Abstract
The phosphorylation of insulin receptor substrate 1 (IRS-1) on tyrosine residues by the insulin receptor (IR) tyrosine kinase is involved in most of the biological responses of insulin. IRS-1 mediates insulin signaling by recruiting SH2 proteins through its multiple tyrosine phosphorylation sites. The phosphorylation of IRS-1 on serine/threonine residues also occurs in cells; however, the particular protein kinase(s) promoting this type of phosphorylation are unknown. Here we report that glycogen synthase kinase 3 (GSK-3) is capable of phosphorylating IRS-1 and that this modification converts IRS-1 into an inhibitor of IR tyrosine kinase activity in vitro. Expression of wild-type GSK-3 or an “unregulated” mutant of the kinase (S9A) in CHO cells overexpressing IRS-1 and IR, resulted in increased serine phosphorylation levels of IRS-1, suggesting that IRS-1 is a cellular target of GSK-3. Furthermore, insulin-induced tyrosine phosphorylation of IRS-1 and IR was markedly suppressed in cells expressing wild-type or the S9A mutant, indicating that expression of GSK-3 impairs IR tyrosine kinase activity. Taken together, our studies suggest a new role for GSK-3 in attenuating insulin signaling via its phosphorylation of IRS-1 and may provide new insight into mechanisms important in insulin resistance.
Insulin affects a wide variety of biological processes including glycogen synthesis, glucose transport, mitogenesis, and protein synthesis (1–3). Most, if not all, of these responses are mediated by the cellular substrates of the insulin receptor (IR), insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) (1), which mediate insulin signaling by recruiting SH2 proteins such as the p85 regulatory subunit of PI3 kinase (4), GRB-2 (5), SH-PTP2 (6), and Nck (7) through its multiple tyrosine phosphorylation sites. Although IRS-1 exhibits insulin-stimulated tyrosine phosphorylation (8–10), in unstimulated cells it is predominantly phosphorylated on serine/threonine residues. In some instances these latter phosphorylations have been shown to antagonize some of the responses induced by insulin. Treatment of 3T3-L1 adipocytes with okadaic acid, a serine/threonine phosphatase inhibitor, results in the hyperphosphorylation of IRS-1 on serine/threonine residues, which correlates with a reduction of the ability of the IR to phosphorylate IRS-1 (10). Tumor necrosis factor α, a key mediator in several insulin resistance models, increases phosphorylation of IRS-1 on serine residues and antagonizes insulin-stimulated tyrosine phosphorylation of IRS-1 and IR in adipocytes or hepatoma cells (11–13). Modification of IRS-1 can thus have a key role in the generation of states associated with insulin resistance; however, the nature of the serine/threonine kinases involved in this processes is not known, and the action of IRS-1 as an inhibitor of IR activity in vivo has not been fully established.
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine kinase that was originally discovered as a result of its ability to phosphorylate and inhibit glycogen synthase (14, 15). Later studies implicated the enzyme in protein synthesis (16), modulation of the transcription factors AP-1 and CREB (cAMP response element binding protein) (17–19), cell fate determination in Drosophila (20), and dorsoventral axis formation in Xenopus (21, 22). GSK-3 is constitutively active in resting cells but undergoes rapid inhibition in the presence of growth factors or hormones such as epidermal growth factor and insulin (16, 23, 24). The enzyme is inhibited by the phosphorylation of serine 9 located near the N terminus of the protein, and several protein kinases have been shown to phosphorylate this site. It is believed that protein kinase B (also known as Akt or Rac) (23) and p90RSK (24, 25) are the physiological regulators of the enzyme. Information concerning the immediate down stream targets of GSK-3 in intact cells is still limited, and their identification may shed light on understanding the precise role of this enzyme.
MATERIALS AND METHODS
In Vitro Phosphorylation of IRS-1.
Recombinant IRS-1 (0.1 μg), a purified preparation obtained from Upstate Biotechnology (Lake Placid, NY), was incubated with recombinant rabbit GSK-3 (26) in the presence of 50 mM Tris (pH 7.3), 10 mM magnesium acetate, 0.01% 2-mercaptoethanol, and 50 μM [γ-32P]ATP (0.25 mCi/ml; 1 Ci - 37 GBq) in a final volume of 30 μl at 30°C for 15 min. Reactions were stopped by addition of Laemmli sample buffer, subjected to SDS/PAGE, and autoradiographed. In other set of experiments, IRS-1 was isolated from CHO cells overexpressing IRS-1 (CHO/IRS-1), which were kindly provided by Peter Wilden (University of Missouri). The cells were lysed in buffer G (20 mM Tris, pH 7.3/10 mM β-glycerophosphate/10% glycerol/1 mM EGTA/1 mM EDTA/50 mM NaF/5 mM NaPPi/25 μg/ml leupeptin/25 μg/ml aprotinin/1 mM phenylmethylsulfonyl fluoride/0.5% Triton X-100), and centrifuged at 10,000 × g. IRS-1 was immunoprecipitated from the supernatants with specific antibody against IRS-1 (Santa Cruz Biotechnology). GSK-3 was added to the immunoprecipitates, and phosphorylation reactions were performed as describe above. For comparing the rates of GSK-3-catalyzed phosphorylation reactions using different substrates, 10 pmol of IRS-1, c-jun (Promega), or a synthetic phosphopeptide, p9CREB [ILSRRPS(P)YR, synthesized by Henry Zebrosky, University of Washington), were incubated with recombinant GSK-3 for the indicated times. The reactions in which IRS-1 or c-jun was the substrate were stopped by addition of Laemmli sample buffer, subjected to SDS/PAGE, and autoradiographed; 32P-labeled bands were cut of the gel and counted for radioactivity. When p9CREB peptide was used as a substrate, the reaction mixture was spotted on p81 phosphocellulose paper (Whatman), washed with 10 mM phosphoric acid, and counted for radioactivity.
Phosphoamino Acid Analysis.
Recombinant IRS-1 or IRS-1 in the form of an immunoprecipitate derived from CHO/IRS-1 cells was incubated with recombinant GSK-3 under the conditions described above. Samples were subjected to gel electrophoresis and transferred to polyvinylidene difluoride membrane (Millipore). 32P-Labeled IRS-1 bands were cut from the membrane, digested with 6 M HCL, and subjected to one-dimensional phosphoamino acid analysis (27). Similar procedure was used to determine the phosphoamino amino acid composition of IRS-1 immunoprecipitated from 32P-labeled cells.
In Vitro Effect of IRS-1 Phosphorylation by GSK-3 on IR Tyrosine Kinase Activity.
Recombinant IRS-1 (0.1 μg) was phosphorylated for 15 min by GSK-3 under the same conditions described above except that unlabeled ATP was used. Partially purified IR obtained from CHO cells overexpressing IRs by wheat germ-affinity chromatography (28) was then added to a reaction, or to a control reaction without GSK-3, and incubation was continued for an additional 20 min. The added IR was present in 50 mM Tris (pH 7.3) and 50 μM ATP, so that the concentration of these components remained constant after addition of IR. However, during the second inhibition the magnesium acetate concentration was reduced from 10 to 5 mM, and 5 mM MnCl2 was introduced. The reactions were stopped by addition of Laemmli buffer, boiled, and subjected to SDS/PAGE. Immunoblot analysis was performed using monoclonal antibodies to phosphotyrosine (PY20; Santa Cruz Biotechnology). In a related set of experiments, 10 μg of histone H2B (Boehringer Mannheim) was added along with the IR to GSK-3-treated or untreated IRS-1, in this instance in the presence of [γ-32P]ATP. Phosphorylation of H2B was determined by autoradiography.
Cells and Transient Transfections.
CHO cells expressing IR and IRS-1 (CHO/IR/IRS-1), also provided by Peter Wilden, were grown in F-12 medium supplemented with 10% fetal calf serum. The cells were transiently transfected with pCMV4 expression plasmids encoding wild-type (WT) GSK-3β, or its S9A mutant (26), using an electroporation technique (29). In brief, cells were grown on 15-cm plates to subconfluency, detached by trypsinization, and collected by centrifugation (800 × g). The cell pellets were washed twice with PBS, resuspended in 0.5 ml PBS (GIBCO/BRL), and transferred to a gene pulser cuvette (Bio-Rad). DNA (40 μg) was added to the cells and the mixture put on ice for 5 min. The cells were electroporated (950 μF, 0.34 V), followed by addition of 1 ml media supplemented with 10% fetal calf serum, and put back on ice for 8 min. The transfected cells were then seeded on 10-cm plates and were harvested 48 h posttransfection. In some experiments cells were transfected with “GFP vector” (phGFP-S65T; CLONTECH) and fluorescent cells were counted 48 h posttransfection. Such analyses indicated that 50–60% of the cell population expressed the foreign protein.
GSK-3 Activity Assays.
Transfected cells were washed once with PBS and incubated in F-12 media supplemented with 0.5 mg/ml BSA for 4 h. The cells were then treated with insulin (100 nM) for 5 min and GSK-3 activity was determined as described (24). In brief, cells were washed twice with ice cold PBS and lysed with buffer G. The cell lysates were centrifuged for 20 min at 15,000 × g, and the resulting supernatants were passed through DE52 mini-columns. The flow-through was collected, and equal amount of proteins lysates were incubated with specific antibodies against GSK-3β prebound to protein A Sepharose at 4°C for 3 h. Kinase assays were performed on the immunoprecipitates in reaction mixtures that included 30 mM Tris (pH 7.4), 10 mM magnesium acetate, 0.5 mM DTT, 0.1 mg/ml BSA, 100 μM [γ-32P]ATP, and 60 μM P-GS1 peptide substrate (24) using a 15-min incubation. The mixtures were spotted on phosphocellulose paper (p81; Whatman), washed with 100 mM phosphoric acid, and counted for radioactivity. GSK-3 activity can be assayed with other substrates (as described in previous section).
32P-Phosphate Labeling.
Transfected cells were washed twice with DMEM lacking phosphate and were incubated with the same medium supplemented with 0.5 mg/ml BSA. The cells were metabolically labeled with [32P]orthophosphoric acid (1 mCi/10-cm plate) for 4 h. They were then washed twice with ice cold PBS, lysed with buffer G, and IRS-1 was immunoprecipitated from the lysates using specific antibodies against IRS-1. The immunoprecipitates were washed twice with 50 mM Tris, pH 7.3/0.5 M LiCl, twice with lysis buffer, and analyzed by gel electrophoresis followed by autoradiography.
Immunoblot Analysis.
Transfected cells were treated with insulin (100 nM) for 15 min and lysed with Buffer G as described in previous sections. Equal amount of proteins were subjected to gel electrophoresis and immunoblotted with monoclonal antibodies to phosphotyrosine (PY 20; Santa Cruz Biotechnology).
RESULTS
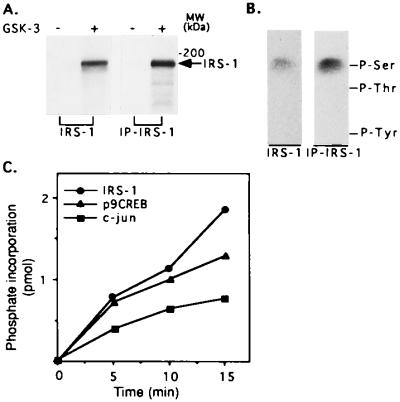
To determine whether IRS-1 might be a target for phosphorylation by GSK-3, in vitro assays were performed using recombinant IRS-1, or immunoprecipitated IRS-1 from CHO cells overexpressing IRS-1 (CHO/IRS-1) (9), as the substrate. Phosphorylation of IRS-1 from either source by GSK-3 was readily detected (Fig. 1A). Phosphoamino acid analysis of the phosphorylated IRS-1 (recombinant IRS-1 or IRS-1 isolated from cells) showed that the protein was phosphorylated by GSK-3 exclusively on serine residues (Fig. 1B). To compare the ability of IRS-1 to serve as a substrate of GSK-3 with that of known substrates of the enzyme, we carried out time courses of the phosphorylation of IRS-1, c-jun, and a synthetic phosphopeptide (p9CREB) patterned after the GSK-3 phosphorylation site in CREB (19). A typical experiment is shown in Fig. 1C. The initial rate of IRS-1 phosphorylation by GSK-3 is higher as compared with that of c-jun (≈2-fold) and similar to that of p9CREB, indicating that IRS-1 is a relatively “good substrate” for GSK-3. Similar results were obtained in three different experiments.
Figure 1.
Phosphorylation of IRS-1 by GSK-3. (A) Recombinant IRS-1 (designated as IRS-1) or IRS-1 immunoprecipitated from CHO cells overexpressing IRS-1 (designated as IP-IRS-1) was incubated with recombinant rabbit GSK-3 in the presence of 50 mM Tris (pH 7.3), 10 mM magnesium acetate, 0.01% 2-mercaptoethanol, and 50 μM [γ-32P]ATP (0.25 mCi/ml) in a final volume of 30 μl at 30°C for 15 min. Reactions were stopped by addition of Laemmli sample buffer, subjected to SDS/PAGE, and autoradiographed. (B) Phosphoamino acid composition of each type IRS-1 phosphorylated by GSK-3 was determined as described. The migration positions of phosphorylated tyrosine (P-Tyr), threonine (P-Thr), and serine (P-Ser) are indicated. (C) IRS-1, c-jun, and p9CREB peptide (see Materials and Methods), 10 pmol of each, were phosphorylated by GSK-3 under the conditions described in A for the indicated times. Reactions with IRS-1 or c-jun as the substrates were stopped by addition of Laemmli sample buffer, subjected to SDS/PAGE, and autoradiographed; radioactive bands were cut from the gels and counted for radioactivity. The reaction mixtures containing p9CREB was spotted on phosphocellulose paper and handled as described. Phosphate incorporation into each substrate is presented.
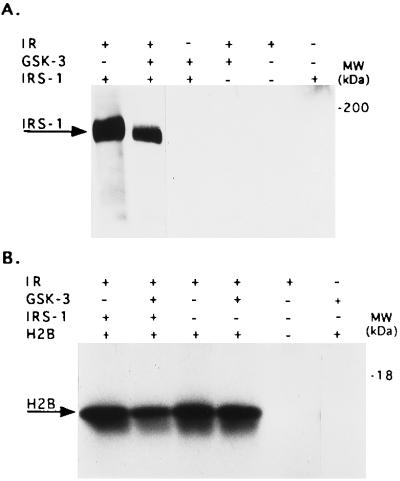
We next determined whether the phosphorylation of IRS-1 by GSK-3 would affect its phosphorylation by the IR in vitro. For this, recombinant IRS-1 was phosphorylated by GSK-3 and compared with untreated IRS-1 for its ability to serve as a substrate for partially purified IR. As seen in Fig. 2A, the ability of IR to phosphorylate GSK-3-phosphorylated IRS-1 on tyrosine residues was significantly lower (by 75 ± 5.5%, n = 3) than its ability to phosphorylate untreated IRS-1 (Fig. 2A, lanes 1 and 2). In this experiment we also verified that GSK-3, which has been reported to be a dual specific enzyme (30), does not phosphorylate IRS-1 on tyrosine (Fig. 2A, lane 3; and also see Fig. 1B). To examine whether the reduction in IRS-1 phosphorylation by IR was due to a reduced ability of serine phosphorylated IRS-1 to serve as a substrate, or to inhibition of IR tyrosine kinase activity, we added an exogenous substrate, histone H2B, to the reaction mixture. Histone H2B, although infrequently used in this capacity, is known to serve as a substrate for IR (31). The phosphorylation of H2B by IR was reduced ≈50% when IR was incubated together with GSK-3 phosphorylated IRS-1 (Fig. 2B, lane 2). IRS-1 itself had no effect on H2B phosphorylation by IR (Fig. 2B. lanes 1 and 3). We confirmed that H2B was phosphorylated exclusively on tyrosine residues (data not shown), and excluded the possibility that GSK-3 itself affects IR activity (Fig. 2B, lane 4) or phosphorylates H2B (Fig. 2B, lane 6). These studies indicate that IRS-1 that has been phosphorylated by GSK-3 inhibits the tyrosine kinase activity of IR.
Figure 2.
Phosphorylation of IRS-1 by GSK-3 inhibits IR tyrosine kinase activity. (A) Recombinant IRS-1 (0.1 μg) was subjected to sequential phosphorylation reactions, the first involved serine phosphorylation by GSK-3 using unlabeled ATP, and the second involved tyrosine phosphorylation catalyzed by purified IR, again using unlabeled ATP. The reactions were stopped by addition of Laemmli buffer, boiled, and subjected to SDS/PAGE. To detect IR-catalyzed tyrosine phosphorylation of the substrate IRS-1, immunoblot analysis was performed using monoclonal antibodies to phosphotyrosine. Experimental detail are provided in Material and Methods. The composition of the various reactions, including controls are presented in the upper panel of the figure. (B) The same as in A except that 10 μg histone H2B was added together with IR and [γ-32P]ATP. In this instance the phosphorylation of H2B was determined by autoradiography.
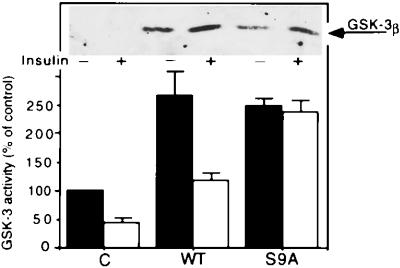
To facilitate studies on the possible regulation of IRS-1 phosphorylation by GSK-3 in intact cells, CHO cells expressing IR and IRS-1 (CHO/IR/IRS-1) (9) were transiently transfected with WT GSK-3 or a mutant GSK-3, S9A, in which serine 9 had been changed to alanine (26), thus preventing inhibition as a result of serine 9 phosphorylation. As determined by Western blot analysis of cell lysates using polyclonal antibodies to GSK-3 β (Fig. 3 Upper), GSK-3 was barely detected in the vector-transfected cells but was readily seen in lysates from GSK-3-transfected cells; usually the GSK-3 protein level seen with WT enzyme was higher than that for S9A. GSK-3 enzyme activity assays (Fig. 3 Lower) revealed that in absence of added insulin both WT and the S9A enzymes exhibited a 2- to 3-fold increase in their kinase activities as compared with the control. The increase in enzymatic activities was not as great in the GSK-3 expressing cells as would have been expected by the increased levels of GSK-3 protein; this is not an uncommon phenomenon and is seen in other cell systems overexpressing an exogenous protein [for example, similar results are seen in cells overexpressing protein kinase C (32, 33)]. Insulin treatment suppressed GSK-3 activity in the control and WT-expressing cells (30–50% of basal activities) but had essentially no effect on GSK-3 activity of the S9A transfected cells, as would be expected if serine 9 is the key regulatory site of inhibition of GSK-3 (25, 34).
Figure 3.
Expression of GSK-3 in CHO/IR/IRS-1 cells. Cells were transiently transfected with pCMV4 expression plasmids encoding WT GSK-3β or its S9A mutant as described. GSK-3 expression levels (Upper) were determined by Western blot analysis of cell lysates using polyclonal antibodies to GSK-3β. GSK-3 was immunoprecipitated from cell lysates and kinase activities were assayed in the immunoprecipitate complex using a peptide substrate P-GS1 (Lower). Kinase activities are presented as percent of control (cells transfected with vector alone), and are mean ± SEM from three experiments.
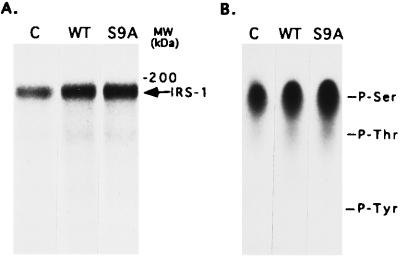
To examine the total phosphorylation of IRS-1 in cells that were overexpressing GSK-3, they were metabolically labeled with [32P]orthophosphoric acid and IRS-1 was immunoprecipitated. As shown in Fig. 4A, IRS-1 total phosphorylation was significantly higher in cells expressing WT or S9A as compared with control cells (2- to 3-fold). Analysis of the phosphoamino acid composition of the 32P-labeled IRS-1 revealed that the increased phosphorylation seen in the cells expressing GSK-3 involves serine residues (Fig. 4B). These results, together with the observation that IRS-1 was exclusively phosphorylated on serine residues by GSK-3 in vitro, support the view that IRS-1 can be phosphorylated by GSK-3 in intact cells. No phosphorylation of IRS-1 on tyrosine residues was observed in the experiments of Fig. 4, which was carried out in the absence of added insulin.
Figure 4.
Phosphorylation of IRS-1 by GSK-3 in intact CHO/IR/IRS-1 cells. Transfected cells were metabolically labeled with [32P]orthophosphoric acid for 4 h, and IRS-1 was immunoprecipitated from the cell lysates. Samples were (A) analyzed by gel electrophoresis followed by autoradiography or (B) transferred to polyvinylidene difluoride membrane followed by acid digestion. Phosphoamino acid analysis was performed on the digested samples as described. The migration positions of phosphorylated tyrosine (P-Tyr), threonine (P-Thr), and serine (P-Ser) are indicated.
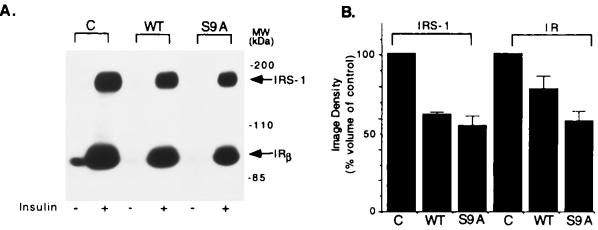
Of critical importance was the question as to whether overexpression of GSK-3 would affect the insulin-induced tyrosine phosphorylation of IRS-1 by IR and autophosphorylation of the latter. Accordingly, cells were stimulated with insulin, and the phosphotyrosine levels of IRS-1 and IR were determined by Western blot analysis using specific antibodies against phosphotyrosine. As shown in Fig. 5, insulin-induced tyrosine phosphorylation of IRS-1 was significantly lower in WT and S9A-expressing cells (62 and 55% of control cells, respectively) as well as tyrosine phosphorylation of IR (78.3 and 58.3% of control cells, respectively; Fig. 5B). Expression levels of IRS-1 and IR were similar in all transfected cells as determined by immunoblot analysis with antibodies against IRS-1 and IR (data not shown). These results indicate that expression of GSK-3 in cells impairs insulin-induced IR tyrosine kinase activity and tyrosine phosphorylation of IRS-1.
Figure 5.
Insulin-induced tyrosine phosphorylation of IRS-1 and IR in GSK-3-expressing cells. (A) CHO/IR/IRS-1 cells expressing WT GSK-3 or the S9A mutant were starved for 4 hr followed by stimulation with insulin (100 nM) for 15 min. Equal amount of protein from cell lysates were subjected to gel electrophoresis, transferred to polyvinylidene difluoride membrane, and immunoblotted with monoclonal antibodies to phosphotyrosine. Tyrosine phosphorylated IRS-1 and the β subunit of IR are indicated. (B) Image density of tyrosine phosphorylated IRS-1 and the β subunit of IR from A. Results are presented as percent of control cells and are the mean ± SE from three independent experiments.
DISCUSSION
GSK-3 was originally discovered as a serine/threonine kinase believed to be involved as a negative regulator of glycogen synthesis through its effect on glycogen synthase activity. Eventually other studies implicated the enzyme as having a role in transcriptional regulation and development (18, 35). Still, little is known about the immediate downstream targets of GSK-3 in intact cells. In these studies we identify IRS-1 as a potentially new target of GSK-3 in vitro and in intact cells. We show that phosphorylation of IRS-1 by GSK-3 in vitro causes inhibition of the the tyrosine phosphorylation of IRS-1 catalyzed by IR. Similarly, cells expressing elevated levels of GSK-3 exhibit decreased insulin-induced tyrosine phosphorylation of IRS-1 and IR. These results suggest that GSK-3 impairs insulin signaling via its phosphorylation of IRS-1. The high serine phosphorylation levels of IRS-1 previously reported to be presented in untreated cells (8–10) can be accounted for at least in part by constitutively active GSK-3. Addition of insulin, which suppresses GSK-3 activity and activates a protein phosphatase (36, 37), would result in a reduction of GSK-3-serine phosphorylated sites on IRS-1, which in turn would diminish its function as an inhibitor of IR. However the complete picture for regulation of IRS-1 function by insulin as a result of changes in serine phosphorylation is probably not this simple. It has been reported (refs. 8–10, and also unpublished results from this laboratory) that insulin actually increases the total level of serine phosphorylation of IRS-1 in intact cells. Protein serine/threonine kinases other than GSK-3 may have a role in IRS-1 phosphorylation, and, in contrast to GSK-3, may be up-regulated by insulin. For example, casein kinase 2 (CK-2) has been shown to phosphorylate IRS-1 (38). Analysis of the phospho-peptides maps of IRS-1 phosphorylated by CK-2 in vitro and IRS-1 phosphorylated in vivo revealed several common peptides, some of which were insulin sensitive (38), suggesting that IRS-1 is a cellular target of CK-2. Moreover, it has been reported by several laboratories, including this one, that insulin and other growth factors stimulate CK-2 activity, but these results have been disputed (reviewed in ref. 39). Our studies also indicate that both protein kinase A and protein kinase C are also capable of phosphorylating IRS-1 in vitro (unpublished data from this laboratory). It will be of great importance to identify the particular serine/threonine sites of IRS-1 that are phosphorylated in resting and in insulin-stimulated cells as well as the serine/threonine kinases responsible for these multiple phosphorylations.
Several studies have indicated that a serine phosphorylated IRS-1 plays a role in the impairment of insulin signaling. Okadaic acid, a serine/threonine phosphatase inhibitor, was shown to induce hyperphosphorylation of IRS-1 on serine/threonine residues, which was associated with a reduction in the ability of IR to phosphorylate IRS-1 (10). Tumor necrosis factor α, an important mediator in the development of insulin resistance in several model systems, was also shown to increase serine phosphorylation of IRS-1 in intact cells (11–13). This modification converted IRS-1 to an inhibitor of the IR autophosphorylation tyrosine kinase activity (12). Although the identity of the kinase activated by tumor necrosis factor α is not known, it was demonstrated clearly that such a kinase may play a role in the impairment of insulin action (12).
In non-insulin-dependent diabetes and obesity, cells are relatively insensitive to insulin. Although the mechanisms important for the development of insulin resistance are poorly understood, it is now clear that a number of factors may contribute to this process. It will be of interest to determine whether or not an increased phosphorylation of IRS-1 by GSK-3 may be one of them.
Acknowledgments
We thank Ms. Erlynda Munar for her assistance in maintaining the tissue culture facilities. This work was supported by a grant (DK 42528) from the National Institutes of Health, a grant from Muscular Dystrophy Association, and a Pilot Project grant from the W. M. Keck Center for Advances Studies of Neural Signaling at the University of Washington. H.E.-F. is a Research Fellow of the Washington State Heart Association.
ABBREVIATIONS
- IR
insulin receptor
- IRS-1
insulin receptor substrate 1
- GSK-3
glycogen synthase kinase 3
- CREB
cAMP response element binding protein
- WT
wild type
References
- 1.Myers M G J, White M F. Annu Rev Pharmacol Toxicol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- 2.Czech M P. Annu Rev Nutr. 1995;15:441–471. doi: 10.1146/annurev.nu.15.070195.002301. [DOI] [PubMed] [Google Scholar]
- 3.Saltiel A R. Am J Physiol. 1996;33:E375–E385. doi: 10.1152/ajpendo.1996.270.3.E375. [DOI] [PubMed] [Google Scholar]
- 4.Myers M G J, Backer J M, Sun X J, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White M F. Proc Natl Acad Sci USA. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolnik E Y, Batzer A, Li N, Lee C H, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 6.Lechleider R J, Freeman R M, Jr, Neel B G. J Biol Chem. 1993;268:13434–13438. [PubMed] [Google Scholar]
- 7.Lee C H, Li W, Nishimura R, Zhou M, Batzer A G, Myers M G, Jr, White M F, Schlessinger J, Skolnik E Y. Proc Natl Acad Sci USA. 1993;90:11713–11717. doi: 10.1073/pnas.90.24.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Nature (London) 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 9.Sun X J, Miralpeix M, Myers M G J, Glasheen E M, Backer J M, Kahn C R, White M F. J Biol Chem. 1992;267:22662–22672. [PubMed] [Google Scholar]
- 10.Tanti J F, Grémeaux T, van-Obberghen E, Le-Marchand-Brustel Y. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 11.Hotamisligil G S, Murray D L, Choy L N, Spiegelman B M. Proc Natl Acad Sci USA. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil G S, Peraldi P, Budavari A, Ellis R, White M F, Spiegelman B M. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 13.Kanety H, Feinstein R, Papa M Z, Hemi R, Karasik A. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P. Eur J Biochem. 1985;151:439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- 15.Roach P J. The Enzymes. Orlando, FL: Academic; 1986. [Google Scholar]
- 16.Welsh G I, Proud C G. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle W J, Smeal T, Defize L H, Angel P, Woodgett J R, Karin M, Hunter T. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 18.Plyte S E, Hughes K, Nikolakaki E, Pulverer B J, Woodgett J R. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 19.Fiol C J, Williams J S, Chou C H, Wang Q M, Roach P J, Andrisani O M. J Biol Chem. 1994;269:32187–32193. [PubMed] [Google Scholar]
- 20.Siegfried E, Chou T B, Perrimon N. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 21.He X, Saint J J P, Woodgett J R, Varmus H E, Dawid I B. Nature (London) 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 22.Pierce S B, Kimelman D. Development (Cambridge, UK) 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- 23.Cross D A, Alessi D R, Vandenheede J R, McDowell H E, Hundal H S, Cohen P. Biochem J. 1994;303:21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldar-Finkelman H, Seger R, Vandenheede J R, Krebs E G. J Biol Chem. 1995;270:987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- 25.Stambolic V, Woodgett J R. Biochem J. 1994;303:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eldar-Finkelman H, Agrast G M, Foord O, Fischer E H, Krebs E G. Proc Natl Acad Sci USA. 1996;93:10228–10233. doi: 10.1073/pnas.93.19.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamps M P, Sefton B M. Anal Biochem. 1989;176:22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- 28.Pike L J, Eakes A T, Krebs E G. J Biol Chem. 1986;261:3782–3789. [PubMed] [Google Scholar]
- 29.Yamauchi K, Pessin J E. J Biol Chem. 1994;269:31107–31114. [PubMed] [Google Scholar]
- 30.Wang Q M, Fiol C J, DePaoli R A A, Roach P J. J Biol Chem. 1994;269:14566–14574. [PubMed] [Google Scholar]
- 31.Wente S R, Villalba M, Schramm V L, Rosen O M. Proc Natl Acad Sci USA. 1990;87:2805–2809. doi: 10.1073/pnas.87.7.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldar H, Zisman Y, Ulrich A, Livneh E. J Biol Chem. 1990;265:13290–13296. [PubMed] [Google Scholar]
- 33.Housey G M, Johnson M D, Hsiao W L W, O’Brian C A, Murphy J P, Kirschmeier P, Weinstein I B. Cell. 1988;52:343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland C, Leighton I A, Cohen P. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welsh G I, Wilson C, Proud C G. Trends Cell Biol. 1996;6:274–279. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 36.Bollen M, Dopere F, Goris J, Merlevede W, Stalmans W. Eur J Biochem. 1984;144:57–63. doi: 10.1111/j.1432-1033.1984.tb08430.x. [DOI] [PubMed] [Google Scholar]
- 37.Olivier A R, Ballou L M, Thomas G. Proc Natl Acad Sci USA. 1988;85:4720–4724. doi: 10.1073/pnas.85.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanasijevic M J, Myers M G J, Thoma R S, Crimmins D L, White M F, Sacks D B. J Biol Chem. 1993;268:18157–18166. [PubMed] [Google Scholar]
- 39.Litchfield D W, Dobrowlsa G, Krebs E G. Cell Mol Biol Res. 1994;40:373–381. [PubMed] [Google Scholar]