats failed to recover. Surviving PGE1 rats had a mean weight loss (−1.5 ± 5.3 g) after 24 days and ALN-β-CD rats gained weight (5.0 ± 5.0 g).
Apparently, this is not what we have anticipated in the design of the delivery system. It seems that ALN-β-CD/PGE1 complex quickly releases PGE1 after local administration, causing the apparent PGE1 side effect. More importantly, the newly found anabolic effect of ALN-β-CD needs to be explained. Some reports suggesting that local applications of bisphosphonates at weight bearing sites may be anabolic [22]. It has also been known that mechanical stimuli (e.g. poking by needle) to the periosteum might lead to a bone anabolic reaction. To delineate if the anabolic reaction observed with ALN-β-CD is due to either of these two mechanisms, ALN (4.1 mg in 50 μL saline) vs. saline and saline vs. no treatment were administered by injection as additional control groups. As shown in Figure 6 and Table 1, direct injection of saline to the mandibular surface did not cause any bone anabolic response. In agreement with previous reports, local injection of ALN does initiate new bone formation. However, the newly formed bone is widely distributed peripheral to the site of injection (Figure 6, D–1), which is clearly different from the anabolic reaction to ALN-β-CD treatment (localized at the injection site). While histology sections provide valued detail of selected locations of the mandible, it could not provide a panoramic view of the entire mandible after treatment. To overcome this limitation, we used micro-computed tomography (μ-CT) to analyze the mandible bone. As can be seen in Figure 7, the μ-CT data shows that the bone anabolic response to ALN-β-CD administration is centered right at the site of the injection. For ALN injection, however, large new bone formation was found at multiple locations peripheral to the injection site. One explanation is that ALN may promote differentiation of mesenchymal stem cells into osteoblasts [23]. As another possibility, this ALN-mediated bone formation could be the direct result of tissues inflammation associated with ALN local administration [24]
Figure 7.
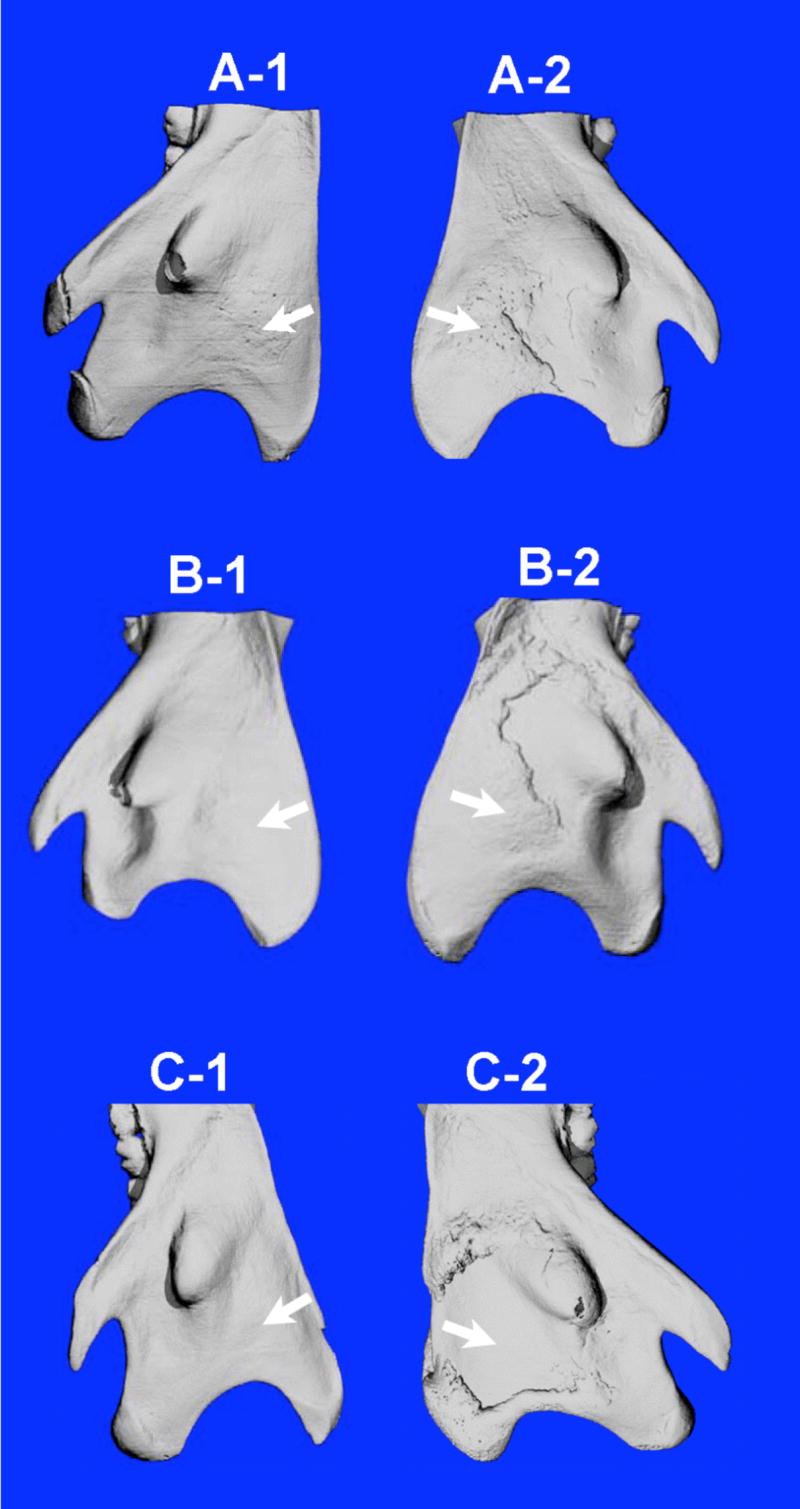
Representative micro-CT images of the lateral aspect of rat mandibular bones 24 days after treatments with different compounds. HP-β-CD/PGE1 (A-1) vs. ALN-β-CD/PGE1 (A-2); HP-β-CD (B-1) vs. ALN-β-CD (B-2); saline (C-1) vs. ALN (C-2). Three rats/groups were used in this study. Arrow indicates the approximate site of injection. The pattern of the new bone generated from ALN-β-CD treatment (B-2) is centered at the site of injection, while the new bone formed by ALN treatment (C-2) is only peripheral to the injection site.
Since CDs are generally known as biologically inert, it is quiet a surprise to find that ALN-β-CD is bone anabolic. Further literature search found that the only known application of a CD derivative as an active therapeutic agent is sugammadex or Org 25969 (per-6-(2-carboxyethylthio)-per-6-deoxy-γ-cyclodextrin), which was developed as a lead compound for antagonism of prolonged rocuronium-induced neuromuscular block [25–27]. While highly water-soluble sugammadex does not have any direct or indirect action on any component of cholinergic transmission, it acts as a solubilized molecular host that complexes with rocuronium and facilitates its movement from the neuromuscular junction back into the plasma, which results in a fast recovery of neuromuscular function.
Based on the working mechanism of sugammadex, we hypothesize that the accidentally discovered bone anabolic effect of ALN-β-CD is probably due to local sequestration of endogenous bone anabolic factors mediated by the ALN-β-CD that immobilized on the bone surface. The bisphosphonate group in the conjugate allows its anchoring to the bone and the hydrophobic β-CD annulus provides a mechanism of molecular complexation and local sequestration of endogenous bone anabolic factors, which result in a bone anabolic reaction. Due to the versatile complexation ability of β-CD, many endogenous bone active lipophilic compounds (e.g. PGE2, lipids, steroids, vitamin D, cholesterol, etc.) may be considered as the potential candidates that would complex with ALN-β-CD immobilized on bone and induce local bone anabolic reactions.
4. Conclusions
We accidentally discovered that ALN-β-CD, an osteotropic cyclodextrin derivative designed as a drug delivery carrier, has bone anabolic effects. The mechanism of the anabolic effect has not been clearly elucidated but is independent of mechanical stimuli to the periosteum, and it does not occur with ALN alone. Potentially, it may act as an immobilized molecular host in bone to sequester local endogenous bone anabolic agents (e.g. PGE2), which directly mediate the bone formation. Further experiments need to be done to confirm this hypothesis. As a potential therapeutic strategy to treat skeletal defect, the bone anabolism of ALN-β-CD needs further validation on animal models of bone metabolic diseases, such as osteoporosis.
Acknowledgments
We acknowledge the financial supports from the College of Pharmacy, University of Nebraska Medical Center, the Technology Advancement Group (TAG) of UNeMed Corporations and NIH Grant AR053325. ATW was supported by a fellowship from Nebraska Society of Periodontology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mussano F, Ciccone G, Ceccarelli M, Baldi I, Bass F. Bone morphogenetic proteins and bone defects: a systematic review. Spine. 2007;32:824–830. doi: 10.1097/01.brs.0000259227.51180.ca. [DOI] [PubMed] [Google Scholar]
- 2.Wan DC, Nacamuli RP, Longaker MT. Craniofacial bone tissue engineering. Dent Clin North Am. 2006;50:175–190. doi: 10.1016/j.cden.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Lane JM. Bone morphogenic protein science and studies. J Orthop Trauma. 2005;19:S17–22. doi: 10.1097/00005131-200511101-00006. [DOI] [PubMed] [Google Scholar]
- 4.Miller SC, Marks SC., Jr Local stimulation of new bone formation by prostaglandin E1: quantitative histomorphometry and comparison of delivery by minipumps and controlled-release pellets. Bone. 1993;14:143–151. doi: 10.1016/8756-3282(93)90241-2. [DOI] [PubMed] [Google Scholar]
- 5.Jee WS, Ke HZ, Li XJ. Long-term anabolic effects of prostaglandin-E2 on tibial diaphyseal bone in male rats. Bone Miner. 1991;15:33–55. doi: 10.1016/0169-6009(91)90109-d. [DOI] [PubMed] [Google Scholar]
- 6.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Sakai A, Uchida S, Tanaka S, Nagashima M, Katayama T, et al. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone. 2004;34:940–948. doi: 10.1016/j.bone.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Ke HZ, Crawford DT, Qi H, Simmons HA, Owen TA, Paralkar VM, et al. A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Miner Res. 2006;21:565–575. doi: 10.1359/jbmr.051110. [DOI] [PubMed] [Google Scholar]
- 9.Mann T. Secretory function of the prostate, seminal vesicle and other male accessory organs of reproduction. J Reprod Fertil. 1974;37:179–188. doi: 10.1530/jrf.0.0370179. [DOI] [PubMed] [Google Scholar]
- 10.Vrotsos Y, Miller SC, Marks SC., Jr Prostaglandin E--a powerful anabolic agent for generalized or site-specific bone formation. Crit Rev Eukaryot Gene Expr. 2003;13:255–263. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.170. [DOI] [PubMed] [Google Scholar]
- 11.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 12.Liu XM, Lee HT, Reinhardt RA, Marky LA, Wang D. Novel biomineral-binding cyclodextrins for controlled drug delivery in the oral cavity. J Control Release. 2007;122:54–62. doi: 10.1016/j.jconrel.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi T, Connors KA. In: Advances in analytical chemistry and instrumentation. Reilly CN, editor. Vol. 4. New York: Wiley-Interscience; 1965. pp. 117–212. [Google Scholar]
- 14.Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, et al. Local simvastatin effects on mandibular bone growth and inflammation. J Periodontol. 2005;76:1861–1870. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH, Russell G, Gertz B. Pharmacokinetics of alendronate: an overview. Int J Clin Pract Suppl. 1999;101:18–26. [PubMed] [Google Scholar]
- 17.Papapoulos SE. Bisphosphonates: structure-activity relations from a clinical perspective. Medicina (B Aires) 1997;57(Suppl 1):61–64. [PubMed] [Google Scholar]
- 18.Yamamoto M, Hirayama F, Uekama K. Improvement of stability and dissolution of prostaglandin E1 by maltosyl-beta-cyclodextrin in lyophilized formulation. Chem Pharm Bull (Tokyo) 1992;40:747–751. doi: 10.1248/cpb.40.747. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama F, Kurihara M, Uekama K. Improving the aqueous stability of prostaglandin E2 and prostaglandin A2 by inclusion complexation with methylated-beta-cyclodextrins. Chem Pharm Bull (Tokyo) 1984;32:4237–4240. doi: 10.1248/cpb.32.4237. [DOI] [PubMed] [Google Scholar]
- 20.Gu FG, Cui FD, Gao YL. Preparation of prostaglandin E1-hydroxypropyl-beta-cyclodextrin complex and its nasal delivery in rats. Int J Pharm. 2005;290:101–108. doi: 10.1016/j.ijpharm.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Stella VJ, Rao VM, Zannou EA, Zia VV. Mechanisms of drug release from cyclodextrin complexes. Adv Drug Deliv Rev. 1999;36:3–16. doi: 10.1016/s0169-409x(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 22.von Knoch F, Eckhardt C, Alabre CI, Schneider E, Rubash HE, Shanbhag AS. Anabolic effects of bisphosphonates on peri-implant bone stock. Biomaterials. 2007;28:3549–3559. doi: 10.1016/j.biomaterials.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Duque G, Rivas D. Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. J Bone Miner Res. 2007;22:1603–1611. doi: 10.1359/jbmr.070701. [DOI] [PubMed] [Google Scholar]
- 24.Goldring SR, Gravallese EM. Bisphosphonates: environmental protection for the joint? Arthritis Rheum. 2004;50:2044–2047. doi: 10.1002/art.20383. [DOI] [PubMed] [Google Scholar]
- 25.Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J Med Chem. 2002;45:1806–1816. doi: 10.1021/jm011107f. [DOI] [PubMed] [Google Scholar]
- 26.Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005;103:695–703. doi: 10.1097/00000542-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson WT, Sprung J, Jankowski CJ. Sugammadex: a novel agent for the reversal of neuromuscular blockade. Pharmacotherapy. 2007;27:1181–1188. doi: 10.1592/phco.27.8.1181. [DOI] [PubMed] [Google Scholar]