Abstract
Eighteen monoclonal Bence–Jones proteins (BJPs) were examined for their effects on cultured LLC‐PK1 (porcine kidney proximal tubule) cells as well as for their amidase and DNase activities. Five proteins were found to enter the cell and to gain access to the nucleus without degradation of epitopes. Intranuclear BJPs ultimately induced DNA fragmentation and cell death. BJPs with relatively high amidase activity were cytotoxic. On the other hand, three of four BJPs with DNase activity had a cytocidal effect on cultured cells; the remaining BJP, which had a relatively high DNase activity but a very low amidase activity, failed to enter the cell and was not cytotoxic in vitro. These results suggest that catalytic and cytotoxic activities of some BJPs may make a significant contribution, in a substantial proportion of myeloma patients, to the development and/or deterioration of the disease.
Introduction
There is increasing evidence that some autoantibodies can hydrolyse their own antigens.1,2 For example, human vasoactive intestinal peptide (VIP),3 thyroglobulin4 and DNA5 were shown to be cleaved by some of their respective autoantibodies. Catalytic autoantibodies themselves were much less active than their light chains,1–3 and most Bence–Jones proteins (BJPs) were capable of detectable cleavage of one or more chromogenic amidase substrates.3,6–8 Although the amidolytic activities of BJPs were generally very weak, several lines of evidence indicate that this was not the result of proteinase contamination.1–9 The catalytic activities reside in the region of immunoglobulins that includes the complementarity‐determining regions (CDRs), and thus differ greatly among individual immunoglobulins.1,2,9 These results suggest that catalytic antibodies may be a relatively standard component of natural immune responses1 and that the catalytic activity of natural immunoglobulins or BJPs, albeit very weak, may affect clinical processes of patients with autoimmune diseases (who produce immunoglobulins with a high catalytic potential) or with multiple myeloma (who excrete massive levels of BJP).8 In a previous study, we showed that four out of 18 BJPs examined had DNA‐nicking activity and that patients excreting DNase‐active BJPs showed somewhat severe clinical symptoms.10 Recently, it was shown that when added to cultured LLC‐PK1 (porcine kidney proximal tubule) cells, some anti‐DNA antibodies traverse the cytoplasm and enter the nucleus in a time‐ and temperature‐dependent manner11 and that when injected into normal mice, some anti‐DNA monoclonal antibodies (mAbs) produced intracellular immunoglobulin deposits in multiple organs.12 It remains to be determined, however, whether BJPs behave in a manner similar to these intact autoantibodies. The present study was undertaken to clarify this issue. It is shown that some, but not all, BJPs actually enter the LLC‐PK1 cell and reach the nucleus in a manner similar to anti‐DNA antibodies, and that the intranuclear BJPs ultimately induce DNA fragmentation and cell death.
Materials and methods
Materials
Monoclonal BJPs were purified to homogeneity from the urine of patients with multiple myeloma, as described previously.6 Virtually all preparations had detectable amidase activity6 whereas only four of 18 monoclonal BJPs examined had DNA‐nicking activity.10
Determination of enzymatic activities
The amidase6 and DNA‐nicking10 activities of BJPs were determined as described previously.
Cell growth
LLC‐PK1 cells (CRL 1392; American Type Culture Collection, Rockville, MD) were obtained from Dainippon Pharmaceutical Co. (Osaka, Japan). LLC‐PK1 cells were cultured in Dulbecco's modified Eagles's minimal essential medium (DMEM) supplemented with 5% fetal calf serum (FCS; Gibco, Rockville, MD) and subcultured every 5–7 days.
Determination of cell viability after incubation with BJP
Cell viability was determined according to the method of Mosmann13 with some modifications. LLC‐PK1 cells were distributed in 96‐well plates, at a density of 5 × 104 cells/0·1 ml/well, and incubated at 37° for 24 hr. The medium was then replaced with FCS‐free DMEM containing BJPs at final concentrations of 0, 0·25, 0·50, 0·75, 1·0 and 1·5 μm, and incubation was continued for 24 hr. To each well, 20 μl of 0·5% 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT; Sigma, Indianapolis, IN), in phosphate‐buffered saline (PBS), was added and incubation was continued for a further 4 hr at 37°. Under these conditions, only living cells reduce significant amounts of MTT to form insoluble formazan. After incubation, 0·04 N HCl in isopropanol and 3% sodium dodecyl sulphate (SDS) solution (0·1 ml of each) were added to each well to ensure that dark blue MTT formazan was dissolved. After incubation for a few minutes at room temperature, the absorbance was read at 590 nm using a microplate reader. The cell survival rate was expressed as a percentage of the control that contained no BJP.
Dual staining of cells
LLC‐PK1 cells were incubated with BJPs, essentially as described above, and then double stained with Hoechst 33342 (Calbiochem, La Jolla, CA) and propidium iodide (PI; Calbiochem), as previously described by Singhal et al.14 Under these conditions, PI is taken up only by damaged cells, while Hoechst 33342 stains both living and dead cells.14
Immunofluorescence staining of intracellular BJPs
LLC‐PK1 cells were placed onto cover glass and allowed to proliferate overnight. They were then washed with FCS‐free DMEM, and incubated for 10 hr with DMEM in the presence of different concentrations of BJP, essentially as described above. After incubation, the slides were washed three times with PBS at 4°. The cells were fixed in acetone at – 20° for 15 min, washed once with 0·1% Triton‐X‐100 in PBS, three times with PBS alone and then blocked by incubation with 3% bovine serum albumin (BSA) in PBS for 20 min. After washing with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)‐labelled anti‐human κ‐ or λ‐chain goat immunoglobulin G (IgG) (Boehringer Mannheim Biochemicals, Indianapolis, IN), for 2 hr at 4°, and then washed three times with PBS. The slides were mounted and examined using an Olympus fluorescence microscope (Olympus Co., Tokyo, Japan).
Detection of DNA fragmentation in situ
Fragmented DNA was detected in situ by a modification15 of the terminal deoxynucleotidyltransferase‐mediated dUTP‐biotin nick‐end labelling (TUNEL) method16 using the MEBSTAIN apoptosis kit (Medical & Biological Laboratories Co., Nagoya, Japan). Biotinylated dUTP was omitted in control staining.
Extraction of DNA from cells and electrophoretic detection of the DNA ladder
DNA extraction and detection of the DNA ladder were carried out essentially as described by Sellins & Cohen.17
Radiolabelling of monoclonal BJPs and binding assay
BJP was labelled with 125I by the chemical oxidation method,18 using the IODO‐BEADS iodination kit (Pierce, Rockford, IL) according to the manufacturer's instructions. LLC‐PK1 cells were placed onto cover glass and allowed to proliferate until confluent. They were then washed three times with FCS‐free DMEM, and incubated at 4° for 2 hr with DMEM containing 125I‐labelled BJP (1 × 104 counts per minute [c.p.m.]). BSA and non‐labelled BJP at 10‐, 100‐, and 1000‐fold concentrations were used as non‐specific binding controls. After washing with FCS‐free DMEM, cells were lysed with 1 N NaOH, and radioactivity was measured in a scintillation counter.
Results
Cytotoxic effect of BJPs
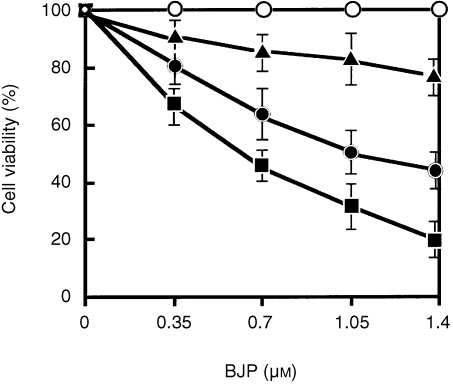
While studying the effect of BJPs on LLC‐PK1 cells, we observed that BJPs sometimes induced cell death. We therefore studied the effects of individual BJPs on cell viability. Of 18 BJPs examined, five induced gradual cell death in a concentration‐dependent manner (Fig. 1). The remaining 13 BJPs had virtually no effect on cell viability.
Figure 1.
Effects of Bence–Jones proteins (BJPs) on cell viability. LLC‐PK1 cells were preincubated with different concentrations of BJP, and the cell viability was determined as described in the text. Amidase and DNase activities of individual BJPs are shown in Fig. 7, and the same symbols are used for the same BJPs in both figures:▪, patient c in Fig. 7; •, patients d–f in Fig. 7; ▴, patient a in Fig. 7; ○, patient b and all other BJPs that are not indicated by alphabetical characters in Fig. 7. Vertical bars indicate standard deviations of the mean values.
Cytochemical detection of cell death
After incubation of LLC‐PK1 cells with BJPs followed by double staining with Hoechst 33342 and PI, very few dead cells were observed in cultures incubated with non‐cytotoxic BJPs (Fig. 2a). In marked contrast, many dead cells were seen in cultures incubated with cytotoxic BJPs (Fig. 2b). The results are in good agreement with those obtained using Mosmann's tetrazolium method (Fig. 1), namely, the greater the cytotoxicity of BJP, the higher the proportion of cells stained with PI.
Figure 2.
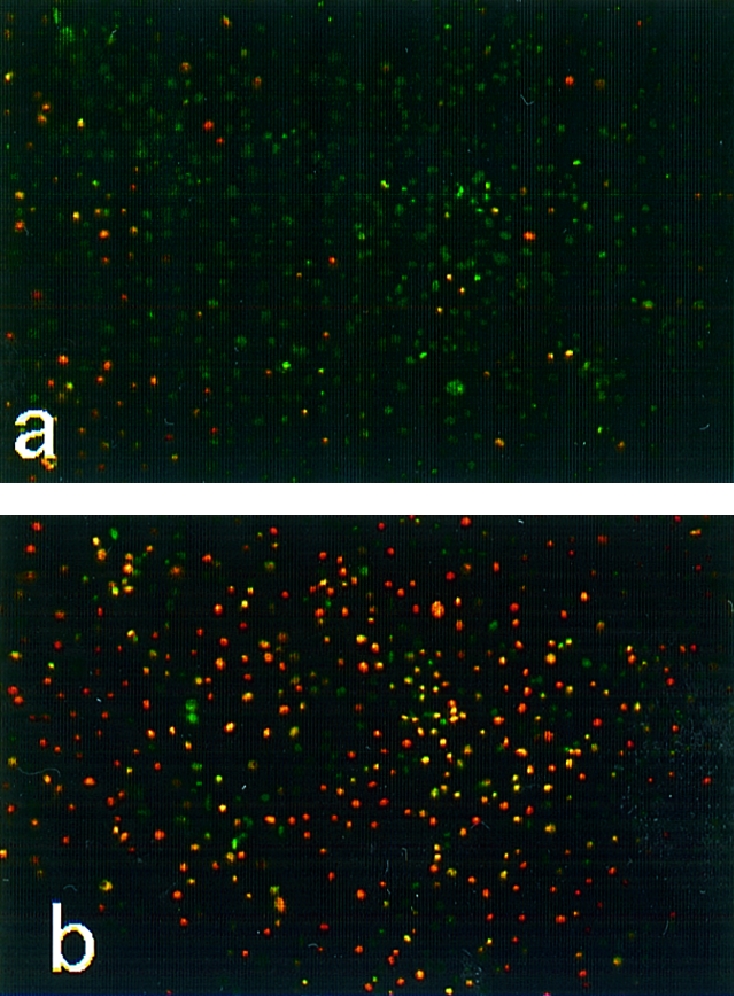
Cytocidal effect of Bence–Jones proteins (BJPs). After incubation of LLC‐PK1 cells with 1·0 μm of non‐cytotoxic (a) or cytotoxic (b) BJP for 1 hr as described in the text, the cells were double stained with Hoechst 33342 (greenish blue) and propidium iodide (reddish yellow), essentially as described previously.14
Incorporation of BJPs into LLC‐PK1 cells
When immunofluorescence staining for BJPs was performed on LLC‐PK1 cells treated with BJPs, there appeared to be some cytoplasmic staining as well as the brighter nuclear staining (Fig. 3b). By contrast, all the non‐cytotoxic BJPs were negative for this staining (Fig. 3a). To further substantiate this result, the cells treated with cytotoxic BJP were lysed and the lysate was subjected to electrophoresis followed by immunoblotting with anti‐κ chain antiserum, as described previously.19 An immunoreactive band with molecular mass corresponding to that of the original BJP was detected (data not shown). By contrast, no positive band was detected in the lysate that had been treated with non‐cytotoxic BJP. These results indicate that a subgroup of BJPs was taken up by LLC‐PK1 cells with little or no degradation of epitopes, whereas the majority of BJPs were degraded without access to the nuclei.
Figure 3.
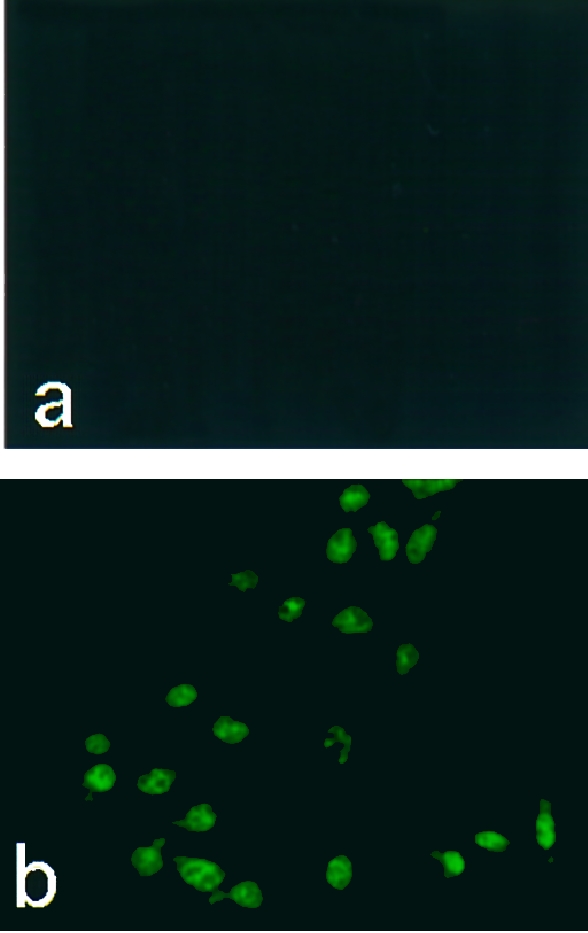
Immunofluorescence staining of intracellular Bence–Jones proteins (BJPs). After incubation of LLC‐PK1 cells with 1·0 μm of non‐cytotoxic (a) or cytotoxic (b) BJP, the cells were stained with fluorescein isothiocyanate (FITC)‐labelled anti‐human κ‐chain goat immunoglobulin G (IgG), essentially as described.19
Binding of BJPs to the surface of LLC‐PK1 cells
The above results indicate that there are two subgroups of BJP: one reaches the nucleus while the other does not. In order to clarify whether or not the specific surface receptor is involved with this intracellular transport, competitive binding experiments were carried out between the two subgroups. As shown in Fig. 4(a), binding of cytotoxic BJP (▪ in Fig. 1) was almost completely inhibited by 1000‐ and 100‐fold (data not shown) concentrations of non‐cytotoxic BJP (○ in Fig. 1), cytotoxic non‐labelled BJP or BSA, suggesting that there is no specific receptor for cytotoxic BJP. Figure 4(b) shows that essentially the same results were obtained at 10‐fold concentrations of these competitors, although a higher level of scatter was observed with this data.
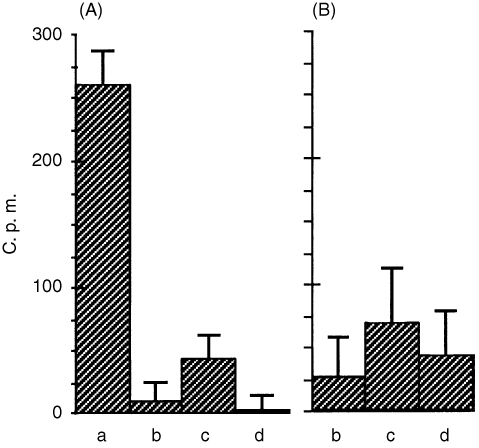
Figure 4.
Binding of radiolabelled cytotoxic BJP to LLC‐PK1 cells in the absence (a) and presence of 1000‐fold (A) and 10‐fold (B) concentrations of non‐cytotoxic BJP (b), BSA (c) and cytotoxic non‐labelled BJP (d). c.p.m., counts per minute.
Cytochemical detection of biotin‐labelled DNA fragmentation
It is known that TUNEL‐positive staining is indicative of DNA fragmentation, which is found not only in histologically defined apoptotic cells but also in morphologically intact cells that are destined to go through cell death.16 As shown in Fig. 5(a), TUNEL staining was negative for cells treated with non‐cytotoxic BJPs. However, TUNEL‐positive staining was detected as green fluorescence on the nuclei of LLC‐PK1 cells that had been treated with cytotoxic BJPs (Fig. 5b). When biotinylated dUTP was omitted from the reaction process, no green fluorescence was detected (data not shown).
Figure 5.
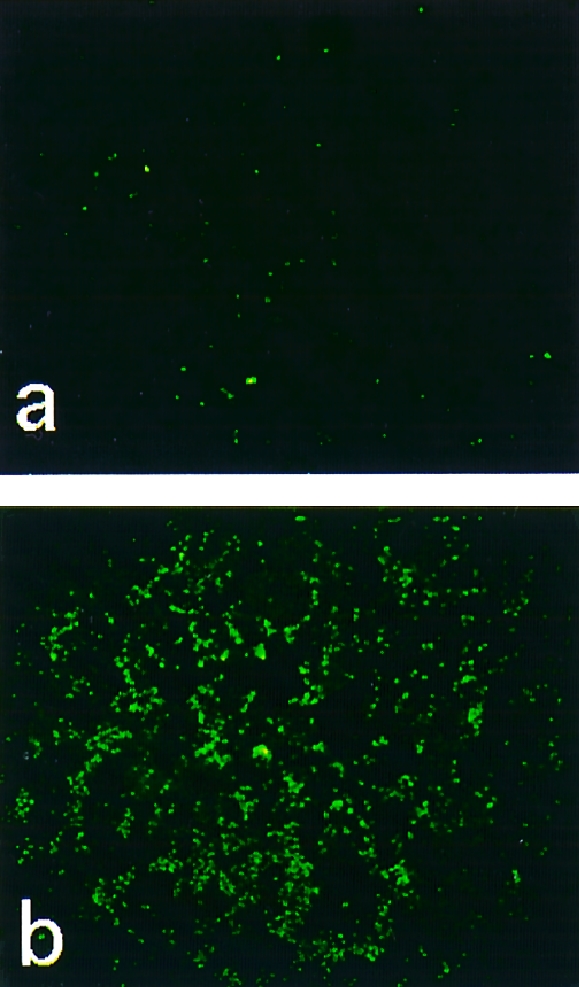
Cytochemical detection of DNA fragmentation. After incubation of LLC‐PK1 cells with 1·0 μm of non‐cytotoxic (a) or cytotoxic (b) Bence–Jones proteins (BJPs), the cells were subjected to terminal deoxynucleotidyltransferase‐mediated dUTP‐biotin nick‐end labelling (TUNEL) staining, as described previously.15
DNA ladder observed by electrophoresis
To further substantiate the above cytochemical results, nuclear DNA was cleaved into oligonucleosome fragments and analysed further, as described below. After treatment of LLC‐PK1 cells with cytotoxic BJP, cells were lysed and the DNA fragments present in the lysate were examined as described in the Materials and methods. Figure 6 shows the presence of fragmented DNA as a ladder‐like prominent smear (lanes 2–4). These DNA ladders were not detected in LLC‐PK1 cells treated with BSA or non‐cytotoxic BJP (lanes 1 and 5).
Figure 6.
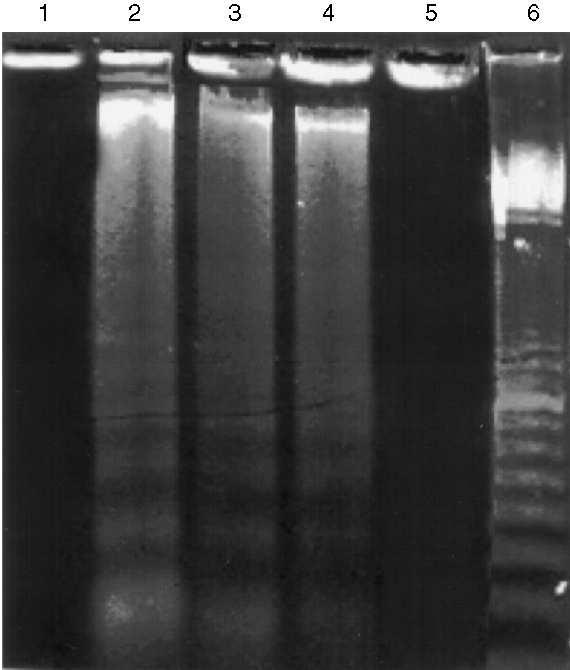
DNA fragmentation in LL‐CPK1 cells after incubation with Bence–Jones proteins (BJPs). LL‐CPK1 cells were incubated with BJPs and the DNA fragments present in the lysate were detected by the method of Hockenbery et al.17 Lane 1, 2 μm of bovine serum albumin (BSA); lanes 2–4, 2, 1 and 0·25 μm of cytotoxic BJP, respectively; lane 5, 2 μm of non‐cytotoxic BJP; lane 6, 100‐bp DNA ladder marker (Amersham Pharmacia Biotech, Tokyo, Japan).
Relationship between cytotoxicity and catalytic activities
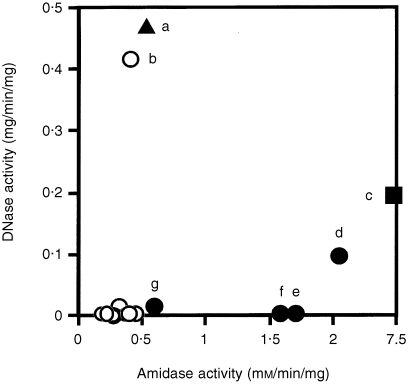
It was shown previously that the majority of BJPs had weak amidase activity1,3,6 while ≈ 20% of BJPs examined had DNA‐hydrolysing activity.10 We therefore studied whether or not the nuclear localization of BJP is related to either enzymic activity. Figure 7 shows that BJPs with relatively high amidase activity (abscissa) were cytocidal (c–f). The most cytotoxic BJP (Fig. 1, ▪) had the highest amidase activity (Fig. 7, ▪), and was secreted by a 63‐year‐old male (patient c) who died of renal failure 4 years after the first diagnosis (average daily BJP excretion, 0·8 g). On the other hand, three patients (e–g) who secreted BJPs with relatively high amidase but no DNase activities exhibited mild symptoms, although their BJPs were moderately cytocidal in vitro (Fig. 1, •). Three out of four BJPs with DNA‐nicking activity (ordinate) were cytotoxic (a, c and d). However, one BJP with a relatively high DNase activity (b) was neither taken up by the cells nor cytotoxic. These results suggest that the amidase activity is more related to cytotoxicity than the DNase activity. It is possible, however, that BJPs have a number of other, as yet unknown, activities and more data are required to confirm this.
Figure 7.
Relationship between amidase, DNase and in vitro cytotoxicity of Bence–Jones proteins (BJPs). Cell viability was determined as described in the text: ≈ 25% (▪), 50% (•), 75% (▴) and 100% (○) viable after 1 hr of incubation with 1 μm of BJP. The symbols correspond to those given in Fig. 1.
Discussion
Among the various symptoms associated with multiple myeloma, clinically significant renal impairment has been reported to occur in 40–60% of patients and to be the second most common cause of death after infection.20 Although the aetiology of renal failure is multifactorial, we highlighted the possibility that the catalytic activity of BJPs may contribute, to some extent, to the clinical process.8 The present results show that a subset of BJPs entered LLC‐PK1 cells, gained access to the nucleus and induced DNA fragmentation. These results lend new support to the above hypothesis. However, the catalytic potential of individual BJPs differs greatly, not only in the degree of enzymic activities but also in the substrate specificity3,8,10 and thus the extent that an individual BJP contributes to the aetiology may be very diverse, ranging from practically null to significant levels. If a BJP is taken up by the cell and has contact with protein(s) or nucleic acid(s) that are necessary for the maintenance of cell function, slow cleavage of a single peptide or nucleotide bond may be sufficient to lead to the gradual loss of function, resulting in cell death.
Madaio and co‐workers11,12,21 showed that some lupus anti‐DNA autoantibodies and their Fab′ fragments entered the cell and gained access to the nucleus in a time‐dependent manner. This suggests that the antigen‐binding region is mainly responsible for both cell entry and nuclear localization. The present results obtained with BJPs are in general agreement with these previous findings obtained for intact anti‐DNA autoantibodies. In the past few years, enormous progress has been made in understanding nuclear protein import.22,23 Nuclear proteins synthesized on free ribosomes in the cytoplasm are translocated efficiently and precisely to the nucleus through the nuclear pore complex present in the nuclear envelope. The pore complex can accommodate the active transport of large molecules. This process is conferred by several nuclear localization signals. Among them, two signals were relatively well characterized: a single basic type consisting of a short stretch of highly basic amino acid residues, such as PKKKRKV; and a bipartite basic type consisting of two stretches of basic amino acid residues separated by a spacer of random amino acid residues, such as KRPAAIKKAGQAKKKK. It is possible that some BJPs have a similar sequence in their CDRs. Foster et al.24 showed that the nuclear localization‐like motifs were present in CDR3 of nuclear localizing anti‐DNA lupus antibodies.
Recently, Yanase et al.21 reported that cellular entry of nuclear localizing anti‐DNA antibodies is mediated by cell‐surface binding to brush border myosin (myosin 1). The initial binding to this receptor provides the subsequent sorting to enter living cells. Imported autoantibodies interact with DNase 1, which was thought to be primarily responsible for apoptosis, and result in the attenuation of apoptosis. These results obtained with the intact anti‐DNA antibodies and their Fab′ fragments were not in agreement, in several areas, with the present study for BJPs. For example, cytotoxic BJPs bound to non‐specific cell‐surface receptors (Fig. 4) and stimulated cell death rather than attenuation of apoptosis (Fig. 1). Furthermore, recent findings suggest that DNases other than DNase 1 are responsible for apoptosis, such as Ca2+/Mg2+‐dependent endonuclease,25 DNase γ26 and caspase‐activated DNase.27 Further elucidation of these conflicting results between anti‐DNA antibodies and BJPs will lead to a better understanding of the pathogenesis of multiple myeloma, as well as autoimmune diseases.
Glossary
Abbreviations
- BJP
Bence–Jones protein
- CDR
complementarity‐determining region
- DMEM
Dulbecco's modified Eagle's minimal essential medium
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- TUNEL
terminal deoxynucleotidyltransferase‐mediated dUTP‐biotin nick‐end labelling
- VIP
vasoactive intestinal peptide
References
- 1.Paul S. Natural catalytic antibodies. Mol Biotechnol. 1996;5:197. doi: 10.1007/BF02900358. [DOI] [PubMed] [Google Scholar]
- 2.Paul S. Antibody catalysis. In: Paul P, editor. Contemporary Immunology: Autoimmune Diseases. Totowa, NJ: Humana Press; 1998. p. 221. [Google Scholar]
- 3.Paul S, Li L, Kalaga RA, et al. Natural catalytic antibodies: peptide‐hydrolyzing activities of Bence Jones proteins and VL fragment. J Biol Chem. 1995;270:15257. doi: 10.1074/jbc.270.25.15257. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Paul S, Tyutyulkova S, et al. Catalytic activity of anti‐thyroglobulin antibodies. J Immunol. 1995;154:3328. [PubMed] [Google Scholar]
- 5.Shuster AM, Gololobov GV, Kvashuk OA, et al. DNA hydrolyzing autoantibodies. Science. 1992;256:665. doi: 10.1126/science.1585181. [DOI] [PubMed] [Google Scholar]
- 6.Matsuura K, Yamamoto K, Sinohara H. Amidase activity of human Bence Jones proteins. Biochem Biophys Res Commun. 1994;204:57. doi: 10.1006/bbrc.1994.2425. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura K, Sinohara H. Catalytic cleavage of vasopressin by human Bence Jones proteins at the arginylglycinamide bond. Biol Chem. 1996;377:587. doi: 10.1515/bchm3.1996.377.9.587. [DOI] [PubMed] [Google Scholar]
- 8.Sinohara H, Matsuura K. Is catalytic activity of Bence Jones proteins an autoimmune effector mechanism in multiple myeloma? In: Paul P, editor. Contemporary Immunology: Autoimmune Diseases. Totowa, NJ: Humana Press; 1998. p. 235. [Google Scholar]
- 9.Kohler H, Paul S. Superantibody activities: new players in innate and adaptive immune responses. Immunol Today. 1998;19:221. doi: 10.1016/s0167-5699(97)01234-6. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura K, Ikoma S, Yoshida K, Sinohara H. DNA‐hydrolyzing activity of Bence Jones proteins. Biochem Biophys Res Commun. 1998;243:719. doi: 10.1006/bbrc.1998.8164. [DOI] [PubMed] [Google Scholar]
- 11.Yanase K, Smith RM, Cizman B, et al. A subgroup of murine monoclonal anti‐deoxyribonucleic acid antibodies traverse the cytoplasm and enter the nucleus in a time‐ and temperature‐dependent manner. Lab Invest. 1994;71:52. [PubMed] [Google Scholar]
- 12.Vlahakos D, Foster MH, Ucci AA, et al. Murine monoclonal anti‐DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation in vivo. J Am Soc Nephrol. 1992;2:1345. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Signhal PC, Sharma P, Sanwal V, et al. Morphine modulates proliferation of kidney fibroblasts. Kidney Int. 1998;53:350. doi: 10.1046/j.1523-1755.1998.00758.x. [DOI] [PubMed] [Google Scholar]
- 15.Mori C, Nakamura N, Okamoto Y, et al. Cytochemical identification of programmed cell death in the fusing fetal mouse palate by specific labelling of DNA fragmentation. Anat Embryol. 1994;190:21. doi: 10.1007/BF00185843. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben‐sasson A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;19:493. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellins KS, Cohen JJ. Gene induction by γ‐irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987;139:3199. [PubMed] [Google Scholar]
- 18.Cheng H, Rukick MJ. A membrane blotting method for following the time course of protein radioiodination background references using IODO‐BEADS. Anal Biochem. 1991;198:191. doi: 10.1016/0003-2697(91)90527-z. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Suzuki Y, Sinohara H. Synthesis of contrapsin and α‐1‐antiproteinase in inflamed and tumor‐bearing mice. Biochem Int. 1988;16:921. [PubMed] [Google Scholar]
- 20.Selby P, Gore M. Myeloma and other plasma cell malignancies. In: Peckham M, Pinego H, Veronesi U, editors. Oxford Textbook of Oncology. Vol. 1. Oxford: Oxford University Press; 1994. p. 852. [Google Scholar]
- 21.Yanase K, Smith RM, Puccetti A, et al. Receptor‐mediated cellular entry of nuclear localizing anti‐DNA antibodies via myosin 1. J Clin Invest. 1997;100:25. doi: 10.1172/JCI119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoneda Y. How proteins are transported from cytoplasm to the nucleus. J Biochem. 1997;121:811. doi: 10.1093/oxfordjournals.jbchem.a021657. [DOI] [PubMed] [Google Scholar]
- 23.Gorlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 24.Foster MH, Kieber‐emmons T, Ohliger M, Madaio MP. Molecular and structural analysis of nuclear localizing anti‐DNA lupus antibodies. Immunol Res. 1994;13:172. doi: 10.1007/BF02918279. [DOI] [PubMed] [Google Scholar]
- 25.Pandey S, Walker OR, Sikorska M. Identification of a novel 97 kDa endonuclease capable of internucleosomal DNA cleavage. Biochemistry. 1997;28:711. doi: 10.1021/bi962387h. [DOI] [PubMed] [Google Scholar]
- 26.Shiokawa D, Ohyama H, Yamada T, Tanuma S. Purification and properties of DNases from apoptotic rat thymocytes. Biochem J. 1997;326:675. doi: 10.1042/bj3260675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enari M, Sakahira H, Yokoyama H, et al. A caspase‐activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]