Abstract
River blindness is a seriously debilitating disease caused by the filarial parasite Onchocerca volvulus, which infects millions in Africa as well as in South and Central America. Research has been hampered by a lack of good animal models, as the parasite can only develop fully in humans and some primates. This review highlights the development of two animal model systems that have allowed significant advances in recent years and hold promise for the future. Experimental findings with Litomosoides sigmodontis in mice and Onchocerca ochengi in cattle are placed in the context of how these models can advance our ability to control the human disease.
Introduction
Infection with Onchocerca volvulus, a filarial nematode, can lead to debilitating skin disease and blindness (river blindness). Adult worms live in subcutaneous nodules; however, the pathology of onchocerciasis is primarily associated with death of microfilariae larvae in the skin and eyes (Figures 1 and 2). It is estimated that 37 million people are infected with O. volvulus [1], over 99% of whom live in West and Central Africa, although there are significant foci in South and Central America. Early attempts at control of onchocerciasis relied on treatment of water courses with insecticides to kill the larvae (larviciding) of the blackfly (Simulium spp.) vectors. Using this approach for over 25 years, the WHO/UNDP Onchocerciasis Control Programme (OCP) reduced the burden of disease in savannah regions of West Africa [2],[3]. In 1987, ivermectin (Mectizan, Merck & Co.) was introduced for mass treatment of onchocerciasis either alone or in combination with larviciding. The OCP closed in December 2002, and control of onchocerciasis now relies on community-based treatment with ivermectin implemented through the African Programme for Onchocerciasis Control (APOC) [4]. The Onchocerciasis Elimination Programme for the Americas similarly distributes Mectizan twice a year in its target countries of Brazil, Colombia, Ecuador, Guatemala, Mexico, and Venezuela [5].
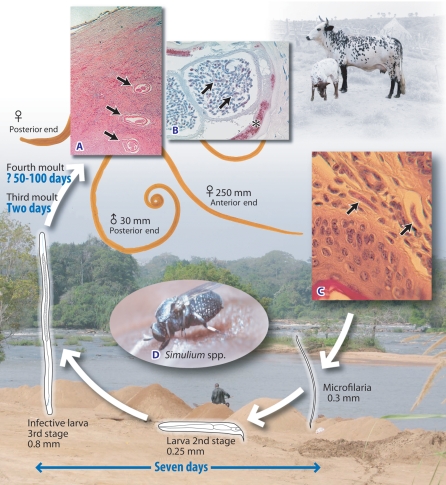
Figure 1. Life cycle of Onchocerca volvulus and Onchocerca ochengi.
Adult female worms initiate the formation of nodules in the skin (onchocercomas) (see Figures 2 and 3) in which their highly coiled bodies can reach a length of approximately 25 cm, while the males are a little over 1/10th that length. Transverse sections of adult female worms in the onchocercoma are shown in (A). Following mating, embryos develop inside the female, which gives birth to motile L1 larvae that are known as microfilaria (MF). A transverse section of an adult female with MF in utero is shown in (B); Wolbachia in lateral hypodermal chords (*) of the adult female and uterine microfilaria (arrows) are stained red. MF migrate into the dermis (shown in [C]), where they are available for transmission to the simuliid blackfly vector (shown in [D]). Within the fly, MF develop further as L1 larvae and molt into second-stage larvae, which molt again to become the infective L3 larvae (7 days). The L3 enter the skin through the wound caused by the feeding fly. The blackfly requires fast moving water to breed and thus infection occurs adjacent to rivers. Adult female worms live for several years and individuals (people or cattle) can remain microfilaraemic for their entire lives if repeatedly exposed to infection. (Photo credits: M. Boussinesq, S. Spetch, J. Allen, O Bain, S. Wanji, S. Uni)
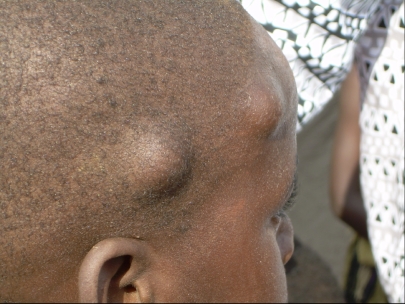
Figure 2. Subcutaneous Nodules on a Child in Ghana.
Photo credit: P. Soboslay.
Ivermectin is very effective at killing microfilariae and has proved successful in reducing morbidity within the community and the risk of severe skin or ocular disease for the individual. However, its macrofilaricidal activity (i.e., efficacy against adult parasites) is, at best, slow and partial, necessitating the use of repeated drug administration for several years [6]–[8]. Furthermore, early hopes that mass treatment with ivermectin would eradicate the disease by breaking transmission have not been realised [2] because of inadequate treatment coverage, migration, and recrudescence of infections in areas where treatment has been suspended. In addition, there is mounting evidence that resistance to ivermectin is emerging [9]–[13]. Such circumstances require development of complementary measures to sustain even the current levels of control, let alone eliminate the disease. What are needed is a safe and effective macrofilaricide and a vaccine.
A major obstacle facing onchocerciasis research and, particularly that concerned with vaccine development, has been the absence of good animal models. Use of mice was limited because they are unable to support cyclical development of filariae species. All rodents are strictly non-permissive to O. volvulus, which can develop only in primates, and thus studies of protective immunity in mice involve implantation of infective stage larvae (L3) into subcutaneous chambers [14]. Mice are somewhat more permissive to Brugia species (causative agents of lymphatic filariasis) but still do not allow natural tissue migration or development of circulating microfilariae. Patent infections with circulating microfilariae can be established in the Mongolian gerbil (Meriones unguiculatus) with Brugia species and Acanthocheilonema viteae; however, the absence of reagents places serious restrictions on immunological investigation. Nonetheless, despite limitations, these models have made significant contributions to our knowledge of filarial infections (reviewed in [14]–[16]) and provided a basis of more recent investigations using two new models.
The first is Litomosoides sigmodontis (Table 1), a natural parasite of the cotton rat (Sigmodon hispidus) that in the early 1990s was found to undergo complete development in BALB/c mice and produce patent infections with circulating microfilariae within 55–60 days post-infection [17]. Development of L. sigmodontis in other inbred strains is restricted. For example, in C57BL/6 mice, filariae are progressively killed and never produce a patent infection. It is now possible to utilise the full power of murine immunology to study the interaction of filarial parasites with their hosts at all stages of the parasite's development from migration of infective larvae to the production of microfilariae. The ability of L. sigmodontis to achieve patency allows a comparison to human studies not possible in other murine models. The data thus far show a striking similarity to human studies, particularly in the context of regulation (discussed below); thus, through experimental manipulation, this model can provide mechanistic explanations of susceptibility and resistance not possible in any other system.
Table 1. General Features of the Biology of O. volvulus, O. ochengi, and L. sigmodontis .
| Filariae | Vector | Time to Patency | Adult | Mf | Disadvantages | Advantages |
| O. volvulus | Blackfly, Simulium spp. | 250–375 days | Subcutaneous nodules | Skin | Experimentation not possible | The target organism |
| O. ochengi | Blackfly, Simulium spp. | From 250 days | Intradermal nodules | Skin | Outbred animals, no pathology | –Very closely related to O. volvulus |
| –Experimentation under natural challenge | ||||||
| –Infection quantifiable | ||||||
| L. sigmodontis | Tropical rat mite, Ornithonyssus bacoti | ∼50 days | Thoracic cavity | Blood | Not skin dwelling, no pathology | –All stages of the life cycle accessible for experimentation |
| –Power of murine immunology | ||||||
| –Protective immunity evoked by vaccination |
The second model is Onchocerca ochengi in cattle (Table 1; Figure 3). This is the closest known relative of the human parasite and is also transmitted by the blackfly, Simulium damnosum sensu lato. O. ochengi is confined to Africa and combines many important features of the human infection [18]. Most importantly, O. ochengi forms nodules that closely resemble those of O. volvulus and which can be enumerated non-invasively or removed for analysis during immunological or chemotherapeutic studies. Furthermore, putative immune animals exist naturally in endemic areas and exhibit demonstrable resistance to infection [19]. The O. ochengi model thus provides the unique opportunity to undertake controlled experiments, both laboratory-based and under natural challenge in the field, that are not possible in humans.
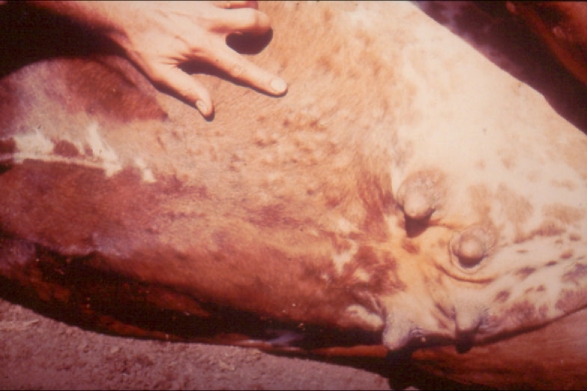
Figure 3. Intradermal Nodules Containing Adult Onchocerca ochengi on Ventral Hide of a Naturally Infected Cow (Bos indicus) in Cameroon.
Photo credit: A. J. Trees.
The main drawback to both of these model systems is that they do not allow the investigation of disease pathology relevant to human onchocerciasis. This work will continue to rely on either human field studies or experimental exposure of mice to Onchocerca antigens in a model of ocular disease [20].
Mechanisms of Parasite Killing
In the L. sigmodontis model, innate responses at the inoculation site are associated with destruction of a majority of L3s in the subcutaneous tissue within 2 days post-infection. However, about one-third of L3 larvae avoid this attack by entering lymphatic vessels [21],[22], a strategy characteristic of many human filariae [23],[24]. The number of larvae that survive this early stage varies depending on sex and strain of the host [25], but is unaffected by the size of the initial inoculum [26]. From Day 4 post-inoculation, surviving L3 begin to appear in the pleural cavity of L. sigmodontis–infected mice. Differences in the pattern of development of the parasites in resistant C57BL/6 and susceptible BALB/c mice appear early and get progressively more apparent [25]. By 30 days post-infection, about one-third of the population in C57BL/6 mice are still at the L4 stage; this contrasts with <15% in susceptible BALB/c mice [27]. Furthermore, worms recovered from the C57BL/6 mice are smaller than those from BALB/c mice. Analysis of cytokine production at this time shows mixed T helper cell type 1 (Th1)-Th2 response in the C57BL/6 mice reminiscent of that observed in putative immune human patients [28]. In BALB/c mice, the cytokine response is more biased towards Th2 (see Box 1).
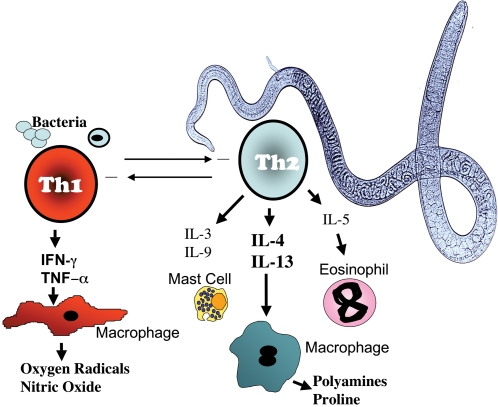
Box 1. Th1 & Th2 Immunity
Helminth parasites are typically associated with the induction of CD4+ T helper 2 (Th2) cells, while microbial pathogens induce Th1 responses. Filarial parasites constitute a unique stimulus to the immune system, as they are worms that (in most cases) contain endosymbiotic bacteria (Wolbachia). The Th1 response functions to activate macrophages to be more efficient at microbial destruction and is essential to survive infection with many intracellular pathogens such as Mycobacteria and Salmonella. The Th2 response is involved in expelling worms from the intestines as well as encapsulating and destroying multicellular parasites. The Th2 response also plays a key role in wound healing and allergic reactions. Macrophages are mediators of both Th1 and Th2 immunity but exhibit different functions. Mast cells and eosinophils are dependent on Th2 cytokines for expansion and recruitment. Th1 and Th2 responses are also associated with differing antibody isotype profiles, with Th1 cytokines promoting cytophilic antibodies while Th2 responses promote antibodies involved in allergic-type responses such as IgE. In addition to T helper cells, T regulatory subsets exist that function primarily to prevent host damage caused by overactive effector responses. These are associated with the production of TGF-β and/or IL-10. Neutrophils, not pictured here, are phagocytic cells of the innate immune system that may become activated prior to Th1/Th2 polarisation, but are also strongly associated with a fourth CD4+ T helper subset: Th17 cells. Th17 cells are strongly pro-inflammatory and have roles in mediating autoimmune disease as well as protection against extracellular bacteria and may exacerbate pathology during helminth infection (for a review of T helper subsets in helminth infection, see Díaz and Allen [87]).
The ability of filarial parasites to induce Th2-type immune responses is well documented, but whether this bias is detrimental or beneficial for the parasite is not always clear. However, infection of IL-4–deficient C57BL/6 mice leads to full parasite development and patency, indicating that a Th2 response is the key determinant of resistance in these non-permissive mice [29]. Consistent with a role for type 2 immunity in parasite killing, partially resistant 129/SvJ mice with a genetic deficiency in either major basic protein or eosinophil peroxidase were found to harbour several times more adult worms than their wild-type littermates [30]. Further, BALB/c mice deficient in IL-4, IL-5, or IL-4Rα (unable to respond to IL-4 or IL-13) present with levels of microfilariae 100 times higher than wild-type controls [31],[32]. This evidence that type 2 cytokines can control microfilarial levels is consistent with studies on Brugia species [33].
Although the data began to build a convincing argument for Th2 control of filarial infections, the picture that emerged proved more complex. The BALB/c IL-4Rα–deficient mice presented a paradox, for although the mice had enormously increased numbers of circulating microfilaria relative to wild-type mice, death of the adult parasites was accelerated. Examination of the effector cells at the site of infection demonstrated that the mice had converted to a Th1 phenotype suggesting that a pro-inflammatory type 1 response was capable of killing the adult parasite (J. Allen and M. G. Nair, unpublished data). Consistent with this, more adult worms are recovered from mice genetically deficient in the type 1 cytokine, IFN-γ [34],[35] Indeed, IFN-γ and IL5 appear to act synergistically to destroy adult parasites [34],[36]. Thus, although Th2 responses seem capable of mediating destruction of the larval stages, both Th1 and Th2 may be needed to contain the more resilient adult stage.
Induction of a Th1 response may be a consequence of the presence of the endosymbiont bacterium Wolbachia found in most human-pathogenic filariae [37]. Filarial-infected humans, cattle, and mice demonstrate significant immune responses to the major surface protein (WSP) of the bacteria [38],[39] (B. Makepeace and A. Trees, unpublished data). Further, WSP as well as the bacteria in total have been shown to stimulate a typical TLR-dependent inflammatory response with induction of IL-6, TNF, etc. by macrophages [40]–[42] and exhibit potent chemotactic activity for neutrophils [41]. Mice with a natural mutation of TLR4 (C3H/HeJ) show a higher degree of susceptibility to L. sigmodontis infection [43]. This is consistent with protection studies in the O. volvulus mouse chamber model that identified a TLR4-dependent larval killing mechanism, albeit with no evidence for Wolbachia involvement [44]. The costs and benefits of symbiosis with Wolbachia for filariae in terms of manipulation of host immune responses have yet to be investigated in depth. However, elimination of Wolbachia from O. ochengi leads to a profound reduction in local neutrophilia in the nodule and a marked infiltration of eosinophils, which degranulate on the cuticle of adult worms prior to parasite death [45]. This is compatible with a potential role for Wolbachia in modulating the anti-nematode response.
Regulation
Although a clearer picture of how mammalian hosts can kill filarial nematodes is emerging, in a successful infection these mechanisms fail. Human studies have long since demonstrated that filarial parasites induce a state of hypo-responsiveness in the host that is associated with the presence of circulating microfilaria [46]. Both the L. sigmodontis and O. ochengi models can mimic this, with Th1 and Th2 cytokines down-regulated coincident with the onset of patency [47],[48]. Intrinsic defects in T cell responses in human filarial infection are linked with expression of the T cell–inhibiting receptor, CTLA-4 [49], and neutralisation of CTLA-4 in mice results in enhanced L. sigmodontis killing [50]. In addition to this intrinsic T cell hypo-responsiveness, T cell responses in humans can be dampened by suppressive antigen-presenting cells [51]. Both mechanisms are operative in the L. sigmodontis model where macrophages that block proliferation of T cells are present at the site of infection prior to patency but become apparent in the draining lymph nodes only following patency [52]. Studies in susceptible BALB/c mice have now directly demonstrated that L. sigmodontis survival is dependent on the induction of a regulatory T cell population that induces hypo-responsiveness [48]. This corroborates the data from human field studies demonstrating that T regulatory (Treg) cells can be isolated from onchocerciasis patients [53], and generalised onchocerciasis is associated with antigen-specific Treg cells that can be found in nodules [54]. These studies demonstrate the utility of the L. sigmodontis model to reveal details of protective and regulatory mechanisms that can help explain observations made in human infections.
The importance of immune regulation in parasite survival is also illustrated by the study of mechanisms that determine microfilarial survival. Different inbred strains of mice differ widely in their capacity to eliminate circulating microfilariae, and these genetically determined differences can be attributed to a single gene locus [55]. However, irrespective of host genetic background, microfilarial density is regulated by the adult female [56]. An immune regulatory environment with interleukin 10 (IL-10) as a key player is induced by the female parasite to facilitate the survival and persistence of her offspring [56]. In the absence of IL-4, normally resistant C57BL/6 mice develop patent infection, but the additional knock-out of IL-10 reverts mice back to a resistant phenotype [57]. This suggests that IL-10 is inhibiting an anti-worm effector response that is redundant when a full Th2 response is in place. In this scenario, wild-type C57BL/6 are non-permissive because Th2 immunity prevents worm development and patency. In the absence of IL-4, patency occurs because Th2-dependent mechanisms are absent but IL-10 is present, suppressing alternative, potentially innate, effector responses. In the absence of both cytokines, IL-10 restraint of innate mechanisms is lifted and once again worms are targeted by the immune response. Consistent with a role for IL-10 in suppressing effector responses, transgenic overexpression of IL-10 in macrophages in genetically resistant FVB mice leads to patency [36]. Design of effective vaccines must take into account that destruction of each parasite stage may require activation of distinct effector pathways and that the parasites themselves induce powerful regulatory networks to modulate these pathways.
Vaccine-Mediated Immunity
The ability of irradiated L3 to generate protection in naïve animals challenged experimentally with normal larvae has been demonstrated in numerous models of filariasis [14],[58], including both the L. sigmodontis [21],[58],[59] and O. ochengi models [19]. The protective efficacy of irradiated L3 has been successfully translated into a field trial using O. ochengi in cattle in which significantly lower worm burdens were observed in vaccinated animals compared to controls after almost 2 years of continuous exposure to intense natural challenge from infected Simulium [19]. This success contrasts with the failure of cattle to develop immunity after drug-abbreviated infections. When naïve, infection-free calves were exposed to sustained and intensive levels of natural challenge, monthly or 3-monthly prophylaxis with macrocyclic lactones completely prevented the development of adult worms. However, when chemotherapy ended but exposure continued, these animals were found to be more susceptible to infection than previously unexposed controls [60] both in terms of adult numbers and microfilarial levels. Similarly, following successful macrofilaricidal treatment of pre-existing patent infections with melarsomine [19] or oxytetracycline [61], cattle were fully susceptible to re-infection. These data suggest that parasite death is an insufficient stimulus for the induction of protective immunity and highlight the importance of defining the mechanisms by which irradiated L3 induce protection.
The L. sigmodontis system allows the careful study of vaccine-mediated protection, including larval migration as early as 6 hours post-infection or challenge, as well as the impact on subsequent development and ability to develop patent infection. Immune protection generated by irradiated L. sigmodontis larvae leads to rapid destruction of the challenge larvae in the subcutaneous tissue [21],[62] and protection is long-lived [63]. Studies with gene-deficient mice showed that vaccination success depends on IL-5 and antibody [22],[59], and this is consistent with observations made using the O. volvulus mouse chamber model [64]. Evidence suggests that the pattern of migration of irradiated L3 does not differ from that of untreated L3 in the first 2 weeks of infection [62]. Further, in normal infections a high proportion of incoming larvae die and yet this does not afford protection to re-infection. These findings argue against protection as a consequence of premature parasite death or aberrant migration. L3 larvae of filarial parasites are known to induce regulatory pathways [65], and irradiated L3 may be failing to produce molecules that initiate downregulatory pathways in the host. Conversely (but not mutually exclusive) irradiated larvae may be failing to shut down the expression of early genes and thus potentially overexpress immunogenic molecules. Powerful genomic and proteomic tools are now available to address this question and to this end, extensive expressed sequence tag (EST) analysis of L. sigmodontis stage-specific genes is well underway [66],[67], which will help to identify both targets of immunity as well as potential immune regulators.
Because disease is associated with the microfilarial stage in onchocerciasis and because this stage is the key to transmission, an anti-microfilarial vaccine also needs to be considered. Indeed, vaccination with microfilariae of O. lienalis in a bovine system was shown some years ago to enhance the clearance of microfilariae subsequently transplanted into the same animal [68]. Moreover, in natural infections of cattle with O. ochengi, skin microfilarial density falls with age in spite of increasing numbers of fecund female worms, which suggests a level of stage-specific microfilarial immunity may develop [69]. Similar experiments using mice as a surrogate host of O. volvulus demonstrated that microfilariae of the human parasite are vulnerable to immune killing and that these responses can be evoked by related species; in this case, O. lienalis [70]. However, what is also clear is that female worms can and do modulate these protective responses [56], and that for any anti-microfilarial vaccine to be effective it must target these parasite regulatory molecules as well as the microfilarial antigens. While there are many reports identifying potential regulatory molecules [71], the search is by no means over. Identification of both parasite-derived immunomodulators and the relevant microfilarial-specific targets is now being facilitated by the filarial genome project [72] and L. sigmodontis EST analysis [66]. The L. sigmodontis and O. ochengi models offer powerful complementary systems to test these candidates in carefully controlled laboratory settings and field settings under natural challenge.
Alternative Treatments Targeting Wolbachia
Control of onchocerciasis in Africa relies on mass distribution of microfilaricidal ivermectin. Given the impossibility of onchocerciasis eradication with ivermectin alone [2] and rising concerns about resistance to this drug [9]–[13], there is a more pressing need to identify complementary therapy using existing drugs. The development of a new drug, apart from the enormous costs, would take 15 years or more to be completed.
Attention has focused on Wolbachia, the bacterial endosymbionts found in most filarial species, as a potential target [73],[74]. Studies with L. sigmodontis have established that both rifampicin [75] and tetracyclines cause growth retardation and sterilisation of adult worms, but in the latter case daily treatment for at least for 4 weeks is required [76]. In cattle, long-term, intermittent antibiotic chemotherapy with oxytetracycline is macrofilaricidal and worm death is preceded by a considerable reduction in Wolbachia [77]. In contrast, short-term, intensive treatment (daily therapy for 2 weeks) induces only transient and inconsequential effects on Wolbachia numbers and is not macrofilaricidal [78]. In humans, 6 weeks of treatment with 100 mg per day of doxycycline has resulted in a complete inhibition of embryogenesis from between 18 [74],[79],[80] and 24 months [73]. Logistical considerations and compliance will demand shorter regimes if tetracyclines are to find their way into routine use against onchocerciasis. One approach will be to identify combination therapies. Given that an added benefit of long-lasting sterilisation of female worms will be interruption of transmission, research in this area should be considered a priority. Importantly, the most recent results show that increasing the dose of doxycycline to 200 mg also exhibits a strong macrofilaricidal effect in human onchocerciasis [81],[82]. However, it must also be recognised that there are restrictions on use of this class of antibiotics in young persons and pregnant women. Nevertheless, these observations have intensified strategies to exploit the Wolbachia genomes for improved antibiotic targeting [83]. In addition, for final local elimination, e.g., in foci in the Americas, anti-wolbachial chemotherapy is being considered [81].
Targeting Wolbachia may also resolve the problem of ivermectin use in areas where onchocerciasis and loiasis are co-endemic and where mass treatment is often discouraged because of severe adverse reactions that result from the rapid destruction of Loa loa microfilariae in the central nervous system [84],[85]. L. loa does not possess endosymbiont Wolbachia [86], and therefore a therapy that targets the bacteria in O. volvulus should have no effect on L. loa. Targeting Wolbachia is arguably the only approach currently available (apart from suramin treatment in a hospital) to treat potentially resistant strains of O. volvulus.
Conclusions
Ten years ago, the mechanisms by which filarial nematodes are killed by the mammalian host were largely unknown. Although fine detail of these processes remain to be determined, the animal models have now allowed us to determine conclusively that Th2 responses drive protective immunity against L3 larvae as well as the microfilarial stage. Bigger weaponry that includes a Th1 pro-inflammatory component may be needed to tackle the adult stage. However, in successful infections all these mechanisms fail because of the ability of the parasite to initiate regulatory pathways. Bypassing this regulation may be the key to development of a vaccine and future disease control. This will require a thorough understanding of how the parasite induces regulation and identification of the targets and processes that mediate a protective but non-pathological response. In the meantime, the prospect of developing new drug regimes using antibiotics to complement ivermectin treatment and to achieve a macrofilaricidal activity may mitigate against problems of emerging drug resistance and offer new therapy in cases where ivermectin is contra-indicated.
Box 2. Methods
Selection of the publications cited was based on four approaches:
Direct knowledge of the authors of this manuscript regarding key papers or unpublished research.
Searches online (predominantly PubMed) for filariasis and a relevant keyword. For example, filarial* and regulat* were used to ensure that we were aware of relevant papers when writing about immune regulation.
Searches on line (predominantly PubMed) for information on a particular topic that may not be recalled by a “filaria*” search (for example, “ivermectin resistance”).
Review of the main Web sites that maintain up-to-date information on disease statistics for river blindness and related diseases. These include:
○ The WHO: http://www.who.int/topics/onchocerciasis/en/
○ The Carter Center: http://www.cartercenter.org/health/river_blindness/index.html
○ Sightsavers: http://www.sightsavers.org/What20We20Do/Eye20Conditions/River20Blindness/World1622.html
○ Global Partnership to Eliminate Riverblindness: http://www.worldbank.org/afr/gper/
Box 3. Learning Points
Although ivermectin has made an immense contribution to onchocerciasis control, it cannot abrogate transmission, and its efficacy is threatened by emerging drug resistance. Therefore, a drug that is effective against adult worms, or a vaccine, is required.
Progress on understanding protective immunity in onchocerciasis has been accelerated by two model systems in particular, Litomosoides sigmodontis in mice and Onchocerca ochengi in cattle.
In both mice and cattle, immunisation with irradiated third-stage larvae (L3) induces significant protection, providing proof-of-principle for a vaccine. In contrast, drug-abbreviated infections fail to induce protective immunity.
Onchocerca volvulus, O. ochengi, L. sigmodontis, and many other filariae contain endosymbiotic bacteria (Wolbachia), which, if depleted by prolonged antibiotic chemotherapy, result in adult worm death. Shorter treatment regimens involving drug combinations are being investigated.
Immunity against filarial parasites is complex, with Th2-type mechanisms driving protection against L3 and microfilariae, whilst both Th1 and Th2 pathways are involved in resistance to adult worms. Parasite survival is achieved by the induction of an immunoregulatory milieu.
Acknowledgments
Special thanks must go to the onchocerciasis patients who graciously participated in our studies over many years and to the Ministries of Health in Ghana, Cameroon, and Togo, and the Ministry for Agriculture, Cameroon, for their support and assistance.
Footnotes
The authors have declared that no competing interests exist.
This work has been supported by the European Union through the STD and INCO-DC programmes (ICA4-CT-1999-10002; ITS3*CT91-0037 SC11*900509; and INCO-CT-2006-032321-SCOOTT); the Edna McConnell Clark Foundation; the Wellcome Trust; UK Medical Research Council; Centre National de la Recherche Scientifique (CNRS), France; the German Research Foundation (DFG); and WHO-TDR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Basanez MG, Pion SD, Churcher TS, Breitling LP, Little MP, et al. River blindness: a success story under threat? PLoS Med. 2006;3:e371. doi: 10.1371/journal.pmed.0030371. doi:10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borsboom GJ, Boatin BA, Nagelkerke NJ, Agoua H, Akpoboua KL, et al. Impact of ivermectin on onchocerciasis transmission: assessing the empirical evidence that repeated ivermectin mass treatments may lead to elimination/eradication in West-Africa. Filaria J. 2003;2:8. doi: 10.1186/1475-2883-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thylefors B, Alleman M. Towards the elimination of onchocerciasis. Ann Trop Med Parasitol. 2006;100:733–746. doi: 10.1179/136485906X112202. [DOI] [PubMed] [Google Scholar]
- 4.African Programme for Onchocerciasis Control. African Programme for Onchocerciasis Control Web site. 2008. Available: http://www.apoc.bf/en/index.htm. Accessed 8 April 2008.
- 5.The Carter Center. IACO 2004 held in Atlanta. Eye of the Eagle volume 6, number 1. Jan, 2005. Available: http://www.cartercenter.org/documents/1969.pdf. Accessed 27 March 2008.
- 6.Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT. The effects of high-dose ivermectin regimens on Onchocerca volvulus in onchocerciasis patients. Trans R Soc Trop Med Hyg. 1999;93:189–194. doi: 10.1016/s0035-9203(99)90305-x. [DOI] [PubMed] [Google Scholar]
- 7.Bronsvoort BM, Renz A, Tchakoute V, Tanya VN, Ekale D, et al. Repeated high doses of avermectins cause prolonged sterilisation, but do not kill, Onchocerca ochengi adult worms in African cattle. Filaria J. 2005;4:8. doi: 10.1186/1475-2883-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke BO. Evidence for macrofilaricidal activity of ivermectin against female Onchocerca volvulus: further analysis of a clinical trial in the Republic of Cameroon indicating two distinct killing mechanisms. Parasitol. 2005;130:447–453. doi: 10.1017/s0031182004006766. [DOI] [PubMed] [Google Scholar]
- 9.Ardelli BF, Prichard RK. Identification of variant ABC-transporter genes among Onchocerca volvulus collected from ivermectin-treated and untreated patients in Ghana, West Africa. Ann Trop Med Parasitol. 2004;98:371–384. doi: 10.1179/000349804225003415. [DOI] [PubMed] [Google Scholar]
- 10.Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT, et al. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:359–370. doi: 10.1179/000349804225003442. [DOI] [PubMed] [Google Scholar]
- 11.Awadzi K, Boakye DA, Edwards G, Opoku NO, Attah SK, et al. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:231–249. doi: 10.1179/000349804225003253. [DOI] [PubMed] [Google Scholar]
- 12.Bourguinat C, Pion SD, Kamgno J, Gardon J, Duke BO, et al. Genetic selection of low fertile onchocercaOnchocerca volvulus by ivermectin treatment. PLoS Negl Trop Dis. 2007;1:e72. doi: 10.1371/journal.pntd.0000072. doi:10.1371/journal.pntd.0000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 14.Lustigman S, MacDonald AJ, Abraham D. CD4+-dependent immunity to Onchocerca volvulus third-stage larvae in humans and the mouse vaccination model: common ground and distinctions. Int J Parasitol. 2003;33:1161–1171. doi: 10.1016/s0020-7519(03)00170-x. [DOI] [PubMed] [Google Scholar]
- 15.Abraham D, Lucius R, Trees AJ. Immunity to Onchocerca spp. in animal hosts. Trends Parasitol. 2002;18:164–171. doi: 10.1016/s1471-4922(02)02245-6. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence R, Devaney E. Lymphatic filariasis: parallels between the immunology of disease in humans and mice. Parasite Immunology. 2001;23:353–361. doi: 10.1046/j.1365-3024.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 17.Petit G, Diagne M, Maréchal P, Owen D, Taylor D, et al. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice; comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp. 1992;67:144–150. doi: 10.1051/parasite/1992675144. [DOI] [PubMed] [Google Scholar]
- 18.Trees AJ, Graham SP, Renz A, Bianco AE, Tanya V. Onchocerca ochengi infections in cattle as a model for human onchocerciasis: recent developments. Parasitology. 2000;120(Suppl):S133–S142. doi: 10.1017/s0031182099005788. [DOI] [PubMed] [Google Scholar]
- 19.Tchakoute VL, Graham SP, Jensen SA, Makepeace BL, Nfon CK, et al. In a bovine model of onchocerciasis, protective immunity exists naturally, is absent in drug-cured hosts, and is induced by vaccination. Proc Natl Acad Sci U S A. 2006;103:5971–5976. doi: 10.1073/pnas.0601385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillette-Ferguson I, Hise AG, Sun Y, Diaconu E, McGarry HF, et al. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect Immun. 2006;74:2442–2445. doi: 10.1128/IAI.74.4.2442-2445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Goff L, Martin C, Oswald IP, Vuong PN, Petit G, et al. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitol. 2000;120:271–280. doi: 10.1017/s0031182099005533. [DOI] [PubMed] [Google Scholar]
- 22.Martin C, Saeftel M, Vuong PN, Babayan S, Fischer K, et al. B-cell deficiency suppresses vaccine-induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infect Immun. 2001;69:7067–7073. doi: 10.1128/IAI.69.11.7067-7073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bain O, Wanji S, Vuong PN, Maréchal P, Le Goff L, et al. Larval biology of six filariae Onchocercinae in a vertebrate host. Parasite. 1994;1:241–254. doi: 10.1051/parasite/1994013241. [DOI] [PubMed] [Google Scholar]
- 24.Wanji S, Tendongfor N, Vuong PN, Enyong P, Bain O. The migration and localization of Loa loa infective and fourth-stage larvae in normal and immunosuppressed rodents. Ann Trop Med Parasitol. 2002;96:823–830. doi: 10.1179/000349802125002220. [DOI] [PubMed] [Google Scholar]
- 25.Graham AL, Taylor MD, Le Goff L, Lamb TJ, Magennis M, et al. Quantitative appraisal of murine filariasis confirms host strain differences but reveals that BALB/c females are more susceptible than males to Litomosoides sigmodontis. Microbes Infect. 2005;7:612–618. doi: 10.1016/j.micinf.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Babayan S, Attout T, Specht S, Hoerauf A, Snounou G, et al. Increased early local immune responses and altered worm development in high-dose infections of mice susceptible to the filaria Litomosoides sigmodontis. Med Microbiol Immunol (Berl) 2004;194:151–162. doi: 10.1007/s00430-004-0226-1. [DOI] [PubMed] [Google Scholar]
- 27.Babayan S, Ungeheuer M-N, Martin C, Attout T, Belnoue E, et al. Resistance and susceptibility to filarial infection with Litomosoides sigmodontis are associated with early differences in parasite development and in localized immune reaction. Infect Immun. 2003;71:6820–6829. doi: 10.1128/IAI.71.12.6820-6829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis–beyond Th1 vs. Th2. Trends Parasitol. 2002;18:25–31. doi: 10.1016/s1471-4922(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 29.Le Goff L, Lamb TJ, Graham AL, Harcus Y, Allen JE. IL-4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int J Parasitol. 2002;32:1277–1284. doi: 10.1016/s0020-7519(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 30.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, et al. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkmann L, Bain O, Saeftel M, Specht S, Fischer K, et al. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Med Microbiol Immunol. 2003;192:23–31. doi: 10.1007/s00430-002-0155-9. [DOI] [PubMed] [Google Scholar]
- 32.Volkmann L, Saeftel M, Fleischer B, Hoerauf A. IL-4 is essential for the control of microfilariae in murine infection with the filaria Litomosoides sigmodontis. Infect Immun. 2001;69:2950–2956. doi: 10.1128/IAI.69.5.2950-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaney E, Gillan V, Wheatley I, Jenson J, O'Connor R, et al. Interleukin-4 influences the production of microfilariae in a mouse model of Brugia infection. Parasite Immunol. 2002;24:29–37. doi: 10.1046/j.0141-9838.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- 34.Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of gamma interferon and interleukin-5 in the control of murine filariasis. Infect Immun. 2003;71:6978–6985. doi: 10.1128/IAI.71.12.6978-6985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeftel M, Volkmann L, Korten S, Brattig N, Al-Qaoud KM, et al. Lack of interferon-γ confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3:203–213. doi: 10.1016/s1286-4579(01)01372-7. [DOI] [PubMed] [Google Scholar]
- 36.Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol. 2005;27:417–429. doi: 10.1111/j.1365-3024.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 37.Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua V, et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Lamb TJ, Le Goff L, Kurniawan A, Guiliano DB, Fenn K, et al. Most of the response elicited against Wolbachia surface protein in filarial nematode infection is due to the infective larval stage. J Infect Dis. 2004;189:120–127. doi: 10.1086/380490. [DOI] [PubMed] [Google Scholar]
- 39.Punkosdy GA, Addiss DG, Lammie PJ. Characterization of antibody responses to Wolbachia surface protein in humans with lymphatic filariasis. Infect Immun. 2003;71:5104–5114. doi: 10.1128/IAI.71.9.5104-5114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Buttner DW, et al. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol. 2004;173:437–445. doi: 10.4049/jimmunol.173.1.437. [DOI] [PubMed] [Google Scholar]
- 41.Brattig NW, Buttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001;3:439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- 42.Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, et al. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol. 2007;178:1068–1076. doi: 10.4049/jimmunol.178.2.1068. [DOI] [PubMed] [Google Scholar]
- 43.Pfarr KM, Fischer K, Hoerauf A. Involvement of Toll-like receptor 4 in the embryogenesis of the rodent filaria Litomosoides sigmodontis. Med Microbiol Immunol (Berl) 2003;192:53–56. doi: 10.1007/s00430-002-0159-5. [DOI] [PubMed] [Google Scholar]
- 44.Kerepesi LA, Leon O, Lustigman S, Abraham D. Protective immunity to the larval stages of Onchocerca volvulus is dependent on Toll-like receptor 4. Infection and Immunity. 2005 doi: 10.1128/IAI.73.12.8291-8297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nfon CK, Makepeace BL, Njongmeta LM, Tanya VN, Bain O, et al. Eosinophils contribute to killing of adult Onchocerca ochengi within onchocercomata following elimination of Wolbachia. Microbes Infect. 2006;8:2698–2705. doi: 10.1016/j.micinf.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Maizels RM, Lawrence RA. Immunological tolerance: the key feature in human filariasis? Parasitol Today. 1991;7:271–276. doi: 10.1016/0169-4758(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 47.Graham SP, Trees AJ, Collins RA, Moore DM, Guy FM, et al. Down-regulated lymphoproliferation coincides with parasite maturation and with the collapse of both gamma interferon and interleukin-4 responses in a bovine model of onchocerciasis. Infect Immun. 2001;69:4313–4319. doi: 10.1128/IAI.69.7.4313-4319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor MD, Le Goff L, Harris A, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 49.Steel C, Nutman TB. CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. J Immunol. 2003;170:1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 50.Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, et al. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 51.Semnani RT, Nutman TB. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol Rev. 2004;201:127–138. doi: 10.1111/j.0105-2896.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 52.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 53.Doetze A, Satoguina J, Burchard G, Rau1 T, Löliger C, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-ß but not by a Th1 to Th2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 54.Satoguina J, Mempel M, Larbi J, Badusche M, Loliger C, et al. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 2002;4:1291–1300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 55.Schulz-Key H, Hoffmann W. Genetic determinants of innate immunity and immunomodulation in nematode infections in mice. 2005. Available: http://www.science.ngfn.de/dateien/NIE-S37T32_Schulz-Key.pdf. Accessed 27 March 2008.
- 56.Hoffmann WH, Pfaff AW, Schulz-Key H, Soboslay PT. Determinants for resistance and susceptibility to microfilaraemia in Litomosoides sigmodontis filariasis. Parasitology. 2001;122:641–649. doi: 10.1017/s0031182001007892. [DOI] [PubMed] [Google Scholar]
- 57.Specht S, Volkmann L, Wynn T, Hoerauf A. Interleukin-10 (IL-10) counterregulates IL-4-dependent effector mechanisms in murine filariasis. Infect Immun. 2004;72:6287–6293. doi: 10.1128/IAI.72.11.6287-6293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storey DM, Al-Mukhtar AS. Vaccination of jirds, Meriones unguiculatus, against Litomosoides carinii and Brugia pahangi using irradiated larvae of L. carinii. Tropenmed Parasit. 1982;33:23–24. [PubMed] [Google Scholar]
- 59.Martin C, Al-Qaoud KM, Ungeheuer MN, Paehle K, Vuong PN, et al. IL-5 is essential for vaccine-induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol (Berl) 2000;189:67–74. doi: 10.1007/pl00008258. [DOI] [PubMed] [Google Scholar]
- 60.Njongmeta LM, Nfon CK, Gilbert J, Makepeace BL, Tanya VN, et al. Cattle protected from onchocerciasis by ivermectin are highly susceptible to infection after drug withdrawal. Int J Parasitol. 2004;34:1069–1074. doi: 10.1016/j.ijpara.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Nfon CK, Makepeace BL, Njongmeta LM, Tanya VN, Trees AJ. Lack of resistance after re-exposure of cattle cured of Onchocerca ochengi infection with oxytetracycline. Am J Trop Med Hyg. 2007;76:67–72. [PubMed] [Google Scholar]
- 62.Le Goff L, Maréchal P, Petit G, Taylor DW, Hoffman W, et al. Early reduction of the challenge recovery rate following immunization with irradiated infective larvae in a filaria mouse system. Trop Med Int Health. 1997;2:1170–1174. doi: 10.1046/j.1365-3156.1997.d01-218.x. [DOI] [PubMed] [Google Scholar]
- 63.Babayan SA, Attout T, Harris A, Taylor MD, Le Goff L, et al. Vaccination against filarial nematodes with irradiated larvae provides long-term protection against the third larval stage but not against subsequent life cycle stages. Int J Parasitol. 2006;36:903–914. doi: 10.1016/j.ijpara.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Abraham D, Leon O, Schnyder-Candrian S, Wang CC, Galioto AM, et al. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect Immun. 2004;72:810–817. doi: 10.1128/IAI.72.2.810-817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gillan V, Devaney E. Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infect Immun. 2005;73:4034–4042. doi: 10.1128/IAI.73.7.4034-4042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Litomosoides sigmodontis ESTs. 2008. Available: http://www.nematodes.org/nematodeESTs/Litomosoides.php. Accessed 27 March 2008.
- 67.Allen JE, Daub J, Guilliano D, McDonnell A, Lizotte-Waniewski M, et al. Analysis of genes expressed at the infective larval stage validate the utility of Litomosoides sigmodontis as a murine model for filarial vaccine development. Infect Immun. 2000;68:5454–5458. doi: 10.1128/iai.68.9.5454-5458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Townson S, Bianco AE. Immunization of calves against the microfilariae of Onchocerca lienalis. J Helminthol. 1982;56:297–303. doi: 10.1017/s0022149x00034684. [DOI] [PubMed] [Google Scholar]
- 69.Trees AJ, Wahl G, Klager S, Renz A. Age-related differences in parasitosis may indicate acquired immunity against microfilariae in cattle naturally infected with Onchocerca ochengi. Parasitology. 1992;104 (Pt 2):247–252. doi: 10.1017/s0031182000061680. [DOI] [PubMed] [Google Scholar]
- 70.Bianco AE, Luty A, Whitworth J, Taylor D. Immunity to Onchocerca volvulus microfilariae in mice and the induction of cross-protection with O. lienalis. Trop Med Parasitol. 1991;42:188–190. [PubMed] [Google Scholar]
- 71.Maizels RM, Yazdanbakhsh M. Regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–743. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 72.Blaxter M, Daub J, Guiliano D, Parkinson J, Whitton C. The Brugia malayi genome project: expressed sequence tags and gene discovery. Trans R Soc Trop Med Hyg. 2002;96:7–17. doi: 10.1016/s0035-9203(02)90224-5. [DOI] [PubMed] [Google Scholar]
- 73.Hoerauf A, Buttner DW, Adjei O, Pearlman E. Onchocerciasis. Brit Med J. 2003;326:207–210. doi: 10.1136/bmj.326.7382.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 75.Volkmann L, Fischer K, Taylor M, Hoerauf A. Antibiotic therapy in murine filariasis (Litomosoides sigmodontis): comparative effects of doxycycline and rifampicin on Wolbachia and filarial viability. Trop Med Int Health. 2003;8:392–401. doi: 10.1046/j.1365-3156.2003.01040.x. [DOI] [PubMed] [Google Scholar]
- 76.Hoerauf A, Nissen-Pahle K, Schmetz C, Henkle-Duhrsen K, Blaxter ML, et al. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J Clin Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langworthy NG, Renz A, Mackenstedt U, Henkle-Duhrsen K, de Bronsvoort MB, et al. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc R Soc Lond B Biol Sci. 2000;267:1063–1069. doi: 10.1098/rspb.2000.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilbert J, Nfon CK, Makepeace BL, Njongmeta LM, Hastings IM, et al. Antibiotic chemotherapy of onchocerciasis: in a bovine model, killing of adult parasites requires a sustained depletion of endosymbiotic bacteria (Wolbachia). J Infect Dis. 2005;192:1483–1493. doi: 10.1086/462426. [DOI] [PubMed] [Google Scholar]
- 79.Hoerauf A, Mand S, Adjei O, Fleischer B, Buttner DW. Depletion of wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet. 2001;357:1415–1416. doi: 10.1016/S0140-6736(00)04581-5. [DOI] [PubMed] [Google Scholar]
- 80.Hoerauf A, Mand S, Volkmann L, Buttner M, Marfo-Debrekyei Y, et al. Doxycycline in the treatment of human onchocerciasis: Kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 2003;5:261–273. doi: 10.1016/s1286-4579(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 81.WHO. Meeting of the International Task Force for Disease Eradication–11 January 2007. Wkly Epidemiol Rec. 2007;82:197–202. [PubMed] [Google Scholar]
- 82.Hoerauf A, Specht S, Buttner M, Pfarr K, Mand S, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med Microbiol Immunol. E-pub ahead of print: 13 November 2007. 2007 doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfarr KM, Hoerauf AM. Antibiotics which target the Wolbachia endosymbionts of filarial parasites: a new strategy for control of filariasis and amelioration of pathology. Mini Rev Med Chem. 2006;6:203–210. doi: 10.2174/138955706775475984. [DOI] [PubMed] [Google Scholar]
- 84.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, et al. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- 85.Wanji S, Tendongfor N, Esum M, Ndindeng S, Enyong P. Epidemiology of concomitant infections due to Loa loa, Mansonella perstans, and Onchocerca volvulus in rain forest villages of Cameroon. Med Microbiol Immunol (Berl) 2003;192:15–21. doi: 10.1007/s00430-002-0154-x. [DOI] [PubMed] [Google Scholar]
- 86.Buttner DW, Wanji S, Bazzocchi C, Bain O, Fischer P. Obligatory symbiotic Wolbachia endobacteria are absent from Loa loa. Filaria J. 2003;2:10. doi: 10.1186/1475-2883-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Díaz A, Allen JE. Mapping immune response profiles: The emerging scenario from helminth immunology. Eur J Immunol. 2007;37:3319–3326. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]