Abstract
Mitogen-activated lymphoblasts isolated from the blood and lymph nodes, but not the spleen, of domestic cats acutely infected with the Petaluma or Glasgow8 isolates of feline immunodeficiency virus (FIV), suppressed the replication of FIV in the MYA-1 T-cell line in a dose-dependent manner. This effect was not limited to the homologous isolate of FIV. The suppressor activity declined with progression to chronic infection, with lower levels of activity detectable only in the lymph nodes. Immunization of domestic cats with whole inactivated FIV vaccine elicited profound suppressor activity in both the blood and lymph nodes. The suppressor activity was associated with the CD8+ T-cell subpopulation, the effect did not appear to be major histocompatibility complex-restricted, and was mediated by a soluble factor(s). This activity may be associated with the control of virus replication during both the asymptomatic stages of FIV infection, and in the protective immunity observed in cats immunized with whole inactivated virus vaccines.
INTRODUCTION
Infection with human immunodeficiency virus (HIV-1) is characterized by the rapid development of HIV-1-specific major histocompatibility complex (MHC) class I-restricted cytotoxic T lymphocytes (CTL), which recognize a variety of structural and non-structural viral antigens.1,2 The association between high levels of virus-specific CTL detected prior to the development of virus-neutralizing antibodies and reduction in early viraemia,3,4 coupled with the observation of a decline in CTL precursor frequency with progression to clinical disease,5,6 has led to the conclusion that the virus-specific CTL response has a pivotal role to play in controlling infection. This belief is reinforced by the detection of virus-specific CTL in seronegative children born to HIV-infected mothers,7–9 and in seronegative sex workers repeatedly exposed to the virus10 and yet who remain uninfected.
CD8+ T cells can also control HIV infection without lysing the infected cell.11,12 This non-cytolytic cellular immune response involves suppression by CD8+ T cells of HIV replication in CD4+ T cells and macrophages.12,13 Such activity has been demonstrated previously either by cocultivating CD8+ T cells with an individual’s own CD4+ T cells, termed the ‘endogenous assay’, or by coculturing the patient’s CD8+ T cells with autologous or allogeneic CD4+ T cells infected in vitro with a known isolate of HIV, termed the ‘acute infection assay’.13 The antiviral activity is not MHC-restricted and involves the release of soluble factors. A number of cytokines have been implicated including the β-chemokines RANTES (regulated on activation, normal T expressed and secreted), macrophage inflammatory proteins -1α and -1β (MIP-1α; MIP-1β,14 macrophage-derived chemokine (MDC)15 and interleukin-16 (IL-16).16 In addition, another soluble factor, which does not share identity with any known cytokine or chemokine, termed CD8+ T-cell antiviral factor (CAF)11,17 has also been described. Several studies have revealed the occurrence of a similar antiviral activity in non-human primate models of acquired immune deficiency syndrome (AIDS).18–21
Unlike simian immunodeficiency virus (SIV) or HIV-2 infection of non-human primates, infection of domestic cats with feline immunodeficiency virus (FIV) represents lentiviral infection in its natural host. This system therefore represents an ideal system in which to characterize the immune responses associated with the control of viral replication during the asymptomatic phase of infection in a natural host.22 Furthermore, the feline model is unique in the ability to induce protective immunity in cats by immunization with whole inactivated virus (WIV) vaccines,23–25 thus allowing the dissection of the vaccinal protective immune response. As in HIV infection, a virus-specific CTL response is observed following experimental infection with FIV prior to the onset of humoral immunity.26,27 Vaccination also elicits both FIV Gag- and Env-specific CTL responses,28 and recent studies suggest that Env-specific CTLs are important in the long-term maintenance of protection.29,30 Therefore, as in HIV and SIV infections, cellular immunity has an important role to play in FIV immunity. However, the contribution played by non-cytolytic CD8+ T cells is less well understood in FIV infection. CD8+ T-cell-mediated antiviral activity has been described in cats chronically infected with the NCSU1 isolate of FIV,31 suggesting that this activity may be involved in the maintenance of the asymptomatic state in these animals.
In the present study, we developed an acute infection assay to measure suppression of FIV replication in vitro. Is suppressor activity involved in the control of virus in infected cats? To address this question we determined the suppression observed in cats acutely and chronically infected with FIV. We examined the lymphoid distribution of this activity, and compared this with the situation in unexposed, non-infected cats. Does suppressor activity contribute to vaccinal protection? Suppressor activity was measured in the lymphoid tissues of cats following immunization with whole inactivated virus vaccines to determine its role in the protection observed. Finally, we demonstrate that this activity is mediated at least in part by a soluble factor(s) secreted by CD8+ T cells which acts in a non-MHC-restricted manner to suppress FIV replication.
MATERIALS AND METHODS
Experimental animals
The 11 adult, outbred, specific pathogen-free domestic cats selected for this study were serologically negative for FIV. Two cats were vaccinated by subcutaneous (s.c.) inoculation on three occasions, 3 weeks apart with 250 μg of cell-free FIV inactivated by treatment with 0·5% paraformaldehyde. A combination of threonyl muramyl dipeptide (tMDP) and SAF-M was used as an adjuvant (kindly provided by Chiron Corporation, Emeryville, CA). This vaccine, prepared from the culture fluid of the FL4 feline lymphoblastoid cell line, which is persistently infected with FIVPET,32 has been previously shown to protect cats from challenge with homologous virus.23 These cats resisted homologous virus challenge and were examined 12 weeks later. Seven cats were experimentally infected with FIV by intraperitoneal (i.p.) inoculation of either 25 cat infective doses 50% (CID50) of FIVPET, 10 CID50 of FIVGL-8, or 4000 CID50 of FIVGL-8. Two adult specific pathogen-free cats which were not exposed to FIV antigens were included as normal controls.
Tissues were prepared from all animals 3–4 months following either vaccination or infection. This corresponded with the acute stage of infection. The two cats infected with 4000 CID50 FIVGL-8 were allowed to develop a chronic infection and were subsequently examined 22 months after challenge.
Preparation of lymphocyte effector cells
Mononuclear cells were prepared from the blood, spleen, and lymph nodes of all cats. At post-mortem examination, blood was collected into an equal volume of Alsevers’ solution (Scottish Antibody Production Unit, Carluke, UK), and mononuclear cells were isolated by centrifugation over Ficoll-Paque (Pharmacia LKB Biotechnology Inc., Piscataway, NJ). Samples of spleen and lymph nodes were also collected, and a single-cell suspension of these tissues was prepared by gentle manual homogenization. Mononuclear cells were again isolated by centrifugation over Ficoll-Paque and were washed twice and stored in liquid nitrogen until use in the assays.
In vitro infection of MYA-1 cells with FIV
MYA-1 cells33 were infected in vitro by incubating 5×105 cells with 1 ml cell-free supernatant from either FIVPET- or FIVGL-8-infected MYA-1 cells, corresponding to one tissue culture infective dose 50% (TCID50) of either isolate of FIV, in a 2054 Falcon tube (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) for 1 hr at 37°. The cells were then washed twice and resuspended in 1 ml fresh RPMI-1640 medium (Gibco Biocult, Paisley, UK) containing 10% fetal bovine serum (Biological Industries Ltd, Cumbernauld, UK), 2 mm l-glutamine, 5×10−5 m 2-mercaptoethanol, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml (complete RPMI-1640 medium) supplemented with 5% culture supernatant from the Ltk−IL-2.23 cell line producing human recombinant IL-2 (a kind gift from T. Miyazawa, University of Tokyo, and M. Hattori, University of Kyoto) for use in the assays.
Detection of antiviral activity in mononuclear cells
The capacity of mononuclear cells from the blood, spleen and lymph nodes of FIV-vaccinated or FIV-infected cats to suppress the replication of FIV in vitro was assessed. The cells were stimulated in vitro with 7·5 μg/ml concanavalin A (Con A; Sigma Chemical Co., Poole, UK) for 72 hr and thereafter maintained in complete RPMI-1640 medium supplemented with 5% culture supernatant from the Ltk−IL-2.23 cell line. Assays were performed by adding the appropriate number of Con A-stimulated lymphoblasts to 5×105 MYA-1 cells previously infected with 1 TCID50 of either FIVPET or FIVGL-8, in flat-bottomed 24-well tissue culture plates (Falcon, Becton Dickinson Labware) to give ratios of lymphoblasts to infected MYA-1 cells of 4:1, 2:1, and 1:1, in a final volume of 2 ml complete RPMI-1640 medium. The cell mixtures were then incubated in a humidified atmosphere containing 5% CO2. At intervals of 3–4 days, 0·5 ml samples of culture supernatant fluids were collected and replaced with fresh medium. Samples were stored at −20° until assayed by enzyme-linked immunosorbent assay (ELISA) for the presence of FIV p24 antigen (PetChek, IDEXX Corp., Portland, ME). Cultures were maintained for up to 14 days.
Depletion of T-cell subsets from effector populations
To determine the phenotype of the cells responsible for the observed suppression of FIV replication by mononuclear cells derived from the lymph nodes, CD4+ or CD8+ T cells were depleted from the effector cell population by incubating lymph node cells with mouse monoclonal anti-fCD4 antibody (vpg 39) or mouse monoclonal anti-fCD8 antibody (vpg 9)34 for 30 min at 4°. Labelled cells were then washed once, and the CD4+ or CD8+ T-cell fraction was removed by incubation with magnetic beads coated with sheep anti-mouse immunoglobulin G1 (IgG1) antibody (Dynabeads M-450, Dynal AS, Oslo, Norway).
Detection of soluble antiviral factors
To assess the involvement of soluble factors in the antiviral activity observed in the lymph nodes, Con A-stimulated lymphoblasts were cocultivated with FIV-infected MYA-1 cells in 24-well tissue culture plates in which the two cell populations were separated by a 0·4-μm high pore density polyethylene terephthalate track-etched membrane (Falcon, Becton Dickinson Labware) in a total volume of 2 ml complete RPMI-1640 medium supplemented with 5% culture supernatant from the Ltk−IL-2.23 cell line. The cultures were incubated at 37° in a humidified atmosphere containing 5% CO2, and the culture supernatant fluids were collected and assayed by ELISA for the presence of FIV p24 antigen.
RESULTS
Lymphocytes from acutely infected cats suppress FIV replication in vitro
To assess the capacity of the mitogen-activated lymphoblasts obtained from different lymphoid organs during the acute stage of FIV infection to suppress the replication of FIV in vitro, mononuclear cells were prepared from the blood, spleen and lymph nodes of three cats, 12 weeks following experimental infection with 25 CID50 FIVPET. The cells were stimulated in vitro for 72 hr with Con A and were then cocultured with FIVPET- or FIVGL-8-infected MYA-1 cells. Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA.
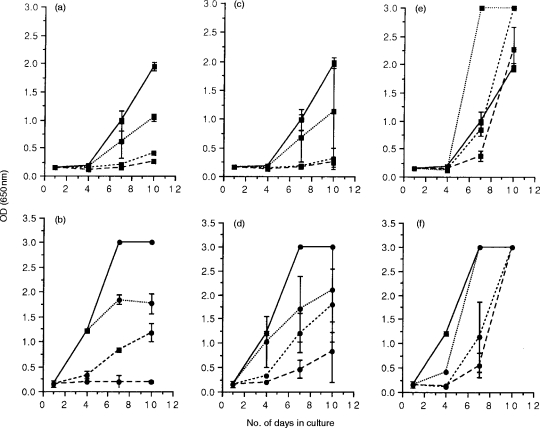
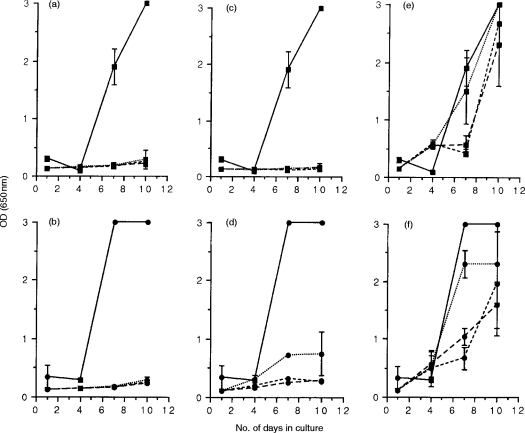
Viral replication was initially observed at day 7 in control cultures of MYA-1 cells infected in vitro with FIVPET and cultured alone (Fig. 1a, c and e). Co-cultivation of FIVPET-infected MYA-1 cells with mitogen-activated lymphoblasts from blood or lymph nodes of FIVPET-infected cats suppressed FIV replication. The degree of suppression varied depending on the effector to target ratio (E:T) examined, with complete suppression at the highest E:T ratios (4:1 and 2:1; P<0·05 on day 7 and 10 by two way t-test) and partial suppression at the lowest E:T ratio (1:1) as indicated by the detection of FIV p24 in the culture supernatants. By day 7 following in vitro infection, viral replication was observed at the lowest E:T ratio (1:1), although the optical density (OD) values measured were lower than those control cultures indicating that virus replication was suppressed by up to 46% at this E:T ratio. However, this effect was not observed when splenic lymphoblasts were co-cultivated with FIVPET-infected cells. In these cultures, viral replication was observed at similar levels to that in control cultures. By day 10, higher levels of FIV p24 were detectable in cultures containing splenocytes suggesting that FIV replication was enhanced in the presence of splenic lymphoblasts.
Figure 1.
FIV-specific suppressor activity in acutely infected cats. Mitogen-activated lymphoblasts were prepared from the blood (PBMC; a and b), lymph nodes (LNC; c and d), and spleen (e and f) of three cats, 12 weeks postinfection with FIVPET, and were cocultured with either FIVPET-infected (▪) or FIVGL-8-infected (•) MYA-1 cells at ratios of 4:1 (---), 2:1 (– – –), and 1:1 (· · ·). Control cultures contained FIV-infected MYA-1 cells alone (——). Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the OD values at 650 nm for the lymphoid tissues of each individual cat. Control cultures with FIV-infected MYA-1 cells were performed in duplicate, and are represented as the mean OD values at 650 nm for the three cats ± 2 SD, error bars are not discernible where the SD is small.
To assess the specificity of the suppressor activity, mitogen-activated lymphoblasts from the FIVPET-infected cats were cocultivated with FIVGL-8-infected MYA-1 cells. Replication of FIVGL-8 was detected earlier (day 4) in control cultures containing MYA-1 cells infected in vitro with FIVGL-8, compared to similar control cultures containing FIVPET-infected MYA-1 cells (day 7, see above), and higher levels of p24 were detected in the culture supernatant indicative of increased viral replication (Fig. 1b,d,andf). Despite the higher levels of viral replication observed in the FIVGL-8-infected cultures, cocultivation with lymphoblasts from the blood or lymph nodes of FIVPET-infected cats did significantly down-regulate FIV replication in a dose-dependent manner (P<0·05 at all E:T ratios on day 7, and at highest E:T ratio on day 10 by two way t-test). This effect became less noticeable at the lowest E:T ratio, presumably reflecting the greater viral burdens in the cultures. In contrast to the experiments using FIVPET-infected MYA-1 cells, splenic lymphoblasts were capable of suppressing FIVGL-8 replication at days 4 and 7 by 90% and 81%, respectively, at an E:T ratio of 4:1. This effect was lost by day 10 in culture.
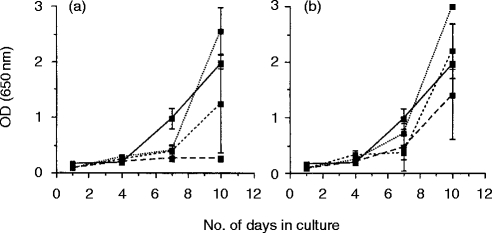
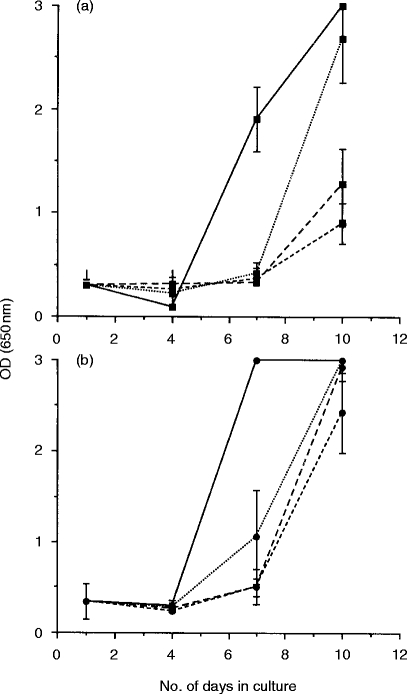
Control experiments were designed to determine whether the observed suppression of FIV replication by blood and lymph node lymphoblasts was elicited by FIVPET infection, or whether it represented an innate form of immunity. Thus, lymphoblasts were prepared from the blood and lymph nodes of two age-matched uninfected cats which had not been exposed to FIV antigens. Suppression was only observed in cultures containing blood lymphocytes at the highest E:T ratio of 4:1, and this was not statistically significant (Fig. 2a). In all other cultures, including those containing lymph node lymphoblasts, FIV replication was comparable to control cultures containing FIVPET-infected MYA-1 cells alone, indicating that although suppressive activity was observed in lymphoblasts prepared from uninfected cats, the activity was lower than that observed in the acutely infected cats. These results suggest that there may be an up-regulation of the suppressive activity associated with prior exposure to FIV.
Figure 2.
FIV-specific suppressor activity in normal cats. Mitogen-activated lymphoblasts were prepared from the blood (a) and lymph nodes (b) of two normal, specific pathogen-freecats which had never been exposed to either infectious FIV or FIV antigens, and were cocultured with FIVPET-infected MYA-1 cells at ratios of 4:1 (---), 2:1 (– – –), and 1:1 (· · ·). Control cultures contained FIVPET-infected MYA-1 cells alone (——). Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the mean OD values at 650 nm for the two cats ± 2 SD, error bars are not discernible where the SD is small.
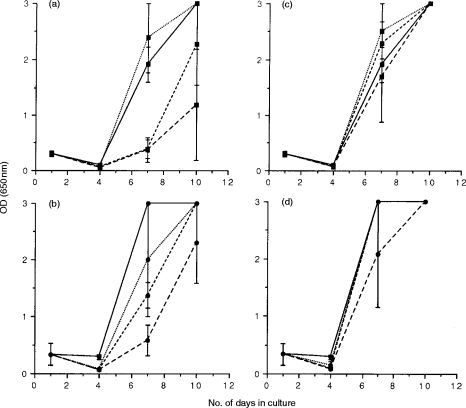
To determine whether the observed suppression of FIV replication was a feature of FIVPET infection alone, experiments were performed in which blood and lymph node lymphoblasts from two FIVGL-8-infected cats prepared at 16 weeks following experimental infection, were cocultivated with FIVGL-8-or FIVPET-infected MYA-1 cells (Fig. 3). In cultures of PBMC, suppression of FIVPET replication was observed at the highest E:T ratios (4:1 and 2:1; P<0·05 at E:T ratio of 4:1, but not significant at E:T ratio of 2:1 by two way t-test) at day 7 (87% suppression) and day 10 (61% suppression), and suppression of FIVGL-8 was observed at the highest E:T ratios on day 7 (81% suppression at 4:1). However, no suppression was observed in cultures of lymph node cells with either FIVPET or FIVGL-8-infected MYA-1 cells. This is in marked contrast to the results observed with lymph node cells from cats acutely infected with FIVPET, where suppression of both FIVPET and FIVGL-8 was observed (Fig. 1).
Figure 3.
FIV-specific suppressor activity during acute infection with FIVGL-8. Mitogen-activated lymphoblasts were prepared from the blood (a and b) and lymph nodes (c and d) of two cats, 16 weeks postinfection with FIVGL-8, and were cocultured with either FIVPET-infected (▪) or FIVGL-8-infected (•) MYA-1 cells at ratios of 4:1 (---), 2:1 (– – –), and 1:1 (· · ·). Control cultures contained FIV-infected MYA-1 cells alone (——). Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the mean OD values at 650 nm measured for the two cats ± 2 SD, error bars are not discernible where the SD is small.
Suppressor cell activity in chronically FIV-infected cats
The previous experiments clearly demonstrate that a population of cells capable of down-regulating FIV replication in MYA-1 T cells in vitro is present in the blood and lymph nodes of cats, and that this activity is increased during the acute stage of FIV infection.This effect is dose-dependent, is not MHC-restricted, and the activity is not limited to the homologous isolate of the virus. To determine whether this cell population persisted during the chronic, asymptomatic phase of infection, lymphoblasts were prepared from the blood, spleen and lymph nodes of two cats 22 months following experimental infection with FIVGL-8 and analysed for their FIV suppressor activity.
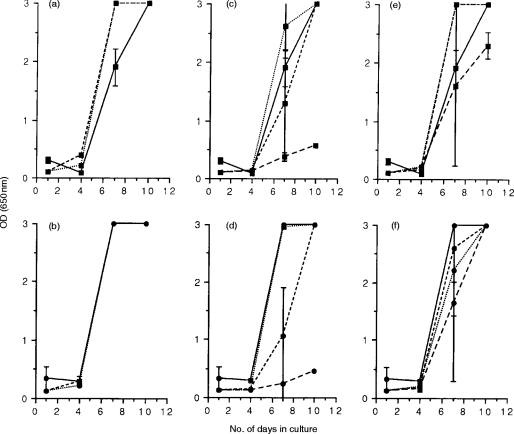
Lymph node lymphoblasts from chronically infected cats were capable of suppressing FIVPET and FIVGL-8 replication at the highest E:T ratio on day 7 (80% suppression of FIVPET, 92% suppression of FIVGL-8) and day 10 (81% suppression of FIVPET, 85% suppression of FIVGL-8). In contrast, suppression of FIV replication was not observed in cultures of lymphoblasts derived from either the blood or spleen, using either FIVPET or FIVGL-8-infected MYA-1 cells as targets (Fig. 4). Interestingly, co-cultivation with splenic lymphoblasts did not induce the enhancement of FIV replication observed during acute FIVPET infection (Fig. 1e,f).
Figure 4.
FIV-specific suppressor activity in chronically infected cats. Mitogen-activated lymphoblasts were prepared from the blood (a and b), lymph nodes (c and d), and spleen (e and f) of two cats, 22 months following infection with FIVGL-8, and were cocultured with either FIVPET-infected (▪) or FIVGL-8-infected (•) MYA-1 cells at ratios of 4:1 (---), 2:1 (– – –), and 1:1 (· · ·). Control cultures contained FIV-infected MYA-1 cells alone (——). Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the mean OD values at 650 nm for the two cats ± 2 SD, error bars are not discernible where the SD is small.
WIV vaccination elicits FIV-specific suppressor cells
To determine whether WIV vaccination could elicit similar suppressor cell activity to that observed during acute FIV infection, the experiments were repeated with lymphoid tissues from 2 WIV vaccinated cats 15 weeks following the final immunization. Lymphoblasts from both the blood and the lymph nodes of the WIV-vaccinated cats suppressed the replication of either FIVPET or FIVGL-8 in vitro (Fig. 5). Lymphoblasts derived from the blood completely suppressed FIVPET replication, with no FIV p24 detected in the culture supernatants at any time during the study, at any E:T ratio, P<0·05 at E:T=4:1 and 2:1 on day 10. Replication of FIVGL-8 was also suppressed, although low levels of FIV p24 could be detected in the lowest E:T ratio of 1:1, nevertheless this suppression was statistically significant (P<0·05 by two-tailed t-test). However, FIVPET replication was not down-regulated by splenic lymphoblasts (Fig. 5e), but partial suppression of FIVGL-8 was observed at the highest E:T ratios on day 7 (65% suppression, E:T=4:1) and day 10 (47% suppression, E:T=4:1).
Figure 5.
FIV suppressor cell activity in WIV-vaccinated, protected cats. Mitogen-activated lymphoblasts were prepared from the blood (a and b), lymph nodes (c and d), and spleen (e and f) of two cats immunized with whole inactivated virus vaccine on three occasions, 3 weeks apart, 15 weeks following the final inoculation, and were cocultured with either FIVPET-infected (▪) or FIVGL-8-infected (•) MYA-1 cells at ratios of 4:1 (---), 2:1 (– – –), and 1:1 (· · ·). Control cultures contained FIV-infected MYA-1 cells alone (——). Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the mean OD values at 650 nm measured for the two cats ± 2 SD, error bars are not discernible where the SD is small.
Suppression of FIV replication is associated with CD8+ T cells
In the preceding experiments, inhibition of virus replication was consistently observed when lymph node cells from acutely infected cats and WIV vaccinates were cocultivated with MYA-1 T cells infected with either FIVGL-8 or FIVPET. To determine the cell phenotype responsible for the suppressor activity, the experiments were repeated using lymphoblasts derived from cats either acutely infected with FIVPET, or immunized with WIV vaccine from which either the CD4+ or CD8+ T-cell subpopulations had been depleted by immunomagnetic bead separation.
Co-cultivation of lymph node lymphoblasts with FIV-infected MYA-1 cells again suppressed the replication of FIV when compared to control cultures containing only FIV-infected MYA-1 cells. Depletion of CD8+ T cells from the effector cells abrogated the suppressor effect (Fig. 6), whereas suppression was still observed in cultures depleted of CD4+ T cells indicating that CD8+ T cells were mediating the suppression. This effect was observed with both FIVPET or FIVGL-8 infected target MYA-1 T cells.
Figure 6.
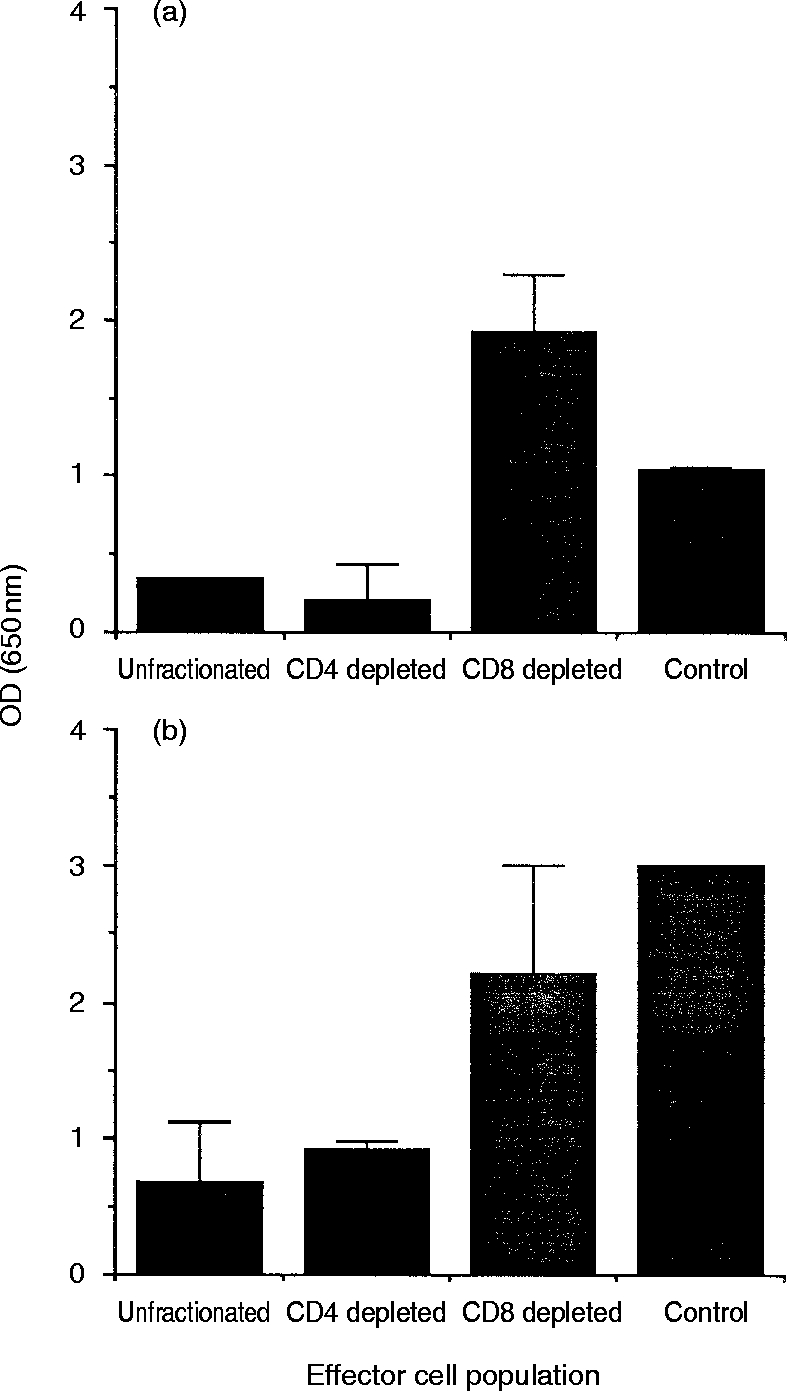
FIV-specific suppression is associated with CD8+ T cells. Mitogen-activated lymphoblasts were prepared from the lymph nodes of three cats, 12 weeks postinfection with FIVPET(a), and two WIV-vaccinated, protected cats (b). To determine the cell phenotype responsible for the virus-specific suppression, the CD4+ or CD8+ T-cell subpopulation was depleted by labelling with appropriate monoclonal antibodies and immuno-magnetic bead separation. Control cultures contained FIV-infected MYA-1 cells alone. Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the mean OD values at 650 nm measured in individual samples for either the three infected cats or two vaccinated cats, respectively, ± 2 SD, error bars are not discernible where the SD is small.
Suppressor effect is mediated by a soluble factor
Our previous studies have shown that MHC class I-restricted FIV-specific CTL responses mediated by CD8+ T cells are elicited in cats following either experimental infection or immunization with WIV vaccines.28,29 Although the activity described in the present report also appears to be mediated by CD8+ T cells, it does not appear to be MHC-restricted since the MYA-1 T cells are unlikely to be histocompatible with all of the cats examined in this study. To formally exclude the involvement of FIV-specific CTL in the suppression of FIV replication described in this report, experiments were performed in which the effector lymphoblasts and the FIV-infected MYA-1 T cells were separated by a 0·4-μm semi-permeable membrane allowing the free passage of soluble molecules, but preventing direct cell-to-cell contact required for cytolysis by CTL.
Suppression of FIVPET and FIVGL-8 replication was observed with lymph nodes from cats acutely infected with FIVPET. Suppression of FIV replication, in a dose-dependent manner, was still observed following separation of the effector and target cells by a semi-permeable membrane, implicating the involvement of a soluble factor in the observed down-regulation of virus replication (Fig. 7). The inhibition of FIVGL-8 replication was lost at all E:T ratios examined by day 10 of culture, whereas replication of FIVPET was still suppressed at the higher E:T ratios (4:1 and 2:1) at this time-point.
Figure 7.
FIV-specific suppressor effect is mediated by a soluble factor. Mitogen-activated lymphoblasts were prepared from the lymph nodes of three cats, 12 weeks postinfection with FIVPET, and were cocultured with either FIVPET-infected(a) or FIVGL-8-infected (b) MYA-1 cells at ratios of 4:1 (---), 2:1 (– – –), and 1:1 (· · ·), separated by a 0·4-μm semi-permeable membrane. Control cultures contained FIV-infected MYA-1 cells alone (——). Replication of FIV was indicated by the detection of viral p24 in the culture supernatant by ELISA. The results shown represent the mean OD values at 650 nm measured for the three cats ± 2 SD, error bars are not discernible where the SD is small.
DISCUSSION
Animal models of human lentivirus infection have an important role to play in the determination of the immune correlates of vaccine-induced protection, and in the control of virus replication during the asymptomatic stage of infection. Studies of FIV in domestic cats have revealed that both humoral immune responses, such as virus neutralizing antibodies,23 and cell-mediated immunity, such as virus-specific CTL,28–30 represent important components of the host’s protective immune response. Recently, another cell-mediated immune response, non-cytolytic antiviral CD8+ T cells, has been implicated in the control of FIV replication during the asymptomatic phase of infection.31 In the present study we developed an acute infection assay based in the in vitro infection of the feline T lymphoblastoid cell line, MYA-1, with equivalent amounts of either FIVPET or FIVGL-8. Using this assay we investigated the contribution of suppressor activity to the control of viral replication during the asymptomatic stage of infection by comparing the capacity of mitogen-stimulated CD8+ T cells from cats acutely or chronically infected with FIV to suppress virus replication in vitro. These responses were compared with cats immunized with whole inactivated virus vaccines to determine the contribution of suppressor activity to vaccinal immunity.
Following acute experimental infection with FIVPET and, to a lesser extent FIVGL-8, a suppressor cell population was demonstrated in the blood and lymph nodes capable of inhibiting FIV replication in vitro. A similar phenomenon has been observed in asymptomatic HIV-infected individuals12 with dose-dependent suppression of virus replication mediated by CD8+ T cells. That activity was associated with a block in virus production rather than destruction of the virus-infected CD4+ T lymphocytes and macrophages themselves. Furthermore, CD8+ lymphocytes from SIV-infected, but not uninfected, rhesus macaques block SIV replication in peripheral blood lymphocytes.21 The inhibition of FIV replication observed in the acutely infected cats was not limited to the homologous isolate of FIV, with suppression of both FIVPET and FIVGL-8 by the lymphoblasts from FIVPET-infected cats. When splenic lymphoblasts were examined they were unable to to suppress FIV replication, and enhancement was observed in some situations. At present, the reason for the observed enhancement of FIV replication with splenic lymphoblasts is unknown, although the spleens of domestic carnivores do contain high numbers of γδ T cells, with up to 33% of T cells in the canine spleen expressing the γδ phenotype (P. Moore, personal communication), and it is possible that these two observations may be linked.
In the present study, the suppressor activity measured during the acute stage of infection did not appear to persist into the more chronic stage of infection. Twenty-two months after infection with FIVGL-8, suppressor activity was no longer observed in the blood, and only the highest E:T ratios of lymph node lymphoblasts demonstrated high levels (80–92%) of suppression. This observation may reflect the much higher viral loads achieved following infection with FIVGL-8 compared to FIVPET (M.J. Hosie, personal communication), and also the more rapid replication rate of FIVGL-8 in vitro (Fig. 1). The decline in antiviral activity with disease progression following FIVGL-8 infection suggests that this activity may contribute to the control of viraemia during asymptomatic infection. This would agree with observations of HIV-infected individuals, where CD8+ T-cell antiviral activity appears during the acute stage of disease, persists during the asymptomatic phase of disease, and then deteriorates with progression to clinical illness.12,35,36 However, our results contrast with those of Jeng and co-workers who were unable to detect CD8+ T-cell anti-FIV activity in the blood of cats acutely infected with the NCSU1 isolate of FIV. Such activity only became detectable during the asymptomatic phase of infection at 22–44 weeks, and the activity persisted in a proportion of the long-term infected cats (95–105 weeks p.i.).31 The differences between these two studies may be attributed to the different FIV isolates, which have different characteristics in vitro and in vivo (M.J. Hosie, personal communication), used to infect the cats.
It is of interest that we observed antiviral activity prior to infection or exposure to viral antigens (Fig. 2). Our data suggest that the CD8+ T cell antiviral activity described may reflect an innate form of antiviral immunity which is up-regulated following exposure to viral antigens. However, since we have not characterized the factor(s) responsible for the observed down-regulation of FIV replication observed in this study, it is not clear whether the same mechanisms are involved in uninfected, unexposed cats as in FIV-infected or vaccinated, protected cats. Certainly CD8+ anti-HIV activity has been observed in uninfected, unexposed humans using both endogenous13 and acute infection assays,37 although higher ratios of CD8+ cells to target cells are required in vitro to demonstrate this activity compared to CD8+ cells from infected individuals. Likewise non-human primates, including baboons and chimpanzees, also possess a constitutive cell-mediated immune mechanism for the control of HIV replication.14,18
Previous studies on cell-mediated immunity to FIV have focussed on virus-specific cytotoxic CD8+ T cells. From these studies we know that FIV-specific cytotoxicity is detected in the blood as early as 2 weeks following experimental infection26,27 preceding seroconversion and the clearance of circulating virus. As infection progresses, CTL activity appears in the lymph nodes and declines in the peripheral blood, presumably reflecting the compartmentalization of the virus to these sites during infection.29 Therefore, non-cytolytic and cytolytic immune responses may both be involved in the control of FIV replication, and both are mediated by CD8+ T cells. It is possible, however, that the two activities may be associated with different subpopulations within the CD8+ T-cell fraction. The rapid expansion of CD8low T cells has been documented following FIV infection.34,38,39 This T-cell subpopulation shares some features with natural killer cells, and has recently been shown to express either CD8αα homodimers or, or to have decreased expression of the CD8β chain.40 Our future studies will focus on the potential antiviral properties of these CD8low T cells.
To formally exclude the possibility that the suppression of FIV replication observed in vitro was not associated with either virus-specific lymphocytoxicity or a mixed lymphocyte reaction (MLR), both of which require intimate cell-to-cell contact, the CD8+ effector T cells were separated from the virus-infected target cells by a 0·4-μm permeable membrane. Separation of the cell populations failed to block the antiviral activity (Fig. 7) indicating the involvement of a soluble factor. Furthermore, the activity was not MHC-restricted, since all of the cats used in the study were outbred, and the MYA-1 cells did not share a common source with any of the experimental cats. In contrast, all of the CD8+ CTL activity described to date appears to be MHC-restricted.26–30
No attempt was made to characterize the soluble factors associated with the antiviral activity described in this report. However, a number of molecules have been shown to be associated with this activity in HIV-infected individuals, including MIP-1α, MIP-1β and RANTES,14 MDC,15 IL-16,16 and another unidentified soluble factor, CAF.11 Indeed it has been shown that CD4+ T cells from individuals that produce higher levels of the C-C chemokines MIP-1α, MIP-1β and RANTES are relatively more resistant to HIV-1 infection.41
One unique feature of the FIV model is the ability to induce virus-specific protection by immunization with WIV vaccines, thus allowing elucidation of the immune correlates of protection. Recent studies have revealed that WIV vaccination elicits FIV-specific CTL activity28 in addition to virus-neutralizing antibodies.23 FIV Env-specific CTLs appear also to be involved in the long-term protection induced by vaccination.29,30 In this report we demonstrate that WIV vaccination also elicits potent antiviral suppressor cell activity in the blood and lymph nodes (Fig. 5), which may represent an invaluable component of the host’s protective immunity, and indicate that WIV vaccination elicits a broad spectrum of virus-specific immune reactivity resulting in protection of which noncytolytic CD8+ T cells represent a fraction.
In summary, experimental FIV infection of domestic cats is associated with the induction of CD8+ T cells capable of suppressing viral replication in vitro in a non-MHC-restricted manner. The effect is not limited to the homologous viral isolate and is mediated, at least in part, by a soluble factor(s). Progression to later disease is accompanied by a decline in suppressor activity. Inoculation of cats with WIV vaccines elicits potent suppressor cell activity in the blood and lymph nodes, suggesting that this activity may represent an important component of the host’s protective immunity. Future studies will focus on the kinetics of the suppressor activity following experimental infection and attempt to correlate this activity with the induction of virus-specific CTL responses, and viral loads in vivo, allowing rational decisions to be made on vaccine design based on a comprehensive understanding of the protective cellular immune mechanisms in vivo.
Acknowledgments
This work was supported by a Program Grant from the Medical Research Council, UK.
References
- 1.Lamhamedi-Cherradi S, Culmann-Penciolelli B, Guy B, et al. Qualitative and quantitative analysis of human cytotoxic T-lymphocyte responses to HIV-1 proteins. AIDS. 1992;6:1249. doi: 10.1097/00002030-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Nixon DF, Broliden K, Ogg G, Proliden P-A. Cellular and humoral antigenic epitopes in HIV and SIV. Immunology. 1992;76:515. [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viraemia in primary human immunodeficiency type 1 syndrome. J Virol. 1994;68:4650. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficeincy virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: Differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldo C, Huang X-L, Fan Z, et al. High levels of anti-human immunodeficiency virus type I (HIV-I) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-I-infected long-term nonprogressors. J Virol. 1995;69:5838. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryson YJ, Pang S, Wei LS, Dickover R, Diagne A, Chen ISY. Clearance of HIV infection in a perinatally infected infant. N Engl J Med. 1995;332:833. doi: 10.1056/NEJM199503303321301. [DOI] [PubMed] [Google Scholar]
- 8.Chenyier R, Langlade-Demoyen P, Marescot M-R, et al. Cytotoxic T lymphocyte responses in the peripheral blood of chilldren born to human immunodeficency virus-1-infected mothers. Eur J Immunol. 1992;22:2211. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 9.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;241:860. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 10.Rowland-Jones SL, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nature Medicine. 1995;1:9. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 11.Levy JA, Mackewicz CE, Andbarker E. Controlling HIV pathogenesis: The role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 12.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing viral replication. Science. 1986;234:1563. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 13.Mackewicz CE, Levy JA. CD8+ cell anti-HIV activity: Nonlytic suppression of virus replication. AIDS Res Hum Retroviruses. 1992;8:1039. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, Devico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Pal R, Garzino-Demo A, Markham PD, et al. Inhibition of HIV-1 infection by the β-chemokine MDC. Science. 1997;278:695. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 16.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIVsuppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 17.Mackewicz CE, Ortega H, Levy JA. Effect of cytokines on HIV replication in CD4+ lymphocytes: Lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153:329. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 18.Blackbourn DJ, Locher CP, Ramachandran B, et al. CD8+ cells from HIV-2-infected baboons control HIV replication. AIDS. 1997;11:737. doi: 10.1097/00002030-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Castro BA, Walker CM, Eichberg JW, Levy JA. Suppression of human immunodeficiency virus replication by CD8+ cells from infected and uninfected chimpanzees. Cell Immunol. 1994;132:246. doi: 10.1016/0008-8749(91)90023-5. [DOI] [PubMed] [Google Scholar]
- 20.Ennen J, Findeklee H, Dittmar MT, Norley S, Ernst M, Kurth R. CD8+ T lymphocytes of African green monkeys secrete an immunodeficiency virus-suppressing lymphokine. Proc Natl Acad Sci USA. 1994;91:7207. doi: 10.1073/pnas.91.15.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannagi M, Chalifoux LV, Lord CI, Letvin NL. Suppression of simian immunodeficency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988;140:2237. [PubMed] [Google Scholar]
- 22.Willett BJ, Flynn JN, Hosie MJ. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 23.Hosie MJ, Osborne R, Yamamoto JK, Neil JC, Jarrett O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J Virol. 1995;69:1253. doi: 10.1128/jvi.69.2.1253-1255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto JK, Hohdatsu T, Olmsted RA, et al. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto JK, Okuda T, Ackley CD, et al. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991;7:911. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- 26.Beatty JA, Willett BJ, Gault EA, Jarrett O. A longitudinal study of feline immunodeficiency virus-specific cytotoxic T lymphocytes in experimentally infected cats, using antigen-specific induction. J Virol. 1996;70:6199. doi: 10.1128/jvi.70.9.6199-6206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song W, Collison EW, Billingsley PM, Brown WC. Induction of feline immunodeficiency virus-specific cytolytic T-cell responses from experimentally infected cats. J Virol. 1992;66:5409. doi: 10.1128/jvi.66.9.5409-5417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn JN, Beatty JA, Cannon CA, et al. Involvement of gag- and env-specific cytotoxic T lymphocytes in protective immunity to feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1995;11:1107. doi: 10.1089/aid.1995.11.1107. [DOI] [PubMed] [Google Scholar]
- 29.Flynn JN, Keating P, Hosie MJ, et al. Env-specific cytotoxic T lymphocytes predominate in cats protected from feline immunodeficiency virus infection by vaccination. J Immunol. 1996;157:3658. [PubMed] [Google Scholar]
- 30.Hosie MJ, Flynn JN. Feline immunodeficeincy virus vaccination: characterization of the immune correlates of immunity. J Virol. 1996;70:7561. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeng CR, English RV, Childers Y, Tompkins MB, Tompkins WAF. Evidence for CD8+ antiviral activity in cats infected with feline immunodeficiency virus. J Virol. 1996;70:2474. doi: 10.1128/jvi.70.4.2474-2480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto JK, Akley CD, Zochlinski H, et al. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 33.Miyazawa TM, Furuya T, Itagaki S, Tohya Y, Takahashi E, Mikami T. Establishment of a feline T-lymphoblastoid cell line highly sensitive for replication of feline immunodeficiency virus. Arch Virol. 1989;198:131. doi: 10.1007/BF01313750. [DOI] [PubMed] [Google Scholar]
- 34.Willett BJ, Hosie MJ, Callanan JJ, Neil JC, Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993;78:1. [PMC free article] [PubMed] [Google Scholar]
- 35.Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;262:1011. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 36.Landay AL, Mackewicz CE, Levy JA. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 37.Rosok B, Voltersvik P, Larsson B-M, Albert J, Brinchmann JE, Asjo B. CD8+ T cells from HIV type 1-seronegative individuals suppress virus replication in acutely infected cells. AIDS Hum Res Retrovirus. 1997;13:79. doi: 10.1089/aid.1997.13.79. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann-Lehmann R, Holznagel E, Aubert A, Ossent P, Reinacher M, Lutz H. Recombinant FeLV vaccine: long-term protection and effect on course and outcome of FIV infection. Vet Immunol Immunopathol. 1995;46:127. doi: 10.1016/0165-2427(94)07012-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehmann R, Beust B, Niederer E, et al. Immunization-induced decrease of the CD4+: CD8+ ratio in cats experimentally infected with feline immunodeficency virus. Vet Immunol Immunopathol. 1992;35:199. doi: 10.1016/0165-2427(92)90132-a. [DOI] [PubMed] [Google Scholar]
- 40.Shimojima M, Miyazawa T, Kohmoto M, et al. Expansion of CD8α+β− cells in cats infected with feline immunodeficiency virus. J Gen Virol. 1998;79:91. doi: 10.1099/0022-1317-79-1-91. [DOI] [PubMed] [Google Scholar]
- 41.Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nature Medicine. 1996;2:412. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]