Abstract
Using binding assays, we discovered an interaction between karyopherin α2 and the nucleoporin Nup153 and mapped their interacting domains. We also isolated a 15-kDa tryptic fragment of karyopherin β1, termed β1*, that contains a determinant for binding to the peptide repeat containing nucleoporin Nup98. In an in vitro assay in which export of endogenous nuclear karyopherin α from nuclei of digitonin-permeabilized cells was quantitatively monitored by indirect immunofluorescence with anti-karyopherin α antibodies, we found that karyopherin α export was stimulated by added GTPase Ran, required GTP hydrolysis, and was inhibited by wheat germ agglutinin. RanGTP-mediated export of karyopherin α was inhibited by peptides representing the interacting domains of Nup153 and karyopherin α2, indicating that the binding reactions detected in vitro are physiologically relevant and verifying our mapping data. Moreover, β1*, although it inhibited import, did not inhibit export of karyopherin α. Hence, karyopherin α import into and export from nuclei are asymmetric processes.
Keywords: binding assays, mapping of interacting domains, digitonin-permeabilized cells, export assay
Macromolecular export from the nucleus has been studied primarily by in vivo assays. The most widely employed of these assays uses microinjection of a reporter substrate into nuclei of intact amphibian oocytes with export of the reporter into the cytoplasm subsequently detected after manual dissection of the oocyte into cytoplasmic and nuclear fractions. Another in vivo assay, primarily employed in yeast, is to detect poly(A) containing mRNA by in situ hybridization with oligo(dT) probes; in various conditional mutants defects in export show up at the restrictive temperature as accumulation of poly(A) containing mRNA in the nucleus (for review see ref. 1). While many important insights have been gained from these in vivo assays they are of limited suitability for a step by step biochemical dissection of the many reactions involved in nuclear export.
So far only one in vitro assay for nuclear export has been described. In this assay, nuclei of digitonin-permeabilized cells were first loaded with a reporter substrate (fluorescently labeled human serum albumin [HSA] coupled to a nuclear localization sequence [NLS]) during a 30 min import reaction, then were washed to remove excess reporter, and finally were incubated again in a 30 min export reaction after which the nuclear fluorescence of the reporter was quantitated. Export of NLS/HSA was observed to be dependent on Ran and on GTP hydrolysis and was inhibited by wheat germ agglutinin (2). However, it is not yet clear, whether export of NLS proteins from the nucleus is physiologically relevant (for contradictory results see refs. 3 and 4). Moreover, although nuclear envelopes of digitonin-permeabilized cells remained intact during the 30 min incubation for import, we recently found that some nuclei became leaky during subsequent manipulations, i.e., either during washing or the 30 min incubation period for export. Leakiness of the envelope was surmised because the normally transport factor-dependent uptake of a fluorescently labeled reporter occurred in the absence of added transport factors (J.M. and G.B., unpublished data).
We therefore modified the previously developed export assay. Instead of assaying for export of an in vitro imported reporter, we assayed for export of an endogenous protein from nuclei of digitonin-permeabilized cells, thereby reducing manipulations to a single 30 min incubation for export during which the nuclear envelope remained intact. Export of the given endogenous nuclear protein was assessed by quantitatively monitoring the reduction of the indirect nuclear immunofluorescence elicited with cognate antibodies. The endogenous nuclear protein that we have chosen for our export studies here is karyopherin α. In the cytoplasm, this protein forms a heterodimeric complex with karyopherin β1. The heterodimer binds to an NLS protein via its α subunit and to a subgroup of peptide repeat containing nucleoporins via its β subunit (5–16). Following Ran and p10-mediated import (17–22), the NLS protein is translocated into the nucleus, along with karyopherin α, whereas karyopherin β1 stays behind at the NPC (8, 16). To mediate another round of import, karyopherin α must be recycled into the cytoplasm. Hence karyopherin α is a protein that shuttles between the cytoplasm and the nucleus and export of endogenous nuclear karyopherin α therefore is a physiologically relevant process.
To learn more about the events that might be involved in export of karyopherin α, we undertook binding studies to identify putative binding partners for karyopherin α. We discovered that karyopherin α2 bound directly to Nup153. This nucleoporin is located on the nucleoplasmic side of the NPC (23) and is associated with fibers that extend from the NPC into the interior of the nucleus (24–26). We mapped the domains of karyopherin α2 and of Nup153 interacting with each other. We also identified a domain of karyopherin β1 that interacts with the repeat domain of Nup98, another nucleoplasmically located nucleoporin. Taking advantage of the fact that peptides representing these domains readily gain access to the nucleus via diffusion across the central tube of the nuclear pore complex (NPC) (27), we investigated their effects on in vitro export of karyopherin α2. We found that export of karyopherin α2 involves its direct interaction with Nup153. In contrast, the domain of karyopherin β1 that interacts with peptide repeat nucleoporins does not affect export, while it completely inhibits import of NLS/HSA.
MATERIALS AND METHODS
Preparation of Recombinant Transport Factors, Transport Factor Segments, and Synthetic Peptides.
The full-length recombinant human proteins: karyopherin α2 (10), karyopherin β1 (13), Ran (28), and p10 (8) were prepared as described. The purified proteins at a concentration of 1 mg/ml of transport buffer (20 mM Hepes⋅KOH, pH 7.3/110 mM KOAc/2 mM Mg(OAc)2/1 mM EGTA/2 mM DTT) were aliquoted, frozen in liquid N2, and stored at −70°C until use.
The recombinant C-terminal fragment of karyopherin α2, termed α2-C, comprising residues 453–529, was prepared by using PCR with primers containing restriction sites for insertion into the pET21b vector; expression in Escherichia coli BL21(DE3) was induced by 1 mM isopropyl β-d-thiogalactoside for 3 hr at 30°C. The His6-tagged protein was purified as described (8).
The recombinant C-terminal fragment of the nucleoporin Nup153, termed Nup153-1, comprising residues 956–1468, was expressed as a His6-tagged protein (see above) and purified from inclusion bodies as described previously for the nucleoporin Nup98 (29).
The following peptides were synthesized, each with an N-terminal cysteine added for coupling purposes: Nup153-C: CGTSVSGRKIKTAVRRKK, representing amino acid residues 1452–1468 of Nup153; Nup153-Cr: CKKRRVATKIKRGSVSTG, representing the reverse of Nup153-C; NLS of simian virus 40 T antigen: CYTPPKKKKRKVED; nuclear export sequence: CLPPLERLTL.
Conjugation of peptides to HSA was done as described (30) using 1 mg peptide per 1 mg activated HSA. As assayed by SDS/PAGE the conjugate was estimated to contain a mean of 10 peptides per HSA. Subsequent coupling of the peptide/HSA conjugate to AffiGel was judged to be 100% effective as no peptide/HSA could be detected in a supernatant after centrifugation.
Nuclear Export Assay.
HeLa cells grown to subconfluency on a coverslip were permeabilized on ice during a 5 min incubation with 1 ml transport buffer containing 35 μg digitonin. The cells were washed once with 1 ml of ice-cold transport buffer. They were then either immediately fixed (see below) or first incubated for 30 min at 20°C in a 20 μl export reaction, washed in 1 ml of ice-cold transport buffer and then fixed. The 20 μl export reaction contained: 1 μl of 20 mg/ml BSA, 1 μl of 20 mM ATP, 1 μl of 20 mM GTP, 1 μl of 100 mM phosphocreatine, and 1 μl of 400 units/ml creatine kinase, various peptides and proteins in transport buffer (as indicated) and transport buffer to adjust the total volume to 20 μl. Cells were fixed by incubation, first for 15 min on ice in 3.7% formaldehyde in transport buffer, and then for 3 min at −20°C in methanol. For indirect immunofluorescence staining, cells were incubated, for 1 hr at 20°C and in 200 μl, first with solution A (2% BSA in PBS), then with rabbit anti-karyopherin α1 antibodies (diluted 1:100 in solution A), washed three times with 1 ml of solution A, incubated with fluorescein isothiocyanate-labeled goat anti-rabbit IgG (Sigma) (diluted 1:100 in solution A), and again washed three times in solution A. In a control experiment, where the incubation with the anti-karyopherin α1 antibodies was omitted, there was no detectable immunofluorescent signal. Fluorescence microscopy and quantitation was done as described (30). For quantitation, the fluorescence of 70 nuclei from 10–12 nonoverlapping, randomly chosen fields was averaged.
Isolation of a Tryptic Fragment of Karyopherin β1 That Binds Nup98.
Karyopherin β1 (7) (200 μg) in transport buffer was incubated with 20 μl immobilized TPCK trypsin (Pierce) at 20°C. Following centrifugation, the supernatant was incubated for 30 min at 20°C with 100 μl of packed AffiGel beads (Bio-Rad) containing 200 μg of Nup98–1 (29). After washing with transport buffer, bound peptide was eluted with 200 μl of 2 M NaCl and analyzed by SDS/PAGE and Coomassie blue staining. The SDS/PAGE-resolved 15-kDa fragment was subjected to partial N-terminal sequence analysis (31).
Other Procedures and Materials.
Nuclear import assays (8), solution binding assays (32, 33), and fluorescent labeling (6) were as described. Rabbit anti-human karyopherin α1 antiserum was kindly provided by Robert O’Neill and Peter Palese.
RESULTS
Export of Endogenous Nuclear Karyopherin α1 and α2 Requires Ran and GTP Hydrolysis.
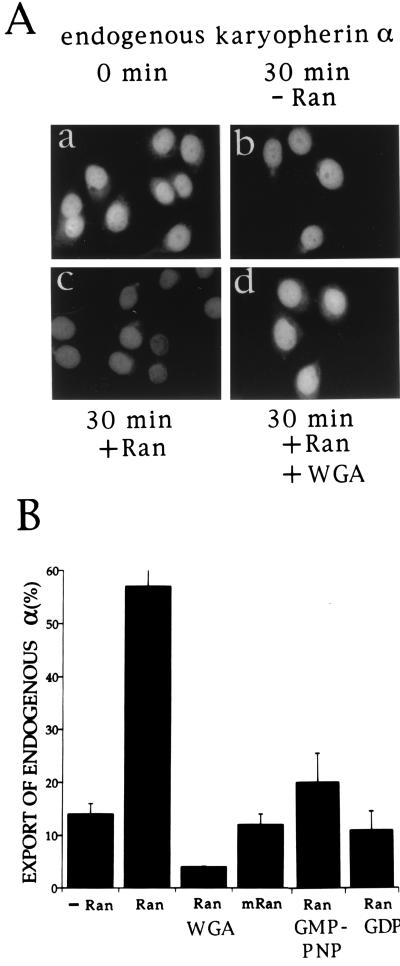
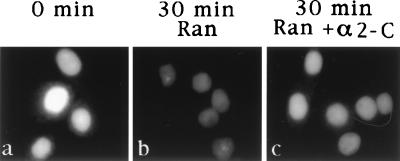
Digitonin-permeabilized HeLa cells were incubated for 30 min at 20°C in an export reaction containing transport buffer, GTP, and an energy-generating system. Export of endogenous karyopherin α was monitored by indirect immunofluorescence with karyopherin α1 antibodies which in addition to karyopherin α1 also recognize karyopherin α2 (34). The endogenous karyopherin α (1 and 2) that is present in nuclei of digitonin-permeabilized cells could be readily visualized by this procedure (Fig. 1Aa). After a 30 min incubation in the export reaction, there was a slight reduction in the immunofluorescent signal (Fig. 1Ab), which amounted to at most 15% (Fig. 1B). However, when Ran was added to the export reaction, there was a dramatic loss of immunofluorescent signal (Fig. 1Ac), amounting to almost 60% of that observed at the zero time point (Fig. 1B). Replacement in the export reaction of GTP by GDP or GMP-PNP (Fig. 1B) or addition of wheat germ agglutinin (Fig. 1Ad) inhibited the Ran-mediated export of karyopherin α. Moreover, when Ran was replaced by a mutant Ran that is unable to hydrolyze GTP (35), there was inhibition of the Ran-mediated export of karyopherin α (Fig. 1B). Together these results indicate that nuclear export of endogenous karyopherin α1 and α2 is stimulated by exogenously added Ran, requires GTP hydrolysis, and is inhibited by wheat germ agglutinin.
Figure 1.
Nuclear export of endogenous karyopherin α requires Ran, and GTP hydrolysis and is inhibited by wheat germ agglutinin. (A) Digitonin permeabilized HeLa cells were either fixed immediately (0 min time point, a); or were first incubated in an export reaction for 30 min either in the absence of Ran (b); or in the presence of 3 μM Ran (c); or in the presence of 3 μM Ran and 4 μg wheat germ agglutinin (d) and then fixed. The fixed cells were indirectly immunostained using antibodies against karyopherin α1 (crossreacting also with karyopherin α2). (B) Quantitation of immunofluorescence (see Materials and Methods) of the experiment shown in A and of additional export reactions. Data were expressed as percentage of the nuclear immunofluorescence of karyopherin α in cells fixed immediately after digitonin permeabilization (see 0 min time point, panel a of (A). Additional export reaction were performed in the presence of either 3 μM mutant Ran that is unable to hydrolyze GTP; or 3 μM Ran and 1 mM GMP-PNP (guanosine 5′-[β,γ-amido]triphosphate); or 3 μM Ran and 1 mM GDP.
The C-Terminal Region of Karyopherin α Binds Directly to the C-Terminal Region of Nup153.
Peptide repeat containing nucleoporins are known to provide docking sites for karyopherin β1 (8, 9, 21, 36) as well as β2 (37–39), β3 (40, 41), and β4 (41). The existence of direct binding sites on mammalian nucleoporins for karyopherin α has so far not been reported. In overlay blots, using SDS/PAGE-separated proteins of rat liver nuclear envelopes, we found Nup153 to be a major karyopherin α2 binding protein (data not shown). To map Nup153’s binding site for karyopherin α2 we prepared a recombinant fragment of Nup153, Nup153–1, that comprises its C-terminal third (Fig. 2). Immobilized Nup153–1 was incubated with recombinant karyopherin α2 in a binding assay and the bound and unbound fractions were analyzed by SDS/PAGE (Fig. 3A). Karyopherin α2 bound to Nup153–1 (Fig. 3A, lane 1). Interestingly, this binding was considerably stimulated in the presence of synthetic NLS peptide (lane 2) (see below and Discussion).
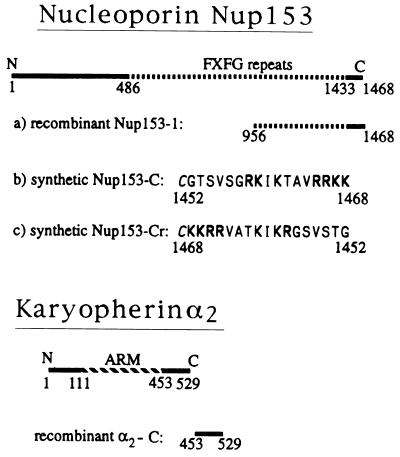
Figure 2.
Recombinant fragments and synthetic peptides of the nucleoporin Nup153 and of karyopherin α2 used in this study. The FXFG repeat containing region of Nup153 is indicated by a dashed line. Basic residues of the synthetic peptide, Nup153-C, and of its reverse peptide, Nup153-Cr, are indicated in bold letters and an added N-terminal cysteine is italicized. ARM, region of 42 residue-long armadillo repeats in karyopherin α2.
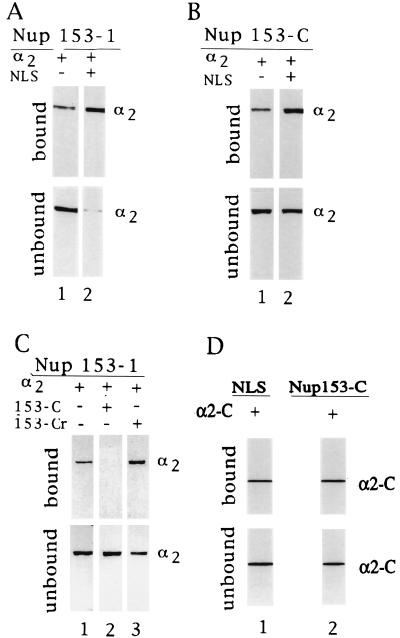
Figure 3.
Karyopherin α2’s C-terminal region binds specifically to the C-terminal region of the nucleoporin Nup153. Underlined are various AffiGel-immobilized recombinant fragments or synthetic peptides, whereby the given amounts were calculated based on a 100% coupling efficiency (see Materials and Methods). The AffiGel-immobilized components were incubated with various soluble components as indicated and the bound and unbound fractions were analyzed by SDS/PAGE and Coomassie blue staining. (A) Immobilized Nup153–1 (2.5 μM) was incubated with either 1 μM karyopherin α2 alone (lane 1) or together with 30 μM of NLS peptide. (B) Immobilized Nup153-C (15 μM) was incubated with 1 μM karyopherin α2 alone (lane 1) or together with 30 μM NLS peptide (lane 2). (C) Immobilized Nup153–1 (2.5 μM) was incubated with 1 μM karyopherin α2 alone (lane 1), or together with 15 μM Nup153-C (lane 2) or 15 μM Nup153-Cr (lane 3). (D) Immobilized NLS peptide (6 μM) or immobilized Nup153-C (6 μM) was incubated with 6 μM karyopherin α2-C, lanes 1 and 2, respectively.
Because yeast karyopherin α was found to bind to a synthetic 18 mer representing the C-terminal region of the yeast nucleoporin, Nup1, and because this region of Nup1 is homologous to the C-terminal region of mammalian Nup153 (M. Rexach and G.B., unpublished data), we tested a synthetic peptide, representing the C-terminal 17 residues of Nup153 (Fig. 2), termed Nup153-C, for its ability to bind karyopherin α2. Indeed, immobilized Nup153-C bound karyopherin α2 and this binding was again stimulated by NLS peptide (Fig. 3B), similar to the observed stimulation of yeast karyopherin α binding to the C-terminal domain of yeast Nup1 (M. Rexach and G.B., unpublished results). Together these data indicate that although Nup153-C shares with NLS a preponderance of basic residues, its binding to karyopherin α2 does not mimic that of an NLS (see below and Discussion).
The binding site for karyopherin α2 on Nup153–1 was indeed localized to Nup153–1’s C-terminal region, as the peptide Nup153-C competed for binding (Fig. 3C, lanes 1 and 2). Moreover, the binding was specific, as a peptide of the reverse sequence, termed Nup153-Cr, rather than competing, actually stimulated binding (lane 3) (see Discussion).
As Nup153-C is strongly basic we reckoned that its cognate binding domain might be located in karyopherin α2’s C-terminal region that contains several stretches of acidic residues. We therefore prepared a recombinant fragment of karyopherin α2 that comprises residues 453–529 and that was termed α2-C (Fig. 2). Indeed, we found that immobilized Nup153-C bound α2-C (Fig. 3D, lane 2). The corresponding C-terminal domain of karyopherin α1 has previously been shown to contain the binding site for NLS protein (32). It was therefore not surprising that immobilized NLS also bound to α2-C. These data indicated that the C-terminal region of karyopherin α2 contained binding sites both for NLS and Nup153. Further mapping of α2-C will determine whether these two sites are separate or whether they overlap.
Specific Inhibition of Karyopherin α Export by Nup153-C and α2-C.
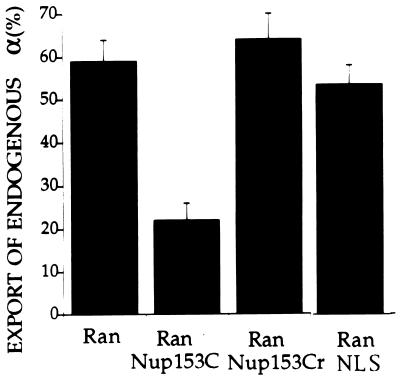
To test whether the interactions between karyopherin α2 and Nup153 are physiologically relevant, we added peptides representing these domains to the export reaction. The Ran stimulated export of karyopherin α was indeed inhibited by Nup153-C but not by its counterpart, Nup153-Cr, containing the sequence in reverse (Fig. 4). As expected, Ran-stimulated export of karyopherin α was not affected when NLS peptide was included in the export reaction (Fig. 4). These data indicated that during export, karyopherin α2 interacted directly and specifically with the C-terminal region of Nup153 and that the NLS peptide did not affect this interaction.
Figure 4.
Inhibition of endogenous karyopherin α export by Nup153-C, but not by Nup153-Cr or NLS peptides. After a 30 min incubation in the export reaction with either 3 μM Ran alone or together with 250 μM Nup153-C, or 250 μM Nup153-Cr, or 250 μM NLS, export of karyopherin α was quantitated as described in Fig. 1A.
The test whether the α2-C fragment affected export of karyopherin α could not be done by the indirect immunofluorescence assay as anti-karyopherin α antibodies also react with α2-C fragment. To circumvent this problem we used a variation of our previously described export assay: nuclei of digitonin-permeabilized cells were first loaded with fluorescently labeled karyopherin α2 in an import reaction, were then washed to remove unimported karyopherin α2, and were then incubated in an export reaction containing Ran and the α2-C fragment. As expected, Ran stimulated export of the in vitro imported karyopherin α2 (compare Fig. 5 A and B). Most importantly, α2-C inhibited the Ran stimulated export of karyopherin α2 (Fig. 5C). We also found that neither Nup153-C nor α2-C inhibited nuclear import of fluorescently labeled karyopherin α2, at least not at the concentrations used in the nuclear export reaction (data not shown). However, α2-C inhibited import of NLS/HSA (data not shown). Taken together these data indicated that export of karyopherin α required interaction of karyopherin α’s C-terminal region with Nup153.
Figure 5.
Recombinant karyopherin α2-C inhibits export of in vitro imported karyopherin α2. 0.5 μM fluorescently labeled karyopherin α2, 0.3 μM karyopherin β1, 3 μM Ran, and 0.2 μM p10 were incubated in a 15 min import reaction with digitonin-permeabilized HeLa cells (8). After washing, cells were either immediatedly fixed (A) or incubated in a 30 min export reaction in the presence of 3 μM Ran (B) or 3 μM Ran and 25 μM karyopherin α2-C (C).
Karyopherin β1 Is Not Directly Involved in Export of Karyopherin α.
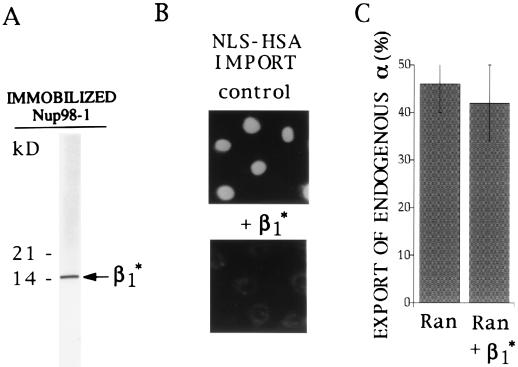
To investigate whether karyopherin β1 might be involved in export of karyopherin α, we mapped a binding site of karyopherin β1 for the peptide repeat region of the nucleoplasmically exposed Nup98. Karyopherin β1 was subjected to limited proteolysis by immobilized trypsin. Following incubation of the tryptic fragments with immobilized Nup98, the bound material was eluted with 2 M NaCl and analyzed by SDS/PAGE and Coomassie blue staining. We obtained a fragment that migrated like a peptide of ≈15 kDa and that was termed β1* (Fig. 6A). Partial N-terminal sequencing of β1* yielded a sequence that was identical to residues Asn-92 to Ser-107 of karyopherin β1. When tested in an import assay, β1* inhibited import of NLS/HSA (Fig. 6B). However, β1* did not inhibit Ran-stimulated export of karyopherin α (Fig. 6C). These data indicated that karyopherin α export did not require interaction of karyopherin β1 with the peptide repeat region of Nup98 and probably also the peptide repeat containing regions of other nucleoporins with which karyopherin β1 is known to interact (8).
Figure 6.
Isolation of a 15-kDa tryptic fragment of karyopherin β1 that binds to the nucleoporin Nup98 and that inhibits import of NLS/HSA but not export of endogenous karyopherin α. (A) After limited trypsin digestion of recombinant karyopherin β1, a karyopherin β1 fragment of an estimated molecular mass of 15 kDa, termed β1*, was isolated by affinity chromatography on immobilized Nup98, eluted by 2 M NaCl and analyzed by SDS/PAGE and Coomassie blue staining. Numbers on the left indicate molecular mass markers. (B) Fluorescently labeled NLS/HSA was imported into nuclei of digitonin-permeabilized HeLa cells in the presence of 0.5 μM karyopherin α2, 0.3 μM karyopherin β1, 3 μM Ran, and 0.2 μM p10 (Upper) and in the additional presence of 40 μM β1*. (C) Export of endogenous karypherin α from digitonin-permeabilized HeLa cells was quantitatively analyzed as in Fig. 1B after a 30 min export reaction in the presence of 3 μM Ran alone or in the presence of 3 μM Ran and 40 μM β1*.
DISCUSSION
We have shown here that in vitro export of endogenous karyopherin α from nuclei of digitonin-permeabilized cells required exogenously added Ran and GTP hydrolysis, as the nonhydrolyzable GTP analog, GMP-PNP, as well as a mutant form of Ran, unable to hydrolyze GTP, inhibited export. These results confirm and reinforce those of our previous experiments in which the export of an in vitro imported reporter (NLS/HSA) was also shown to require exogenously added Ran and GTP hydrolysis (2). Although it is still debated whether NLS proteins undergo export in vivo (3, 4), there can be no doubt about the physiological significance of karyopherin α export, as it belongs to a family of proteins which shuttles repeatedly between the nucleus and the cytoplasm.
By the criterion of extractibility with the nonionic detergent Triton X-100 which disrupts nuclear envelopes, there are two distinct nuclear pools of karyopherin α, one that is extracted by Triton X-100 and that represents ≈50% of the intranuclear pool after digitonin permeabilization, and the other 50% that is not extracted by Triton X-100 (J.M. and G.B., unpublished data). It is likely that the export of karyopherin α from the nucleus into the cytoplasm consists of a series of reactions that begins with mobilization of karyopherin α from intranuclear binding sites, is followed by intranuclear transport from the intranuclear binding sites to the NPC, and is completed by transport across the NPC. It remains to be determined at which of these steps of nuclear export Ran and GTP hydrolysis are required.
To analyze the mechanism of export at the level of the NPC we adopted a strategy of first identifying interactions of karyopherin α2 with known nucleoporins in binding assays, and then to map these interactions to sufficiently small domains of the interacting partners. If these interactions were to involve nucleoporins on the nucleoplasmic side of the NPC, peptides representing these domains and able to diffuse into the nucleus through the central tube of the NPC, should interfere with export and would thus provide strong evidence not only for the physiological significance of the interaction but also for the proper mapping of the interacting domains. This strategy should, in principle, be useful for analyzing any of the intranuclear reactions required for transport of karyopherin α to and across the NPC. It should also be useful for analyzing the export of other proteins or RNPs that need to be exported from the nucleus.
Our data here show that this strategy indeed led to the identification of an intranuclear reaction that is required for nuclear export of karyopherin α2. In overlay assays with SDS/PAGE-separated proteins of a nuclear envelope fraction we found that Nup153 is a major karyopherin α2 binding protein (data not shown). By using binding assays with recombinant proteins, recombinant protein fragments and synthetic peptides, we mapped the interacting domains to the acidic C-terminal region of karyopherin α2 (residues 453–529) and to the basic C-terminal region of Nup153 (residues 1452–1468). Most importantly, peptides representing these regions of Nup153 and of karyopherin α2, Nup153-C and α2-C, respectively, when added to the in vitro export reaction, inhibited export of karyopherin α. These results clearly identified the karyopherin α2-Nup153 interaction as one of the reactions involved in nuclear export of karyopherin α. That export of both karyopherin α1 and α2 was inhibited by the C-terminal region of karyopherin α2 was likely due to the fact that karyopherin α1 also interacted with Nup153 (data not shown). Although we have not yet mapped the domain of karyopherin α1 that interacts with Nup153, the sequence similarity between α1 and α2 provides a likely explanation for the observed inibibition of karyopherin α1 export by α2-C.
Nup153 has been identified as a nucleoplasmically exposed nucleoporin (23). However, its sublocalization to either the terminal ring of the nuclear basket of the NPC (24) or to fibers extending from the NPC to the interior of the nucleus (25, 26) is still controversial. Hence the observed inhibition of karyopherin α export by Nup153-C could affect both intranuclear transport and transport across the NPC.
Our data clearly show that the Nup153-C peptide, although it contains clusters of basic residues characteristic of those in NLS, does not function as an NLS, as Nup153-C binding to karyopherin α2 could not be inhibited by a synthetic NLS peptide. To the contrary, binding of karyopherin α2 to immobilized Nup153-C was stimulated by NLS peptide. Moreover, if Nup153-C would have NLS activity it would be expected to inhibit import of NLS protein in an in vitro import assay, which it did not, at least not when used at the concentration at which it inhibited export (data not shown). Our finding that Nup153’s C-terminal region does not display NLS activity is in agreement with previously reported results on the expression of various Nup153 regions in cultured cells, as an expressed C-terminal region of Nup153, similar to our Nup153–1 fragment, did not enter the nucleus, but remained in the cytoplasm (42). Interestingly, expressed Nup153 fragments located either in the nucleoplasm or the cytoplasm inhibited mRNA export from the nucleus, as long as these fragments contained the C-terminal region of Nup153 (42). It was proposed that inhibition of mRNA export might occur by Nup153’s C-terminal region titrating a soluble “export” factor that is distributed between both the nucleus and the cytoplasm. Karyopherin α might be a candidate for this soluble export factor (see below).
The binding of karyopherin α2 to Nup153-C is clearly specific as it could not be inhibited by a peptide of the reverse sequence, termed Nup153-Cr. To the contrary, Nup153-Cr stimulated binding of karyopherin α2 to Nup153-C. As a similar stimulation of binding was also observed for synthetic NLS, the Nup153-Cr peptide may have acquired NLS-like properties, although we have not tested whether it can mediate import of proteins into the nucleus.
The observed stimulation of binding of karyopherin α2 to Nup153 by NLS peptide is intriguing. We have previously shown that karyopherin α contains both an NLS (at the N-terminal region) and an NLS receptor function (at the C-terminal region) allowing for homophilic interactions (32). We also showed that karyopherin α’s NLS region overlaps with its binding region for karyopherin β. Binding to karyopherin β therefore covers karyopherin α’s NLS and stimulates binding to a heterologous NLS protein (32). Similarly, binding of NLS to karyopherin α may compete with karyopherin α’s homophilic interaction and facilitate exposure of karyopherin α’s binding site to Nup153 and thereby stimulate binding. It remains to be investigated whether the observed NLS-mediated stimulation of karyopherin α binding to Nup153 is physiologically relevant. Although karyopherin α might recycle back into the cytoplasm without cargo, its ability to bind both NLS and Nup153 suggests that it might be able to mediate export of NLS proteins or NLS proteins bound to RNA. If karyopherin α would indeed mediate both NLS protein import and export, NLS containing proteins might undergo futile recycling reactions between the nucleus and the cytoplasm in a potential merry-go-round unless their NLSs are masked, either in the cytoplasm or in the nucleoplasm.
It is likely, that karyopherin α interaction with other nucleoporins is required for its export as well. We found that karyopherin α2 also interacted with the C-terminal region, but not with the peptide repeat containing region of Nup98 (data not shown). Like Nup153, Nup98 is a nucleoplasmically exposed nucleoporin. Interestingly, injection into oocyte nuclei of anti-Nup98 antibodies inhibited export of all RNAs except for tRNA (43), implying that RNP export requires interaction with Nup98 as well. Moreover, in yeast, transfer of Nup120Δ cells to 37°C resulted in nuclear accumulation of karyopherin α (44). Nup120p is a yeast nucleoporin that has no peptide repeats but contains several clusters of basic residues which, like those of yeast Nup1p and Nup2p (M. Rexach and G.B., unpublished data), might interact with karyopherin α.
We also mapped one of karyopherin β1’s binding determinants for the repeat region of Nup98. This was done by limited trypsin digestion of karyopherin β1 and by affinity purification of a tryptic fragment on immobilized Nup98. The purified fragment of about 15 kDa, termed β1*, was partially sequenced and shown to begin with Asn-92 of karyopherin β1. It is likely that β1* represents a determinant for karyopherin β1 binding also to other repeat containing nucleoporins, consistent with our finding that β1* inhibited import of NLS/HSA into nuclei of digitonin-permeabilized cells. In contrast, β1* had no effect on export of karyopherin α. These data indicate that interaction of karyopherin β1 with repeat containing nucleoporins, while required for import of karyopherin α is not required for its export.
Other lines of evidence also suggest that karyopherin α and β1 are exported separately. In yeast, karyopherin α but not karyopherin β1, was found to accumulate in the nucleus, when most of the cellular Ran is likely to be in GDP bound state (45). Moreover, nuclear export of karyopherin β1 appears to be mediated by a nuclear export sequence (46). In contrast, we found that a synthetic nuclear export sequence peptide had no effect on nuclear export of karyopherin α (data not shown). As RanGTP is required for nuclear export of karyopherin α, it is possible that a nuclear RanGTP binding protein of the β karyopherin family may also be involved.
Acknowledgments
We thank Drs. Robert O’Neill and Peter Palese for antibodies to karyopherin α; Karsten Weis and Angus Lamond for karyopherin α2/hSRP1a expressing vector and Stephen Adam for karyopherin β1 expressing vector; and Philip Bernstein for providing us with purified mutant Ran. We thank John Aitchison for critical reading of the manuscript.
ABBREVIATIONS
- HSA
human serum albumin
- NLS
nuclear localization sequence
- NPC
nuclear pore complex
- Nup
nucleoporin
References
- 1.Nakielny S, Fischer U, Michael W M, Dreyfuss G. Annu Rev Neurosci. 1997;20:269–301. doi: 10.1146/annurev.neuro.20.1.269. [DOI] [PubMed] [Google Scholar]
- 2.Moroianu J, Blobel G. Proc Natl Acad Sci USA. 1995;92:4318–4322. doi: 10.1073/pnas.92.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E. Proc Natl Acad Sci USA. 1994;91:7179–7183. doi: 10.1073/pnas.91.15.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michael W M, Choi M, Dreyfuss G. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 5.Adam E J H, Adam S A. J Cell Biol. 1994;125:547–555. doi: 10.1083/jcb.125.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Görlich D, Prehn S, Laskey R A, Hartman E. Cell. 1994;79:767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 7.Moroianu J, Blobel G, Radu A. Proc Natl Acad Sci USA. 1995a;92:2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moroianu J, Hijikata M, Blobel G, Radu A. Proc Natl Acad Sci USA. 1995b;92:6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radu A, Blobel G, Moore M S. Proc Natl Acad Sci USA. 1995a;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis K, Mattaj I W, Lamond A I. Science. 1995;268:1049–1053. doi: 10.1126/science.7754385. [DOI] [PubMed] [Google Scholar]
- 11.Görlich D, Kostka S, Kraft R, Dingwall C, Laskey R A, Hartmann E, Prehn S. Curr Biol. 1995;5:383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- 12.Enenkel C, Blobel G, Rexach M. J Biol Chem. 1995;270:16499–16502. doi: 10.1074/jbc.270.28.16499. [DOI] [PubMed] [Google Scholar]
- 13.Chi N C, Adam E J H, Adam S A. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamoto N, Shimamoto T, Takao T, Tachibana T, Kose S, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. EMBO J. 1995;14:3617–3626. doi: 10.1002/j.1460-2075.1995.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamoto N, Shimamoto T, Kose S, Takao T, Tachibana T, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. FEBS Lett. 1995;368:415–419. doi: 10.1016/0014-5793(95)00699-a. [DOI] [PubMed] [Google Scholar]
- 16.Görlich D, Vogel F, Mills A D, Hartmann E, Laskey R A. Nature (London) 1995;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- 17.Moore M S, Blobel G. Nature (London) 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- 18.Melchior F, Paschal B, Evans J, Gerace L. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore M S, Blobel G. Proc Natl Acad Sci USA. 1994;91:10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paschal B M, Gerace L. J Cell Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rexach M, Blobel G. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 22.Nehrbass U, Blobel G. Science. 1996;272:120–122. doi: 10.1126/science.272.5258.120. [DOI] [PubMed] [Google Scholar]
- 23.Sukegawa J, Blobel G. Cell. 1993;72:29–38. doi: 10.1016/0092-8674(93)90047-t. [DOI] [PubMed] [Google Scholar]
- 24.Panté N, Bastos R, McMorrow I, Burke B, Aebi U. J Cell Biol. 1994;126:603–617. doi: 10.1083/jcb.126.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordes V C, Reidenbach S, Köhler A, Stuurman N, Van Driel R, Franke W W. J Cell Biol. 1993;123:1333–1344. doi: 10.1083/jcb.123.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordes V C, Redenbach S, Rackwitz H R, Franke W W. J Cell Biol. 1997;136:515–529. doi: 10.1083/jcb.136.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rout M P, Wente S R. Trends Cell Biol. 1994;4:357–365. doi: 10.1016/0962-8924(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 28.Floer M, Blobel G. J Biol Chem. 1996;271:5313–5316. doi: 10.1074/jbc.271.10.5313. [DOI] [PubMed] [Google Scholar]
- 29.Radu A, Moore M S, Blobel G. Cell. 1995;8:1–8. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 30.Moore M S, Blobel G. Cell. 1992;69:939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez J, DeMott M, Atherton D, Mische S M. Anal Biochem. 1992;201:255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- 32.Moroianu J, Blobel G, Radu A. Proc Natl Acad Sci USA. 1996;93:6572–6576. doi: 10.1073/pnas.93.13.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moroianu J, Blobel G, Radu A. Proc Natl Acad Sci USA. 1996;93:7059–7062. doi: 10.1073/pnas.93.14.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neill R E, Palese P. Virology. 1994;206:116–125. doi: 10.1016/s0042-6822(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 35.Coutavas E, Ren M, Oppenheim J D, D’Eustachio P, Rush M G. Nature (London) 1993;366:585–587. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- 36.Iovine M K, Watkins J L, Wente S R. J Cell Biol. 1995;131:1669–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollard V, Michael M W, Nakielny S, Siomi M C, Wang F, Dreyfuss G. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 38.Aitchison J D, Blobel G, Rout P R. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- 39.Bonifaci N, Moroianu J, Radu A, Blobel G. Proc Natl Acad Sci USA. 1997;94:5055–5060. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaseen N R, Blobel G. Proc Natl Acad Sci USA. 1997;94:4451–4456. doi: 10.1073/pnas.94.9.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rout M P, Blobel G, Aitchison J D. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 42.Bastos R, Lin A, Enarson M, Burke B. J Cell Biol. 1996;134:1141–1156. doi: 10.1083/jcb.134.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers M A, Forbes D J, Dahlberg J E, Lund E. J Cell Biol. 1996;136:241–250. doi: 10.1083/jcb.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aitchison J D, Blobel G, Rout M P. J Cell Biol. 1995;131:1659–1675. doi: 10.1083/jcb.131.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koepp D M, Wong D H, Corbett A H, Silver P A. J Cell Biol. 1996;133:1163–1176. doi: 10.1083/jcb.133.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iovine M K, Wente S R. J Cell Biol. 1997;137:1–15. doi: 10.1083/jcb.137.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]