Abstract
Changes in the VH-region repertoire of isolator piglets reared for 6 weeks under germ-free (GF) conditions and those colonized (COL) with a defined exclusion flora on the 1st day of life were compared. Although serum immunoglobulin levels were 20–100-fold higher in COL piglets than GF piglets, an analysis of peripheral blood B cells (PBBs) indicated that: GF and COL piglets used the same four VH genes and two DH segments during the 6-week period; proportional usage of VH genes and DH segments was the same as in fetal animals; and VH and DH usage did not differ between COL and GF animals. This pattern differed from the PBBs from 6-week-old conventional (CONV) piglets. When the sequences of 73 splenic CDR3 segments were analysed, DH usage and mutation frequency were the same in sequences from both 6-week-old GF and COL piglets; mutations were infrequent and occurred with the same frequency as in 110-day fetal spleen. However, the median CDR3 length in COL piglets was shifted upward due to 3′ DH N-nucleotide additions. Neither COL nor GF animals made specific serum antibodies to phosphoryl choline given parenterally on a T-cell dependent carrier. In contrast to the near absence of a colonization effect in PBBs and splenic DNA, rearranged variable heavy-chain gene segments (VDJs) recovered from the DNA of mucosal lymphoid tissues of COL piglets showed pronounced differences from those recovered from GF animals in usage of DHA-, DHB-and VHB- and in the frequency of point mutation. The mucosal VDJ transcripts and those from the spleen were similarly affected by colonization. This effect on mucosal lymphoid tissue was consistent with the five-fold selective increase in serum immunoglobulin A (IgA) levels relative to IgM and IgG. Comparison of IgM and IgA transcripts from mucosal tissues suggested that IgA and IgM clones diversify in parallel. Our findings are the first to show that colonization of the gastrointestinal tract of offspring separated from their mothers, differs from ‘conventionalized’ GF animals in that colonization preferentially influences diversification and expansion of the preimmune IgM and IgA repertoire in mucosal lymphoid tissues but not in PBBs and seldom/modestly in VDJs from splenic DNA.
Introduction
The mammalian gastrointestinal tract becomes colonized during the first few days of life with ≈ 500 species of mostly anaerobic, non-pathogenic, indigenous bacteria, often referred to as normal gut flora.1,2 In pigs, concentrations up to 109 bacteria/g and 1011 bacteria/g occur in the small intestine and colon/caecum, respectively.1,3 This normal gut flora is believed to play an important role in the health of the host by competitively inhibiting colonization by pathogenic forms by ‘microbial interference or exclusion’.4,5
Studies comparing germ-free (GF) and conventional (CONV) rodents suggest that normal gut flora also stimulates or plays a regulatory role, in the development of the immune system.6,7 Immunoglobulin M (IgM) responses to the thymus-dependent type 2 (TI-2) antigens of sheep red blood cells and dinitrophenyl (DNP)–Ficoll do not differ between GF and CONV mice, while IgG responses to DNP–bovine serum albumin (BSA) are impaired in GF mice.8 Consistent with this finding, colonization of GF mice significantly increases the serum concentrations of IgG1, IgG2a and IgA whereas IgM levels are highest in GF mice.9 Koopman et al.10 observed that colonization resulted in an increase in mucosal IgA cells that is consistent with the two-fold increase in CD4 T cells, which are otherwise rare in mucosal regions of GF mice.11 While many early studies suggested that cell-mediated immunity was not impaired in GF animals, this has been controversial.7 MacDonald and Carter12 and Woolverton et al.13 showed that the poor cell-mediated responses of GF mice could be restored by bacterial colonization. It has also been shown that colonization is necessary for the proper induction of oral tolerance which is believed to down-regulate T helper type 2 (Th2) cells in the gastrointestinal tract.14 This is consistent with the observation that colonization elevates local interferon- (IFN-) levels and that IFN-γ knockout mice fail to develop oral tolerance.15
Lymphoid development is poor in the appendix of GF rabbits16 and in the ileal Peyer's patches (IPP) of a non-colonized segment of the lamb gut; the latter also shows retarded germinal centre development.17 Smith et al.18 reported that specific pathogen-free animals transferred to a conventional environment showed a substantial increase in M-cell surface area. Mandel et al.19 reported an increase in the number of jejunal Peyer's patch cells expressing swine leucocyte antigen (SLA) class II and membrane immunoglobulin when GF animals were given Nocardia mitogen intragastrically. Interestingly, this mitogenic stimulation prevented death when such animals were given virulent Escherichia coli, suggesting that the antibody repertoire had been expanded to provide protection. In birds, bacteria and bacterial debris can be found in association with the bursa of Fabricius, perhaps suggesting that bacteria also play a role in avian B-cell development by acting in this primary lymphoid organ.20 Recently, Crane et al.21 observed a rapid diversification of the rabbit antibody repertoire between 4 and 8 weeks after birth, which they regarded as corresponding to the period when normal gut flora exerts a significant effect on the adaptive immune response. However compelling these observations, Reynaud et al.22 observed no difference in the degree of somatic hypermutation in the VL genes of conventional and GF lambs. Thus, with a few exceptions, colonization of the gastrointestinal tract with normal flora appears to stimulate diversification of the preimmune repertoire.
Immunoglobulin gene rearrangement and B-cell development begin in utero in an environment normally free of environmental antigen. Thus fetal and newborn mammals have B cells and even small amounts of de novo synthesized antibodies that are encoded by these early rearrangement and developmental events. This often limited repertoire, which develops in the absence of environmental antigenic stimulation, is called the preimmune repertoire. The preimmune and intestinal antibody repertoire appears to have evolved a special relationship to normal gut flora. As much as 90% of the immunoglobulin-secreting cells of the conventional mouse intestine produce ‘background antibodies’ that are absent in GF mice,23 the earliest pup-derived intestinal IgA antibodies recognize normal gut flora24 and at least half of the anaerobic faecal bacteria in humans are coated with IgA.25 In mice, these ‘natural antibodies’ are believed to be derived from B-1 cells.26 These cells develop only early in ontogeny, are self-replenishing and typically produce multireactive antibodies (often autoreactive) which bind their antigens with low affinity.27
Recently we have shown that fetal and newborn piglets utilize primarily four VH genes and two DH segments to generate their preimmune natural antibody repertoire.28,29 Since swine have only a single JH,30 combinatorial diversity is limited to essentially eight combinations. VDJ rearrangement was first observed on day 30 (114 days' gestation) in fetal piglets29 and all major isotypes of immunoglobulins are transcribed in utero28 and can be detected as secreted proteins.31 Like sheep, fetal piglets are immunocompetent for producing antibodies to a broad spectrum of especially TI antigens31 Fetal piglets, like fetal rabbits, show virtually no evidence of hypermutation in their rearranged VDJs29,32 yet 6-month-old animals have VDJs that are so somatically diversified that germline VH and DH usage can be difficult to recognize.33 Thus, extensive somatic diversification of VDJs is a postnatal event that is either intrinsically programmed or driven by environmental factors. Since VH and DH usage in fetal and newborn piglets can be quantitatively monitored by differential polymerase chain reaction (PCR) product hybridization (DPPH),34 we believed if colonization is important in the maturation of the adaptive immune system, the influence of normal gut flora on somatic diversification of VDJ could be conveniently studied in the isolator piglet model. DPPH monitors VH and DH diversification since either decreased usage or somatic mutation of certain VH and DH segments reduces hybridization of amplified VDJs with VH- and DH-specific probes. Thus, a reduction in hybridization is a measure of diversification (see the Materials and Methods). Since junctional diversity in CDR3 accounts for most heavy-chain diversity in fetal and newborn piglets (J. E. P. Butler et al., submitted for publication) we comparatively analysed CDR3 sequences of GF and colonized (COL) piglets that were Colonization could especially affect development of the mucosal immune system;10,25 thus we also compared the level of IgA transcription in different lymphoid tissues and measured the serum levels of IgM, IgA and IgG in COL and GF animals.
The results reported here indicate that VH and DH usage in peripheral blood B cells (PBBs) and diversification of CDR3 in VDJs of splenic DNA, does not differ in the first 6 weeks between GF piglets and COL piglets that were colonized in an isolator with a benign microbial exclusion flora, but differs from CONV 6-week-old piglets. However, this type of isolator colonization results in repertoire diversification, especially in IgM and IgA transcripts in mucosal tissues. Colonization increases the transcription of IgA and selectively elevates serum IgA levels although neither COL or GF animals were able to respond to a T-dependent antigen given parenterally.
Materials and methods
Source and rearing of piglets
All studies on isolator piglets were performed in the isolator piglet facility of the Veterinary Science Department of South Dakota State University in Brookings. Six piglets were recovered by closed hysterectomy, maintained for 6 weeks in rigid tube isolators35 and reared on sterile SPF-Lac. [SPF-Lac formula is skimmed milk, water, vegetable oils, casein, dextrose, glycerl mono-stearate, tricalcium phosphate, sodium bicarbonate, carageenan, choline chloride, iron sulphate, vitamin A supplement, vitamin E supplement, zine sulphate, niacin supplement, calcium pantothenate, vitamin B12 supplement, thiamine hydrochloride menadione sodim disulphate complex (source of vitamin K), pyridoxine hydrochloride, potassium iodide, biotin. Guaranteed analysis: crude protein, 4%; crude fat, 5%; crude fibre, 0%; moisture, 85% maximum; ash, 1·0%; SPF-Lac is manufactured by Pet-Ag Inc., Hampshire, IL.] Animals were monitored twice daily for evidence of clinical disease and rectal swabs were tested bi-weekly for the presence of bacterial contaminants. Two piglets were reared GF (4-1 and 4-2) and two were inoculated with 3 ml of 109 organisms of defined exclusion flora immediately after birth (3-1 and 3-2). The defined flora was provided by Dr David Nesbitt (Food and Feed Safety Research Unit, USDA-ARS, College Station, TX) and had been specifically designed as a competitive exclusion culture for pigs. The culture included Enterococcus faecalis, Streptococcus bovis type 1, Clostridium clostridioforme, Clostridium symbiosum, Clostridium ramosum, Bacteroides frigilis, Bacteroides disasonis, Bacteroides vulgarus, Bacteroides thetaiotamicron and Bacteroides caccae. The second piglet in each treatment group (3-2 and 4-2) was immunized intramuscularly with 5 mg of phosphoryl choline–ovalbumin (PC-OVA) on week 2 and boosted on week 4. As controls, blood was collected from four 6-week-old conventionally reared piglets of the same breed at the Department of Animal Science, Iowa State University. While it is apparent that very few animals were used, each animal was analysed in detail at the molecular level and, as will be shown, differences between COL and GF animals were so large as to not be explainable by animal variation (see Figs 1, 5, 7).
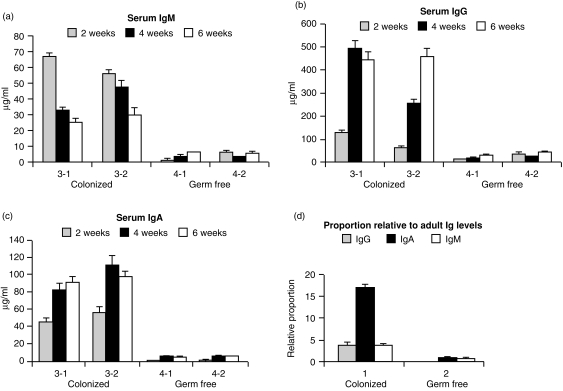
Figure 1.
Serum concentration of IgM, IgG and IgA in colonized (COL) and germ-free (GF) piglets (a–c). Note differences in the scale of the y-axis. All differences between COL and GF piglets are highly significant. The error bars represent the standard deviation of at least nine values in the titration range of the standard curve in the assays used as described for ELISANALYSIS.48,49 (d) Relative proportion of serum immunoglobulins in COL and GF animals compared to adult animals.
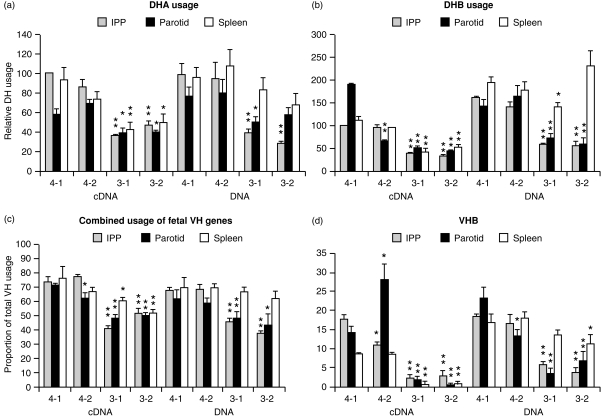
Figure 5.
DPPH values for DH and VH usage in DNA and transcripts from three different lymphoid tissues of 6-week-old GF and COL piglets. (a, b) DHA and DHB, respectively; (c) combined fetal VH gene usage; (d) VHB usage. Animals 4-1 and 4-2 remained GF while 3-1 and 3-2 were COL piglets. In the case of DH values, all data were normalized to values obtained for the IPP of animals 4-1 and this was assigned a value of 100. Mean values for cDNA and DNA were compared by t-test to those for animal 4-1; **Significant at 0·01 level; *significant at 0·05 level. In the case of DPPH values for VH genes, proportional usage is actual and mean values were compared to those for GF animal 4-1.
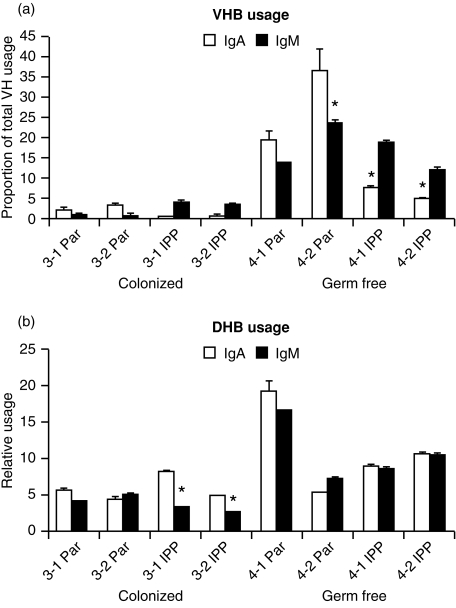
Figure 7.
DPPH analysis of VHB and DHB usage by IgM and IgA transcripts from the parotid gland (Par) and ileal Peyers patches (IPP) of COL and GF isolator piglets. Animals 4-1 and 4-2 remained GF, while 3-1 and 3-2 were COL piglets.
Blood and tissue collection
Blood was collected in 5% ethylenediaminetetraacetic acid (EDTA) on weeks 2, 4 and 6 for the recovery of peripheral blood leucocyte and serum. Animals were killed on week 6 and the following tissues were recovered and stored at − 70°; spleen, liver, parotid gland, IPP, mesenteric lymph nodes, thymus and brain (control tissue).
Preparation of DNA, total RNA and cDNA
RNA and DNA were prepared using TRIzol and DNAzol reagents, respectively, according to the manufacturer's instructions (BRL, Gaithersberg, MD). Studies on relative transcription (see below) utilized first-strand cDNA that was synthesized as previously described33 by simultaneously using an antisense Cµ3 primer (5′-CTG CAC GAA CAC GTC CGC-3′) an antisense 5′-CH3 Cα primer and an antisense β-actin primer (5′-ACA GCG AGG CCA GGA TGG AG-3′). In the case of VDJ transcripts that were studied without respect to isotype, first strain cDNA was routinely prepared using a random hexamer primer.
Relative transcription of IgM and IgA
First-strand cDNA was amplified using primer sets for a 106-base pair (bp) or a 94-bp Cα fragment (the C fragment for the IgAa allotype is 106 and for the IgAb allotype is 94) and a 135-bp β-actin fragment or primers for a 115-bp Cµ fragment and primers for the 135-bp β-actin fragment. For each PCR reaction, the antisense primer was end-labelled with32P, so the radioactive signals generated by the PCR products after their electrophoretic separation, provide a comparative measure of the level of Cα transcription relative to β-actin transcription or Cµ transcription relative to β-actin transcription.36 The simultaneous amplification of β-actin provided a mean of normalizing data for differences in gel loading volume. The reliability of this method has been previously tested and statistically analysed by hierarchical analysis of variance.36
VH and DH usage determined by DPPH
VDJ rearrangements were amplified using a framework 1 (FR1) and antisense JH primer set. VH and DH usage in the amplified product was determined by DPPH34 using VH-, CDR-, or DH-oligonucleotide probes specific for the fetal VH genes (Fig. 2) or DHA or DHB (Fig. 3) when using the probes and hybridization conditions listed in Table 1. The VH- and DH-specific oligonucleotide probes were developed to fully hybridize only to non-mutated CDRs and DH segments. In practice, empirical data indicate that one mutation in CDR1, CDR2 and in DHA or DHB, can be tolerated before a reduction in the hybridization of the different VH or DH probes can be detected. Thus, a reduction in the DPPH values (reported as ‘usage’; see Figs 2, 3, 5, 7) for the fetal VH genes and DH segments can result either from significant mutation of CDR1 or CDR2 (> 1 mutation) or from the use of other VH genes that are unable to hybridize with the VH-specific CDR1 and CDR2 probes. Both represent evidence of repertoire diversification and can only be distinguished by sequence analysis or enumeration of individual hybridizing clones.29 The great value of the DPPH method is that it provides average information on > 30 individual VDJs within each PCR product (estimated by clonotyping) without the need to clone and sequence individual VDJs.29 Proportional VH usage was calculated by comparison to hybridization values obtained with a pan-specific FR2 probe in the manner previously described.34 The pan-specific oligonucleotide FR2 probe hybridizes to a highly conserved region shared by all porcine VH genes that is rarely mutated even in adult swine.33
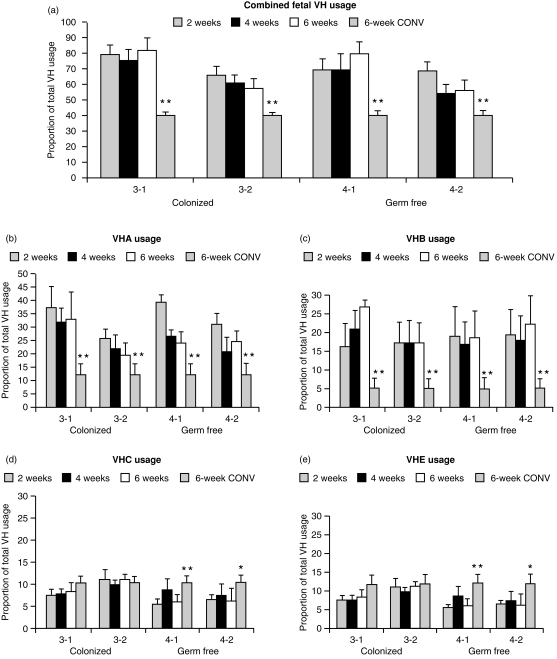
Figure 2.
VH usage in DNA from peripheral blood B cells (PBBs). (a) The combined usage of four fetal VH genes (VHA, VHB, VHC and VHE) as a proportion of total VH usage in four isolator and four conventional piglets (6 weeks CONV). Usage of fetal VH genes in the 6-week-old CONV animals is shown as the righthand bar in each histogram cluster. (b–e) The proportional usage of VHA, VHB, VHC and VHE on weeks 2–6 in GF piglets and COL piglets compared to DPPH usage values from 6-week-old CONV piglets. All data are expressed as mean± SD of four independent measurements. Note differences in the scale of the y-axis. Statistically significant at **P < 0·01 or *P < 0·05 when compared to 6-week-old GF or COL piglets.
Figure 3.
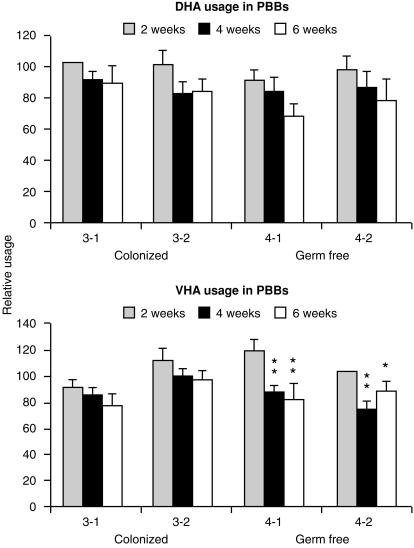
The relative usage of DHA and DHB in the DNA of PBBs from GF and COL piglets. Data are presented for samples collected on weeks 2, 4 and 6 after birth. All values for DHA were normalized to animal 3-1 on week 2 and for DHB, to animal 4-2 on week 2. These were assigned a value of 100. Data are expresses as the mean ± SD of four independent determinations. Mean DPPH values were significantly lower on weeks 4 and 6 than values for week 2 at the 0·01 (**) or 0·05 (*) level for animals 4-1 and 4-2.
Table 1.
Oligonucleotide probes used in DPPH for quantification of VH and DH usage
| Probe | Sequence | Hybridization temp./wash temp. |
|---|---|---|
| VHA | 5′-TGG CAG CTA TTA GTA CTA GT-3′ | 53°/50° |
| VHB | 5′-GAC AAC GCT TTC AGC TGG-3′ | 53°/55° |
| VHC | 5′-TGG CAG GTA TTT ATA GTA GT-3′ | 53°/50° |
| VHE | 5′-TCA GTA GTT ATG CAG TGA GC-3′ | 53°/55° |
| DHA | 5′-TAT AGC TAT GGT GCT AGT TGC TAT-3′ | 53°/55° |
| DHB | 5′-ATA GCG GTT GCT ATA GCG GTT ACG-3′ | 53°/55° |
| FR2 | 5′-CGC CAG GCT CCA GGG AAG-3′ | 53°/50° |
DH usage (relative usage, Fig. 3) is expressed as a ratio of DHA/FR2 or DHB/FR2 and, like DPPH values for VH usage, remains unchanged when DH regions are not highly mutated or heavily trimmed so that the remaining segment is not long enough to fully hybridize. DHA and DHB are used in nearly all fetal VDJ rearrangements29 and in > 90% of the VDJ transcripts from newborn piglets.28
Sequence analyses
VDJs amplified from DNA and first strain cDNA were cloned into EcoRV-digested pBluescript, and individual clones (in XL1 Blue E. coli) randomly selected and transferred to a master plate. For CDR3 analysis, clones were selected at random and their CDR3 regions were amplified using a FR3 and anti-JH primer set. These were sequenced as previously described.29 The CDR3 sequences were compared to those recovered from the spleen of 110-day fetuses and 30 previously cloned and sequenced CDR3s from adults. Sequences were analysed with respect to CDR3 length, DH usage and length, point mutations, deletions, P- and N-nucleotide additions, JH length and JH deletions. CDR3 was defined as the region between the YYCAR-encoded C-terminal consensus sequence of FR3 and the conserved codons for W of FR4. Entire VDJ sequences from DNA and cDNAs recovered from mucosal lymphoid tissues and spleens of 6-week-old piglets were cloned and sequenced in a similar manner. The CDR1 and CDR2 regions of these cloned VDJs were compared to germline sequences of the corresponding VH genes and somatic (non-templated) point mutations were identified. The CDR3 region of these sequences was analysed as described above Data obtained from COL and GF piglets are comparatively presented (Fig. 6). Blots of master plates were subsequently hybridized with VH-specific probes so the VH usage of the randomly selected clones could be determined.
Figure 6.
Comparative analysis of random cDNA transcripts from COL and GF isolator piglets. (a) Proportional VH and DH usage and comparison of CDR3 lengths; (b) frequency of mutations in CDR1, -2 and -3 in transcripts from GF piglets; (c) frequency of CDR mutation among transcripts from colonized piglets. All sequences submitted to GenBank and presented under Accession numbers.
Determination of serum immunoglobulins and antibody activity
IgM, IgG and IgA serum antibody activity to phosphorylcholine (PC) was determined using an enzyme-linked immunosorbent assay (ELISA) -based specific antibody immunoassay (SpAbI) using the sera of sow ‘Analiese’ as a positive reference standard for anti-PC activity and PC-swine albumin as the solid-phase antigen. Samples were analysed as previously described and the results were analysed by elisanalysis.37 The reaction buffer contained 0·05% Tween-20 and 10% Superblock (Pierce Chemicals, Rockford, IL). Specificity to PC was verified by inhibition of a dilution sequence of sera with PC-chloride used at 2 × 10−2 m. Total porcine serum IgM and IgA were determined by sandwich ELISA as previously described38 except that monoclonal anti-IgM (5C9B12) and anti-IgA (1459) were used as the detection antibodies and rabbit anti-mouse IgG conjugated to alkaline phosphatase (Sigma Chemicals, St. Louis, MO) was used as the signal generator. For detection of IgG, the globulin fraction of γ-chain-specific rabbit anti-IgG was used for capture (B85–4+5) and affinity-purified rabbit γ-chain-specific antibody was directlyconjugated to alkaline phosphatase and used for detection. Triplicate dilution sequences of test samples were analyzed by elisanalysis.37,38 Monoclonal anti-IgM (5C9B12) was kindly provided by Dr Prem Paul, Iowa State University and monoclonal anti-IgA (1459) was prepared and provided by Dr Klaus Nielsen, Veterinary Disease Research Laboratory, Nepean, Ontario, Canada.
Results
Colonization elevates serum immunoglobulin levels without diversification of the VH repertoire of PBBs
Weekly testing of rectal and skin samples indicated that while animals 4-1 and 4-2 remained GF, animals 3-1 and 3-2 quickly became colonized. Figure 1(a)–(c) shows that the serum concentrations of IgM, IgA and IgG in COL piglets were significantly elevated. Serum IgM levels in 2-week-old COL piglets were 30-fold higher than in GF piglets. Serum IgM levels in GF animals (4-1 and 4-2) increased slowly during the study and remained below 3 µg/ml. This is < 0·4% of levels in adult swine, and even at 6 weeks, is < 20% of the IgM level in animals that were colonized on the first day of life (3-1 and 3-2 versus 4-1 and 4-2). When serum levels of IgA were measured, GF animals had < 5 µg/ml at any time during the study, whereas COL piglets had levels ranging from 70 to > 100 µg/ml (Fig. 1c). Notably, the increases in serum IgA in COL piglets were inversely related to serum IgM levels; changes in serum IgG levels paralleled those for IgA but ranged up to five-fold higher. Nevertheless, the IgG levels in week 6 in animals colonized at birth were > 10-fold lower than in CONV piglets. (Immunoglobulin levels in conventional piglets at 2 and 4 weeks of age are much higher due to passive immunoglobulin and can exceed 20 mg/ml.)
While serum immunoglobulin levels differed greatly between COL and GF piglets, DPPH values show that the sum of VHA, VHB, VHC and VHE usage,4 i.e. the so-called ‘fetal VH genes of piglets’,29 constitutes ≈ 60–80% of the total VH usage in PBBs during the first 6 weeks of life for both COL and GF piglets (Fig. 2, top, right bar). (Since the degree of hybridization that generates the DPPH values depends on both absolute usage and changes in hybridization due to mutation, absolute usage can only be determined for mutated VDJs by sequencing or enumeration of individual clones; see the Materials and Methods.)
This is the same as in 60–110-day fetal piglets29 and is nearly double that seen in CONV 6-week-old piglets (Fig. 2, top). Combined fetal VH usage in animals 3-2 (colonized) and 4-2 (GF) were lower than in animals 3-1 and 4-1, but this cannot be ascribed to colonization. When data are reported for individual VH gene usage, we observed no consistent differences in DPPH values for any particular VH gene in the DNA of PBBs from COL compared to GF piglets (Fig. 2, bottom). VHA was the most frequently used VH gene (25–35%), consistent with our earlier observations on transcripts from newborn piglets.28 Proportional DPPH values for VHA and VHB in isolator piglets were two- to four-fold higher than in 6-week-old CONV piglets, although usage values for VHC and VHE differed only from CONV animals in 6-week-old GF piglets (Fig. 2, bottom, right bar). Figure 3 shows that none of the differences in DHB usage in PBB between piglets were significant but the GF piglets showed a trend for lowered DHB usage during weeks 4 and 6. However, differences in DHB values were not significant between GF and COL piglets.
Junctional diversity in CDR3 of VDJs from splenic B cells but not mutation frequency, is modestly affected by colonization
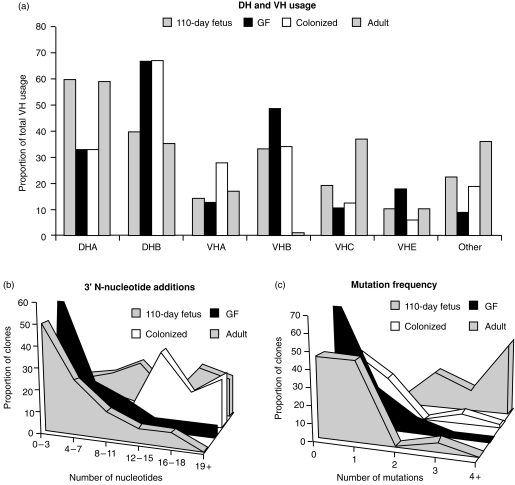
Failure to observe a change in total (Fig. 2, top) or individual fetal VH gene usage (Fig. 2, bottom) or in DH usage (Fig. 3) in PBBs from COL versus GF piglets could overlook changes in junctional diversity or other features of CDR3. Junctional diversity in CDR3 is the main source of heavy-chain repertoire diversity in the fetal piglet (Butler et al., submitted for publication). Thus, we compared the CDR3 sequences of 73 randomly selected VDJs cloned from the spleens of 6-week-old piglets with 17 cloned from the spleen of 110-day fetal piglets and 30 previously described adult sequences.33 Figure 4 summarizes the major findings. DH usage was identical for GF and COL whereas both fetal and adult animals used more DHA (Fig. 4a). Point mutations were almost absent in COL and GF animals, the same as in 110-day fetal spleens (Fig. 4c). While overall CDR3 length was normally distributed in fetal and GF spleen, CDR3 lengths in COL animals were shifted slightly upward (data not shown). Careful analysis indicated that this upward shift was due to greater N-nucleotide additions in the region 3′ of DH (Fig. 4b). One to three 3′ additions of what could be explained as P-nucleotides, were frequent but no differences were seen among COL, GF and fetal animals (data not shown). The VH usage of the randomly selected splenic clones (Fig. 4a) agreed well with DPPH studies on PBBs (Fig. 2). However, COL animals did show a trend toward reduced VHB and VHE usage and increased VHA usage (Fig. 4a). This pattern is also seen in the conventional adults used for comparison although the adults also showed increased VHC usage.
Figure 4.
Summary of sequence analysis of CDR3 segments cloned from 73 splenic DNA clones (randomly selected) from 6-week-old COL and GF piglets and from 17 110-day fetal splenic clones. (a) DH and VH usage in randomly selected splenic clones; (b) 3′ N-nucleotide additions; (c) frequency of point mutation. Comparative data are provided for 30 previously published adult transcripts.
Colonization results in expansion and diversification of the mucosal VH repertoire
Evidence that colonization of isolator piglets with a benign exclusion flora may have preferentially affected the mucosal but not the systemic humoral immune system is suggested when the relationship between the concentration of serum IgM, IgA and IgG in COL piglets was compared to that in an adult reference serum. This comparison shows that the relative IgA concentration in COL piglets was elevated more than five-fold (Fig. 1d). This rather dramatic effect of colonization on serum IgA levels (Fig. 1d), while paralleled only by modest diversification of VDJs amplified from the DNA of spleen (Fig. 4) and no effect on PBBs (Figs 2, 3), suggested that PBBs and splenic B cells may not be representative of the B-cell compartment that is affected by colonization. Rather, these findings hint that colonization may have selectively stimulated diversification and differentiation of IgA+ B cells that had already switched in utero.28,39 In support of this notion, we studied the relative transcription of IgA and IgM in various tissues. We selected for comparison, lymphoid tissue in direct contact with the gut (i.e. IPP), a non-inductive mucosal tissue (parotid) believed to derive its B cells by migration from gut-associated lymphoid tissue40 and the spleen. We found that while relative IgM transcription did not differ among lymphoid tissues of COL and GF piglets, IgA transcription was more than two-fold greater in colonized piglets than in GF piglets (data not shown). To address whether there was repertoire diversification in VDJs recovered from mucosal tissues, cDNA and DNA were prepared from various lymphoid tissues of 6-week-old piglets and comparatively analysed by DPPH using VH- and DH-specific probes. The results of these studies are summarized in Fig. 5.
Data on combined fetal VH usage show that DPPH values from VDJs from DNA and cDNAs recovered from the mucosal tissues of COL piglets were significantly lower than DPPH values for their GF counterparts (Fig. 5c). DPPH values for splenic transcripts from COL piglets were also significantly lower while this was not the case for VDJs from splenic DNA. DHA and DHB values showed a similar pattern (Fig. 5a,b). Transcripts from mucosal tissues of COL piglets gave significantly lower values than their GF counterparts and the same was true for VDJs recovered from mucosal DNA of COL piglets whereas VDJs from splenic DNA were little affected. When individual VH genes were studied, the effect of colonization was most dramatic for VHB and thus data for VHB are presented in Fig. 5(d). In fact, hybridization of a VHB-specific probe with transcripts from animals 3-1 and 3-2 was difficult to detect.
Since DPPH analyses suggest that the CDR1, CDR2 and DH sequences in mucosal transcripts from COL piglets had been mutated or that non-fetal VH or DH genes had been used, we compared the VDJ sequences of randomly selected clones from GF and COL piglets to distinguish between these two possibilities. Figure 6 summarizes the features of the CDR regions of > 30 randomly selected transcripts from GF and colonized piglets. While we found no significant differences in CDR3 length (Fig. 6a) or in the distribution of median length (data not shown), there was greater usage of DHA in colonized piglets (Fig. 6a). VHA accounted for nearly half of all VH gene usage in colonized animals while VHE predominated in GF transcripts. Thus, the much reduced DPPH values for VHB in transcripts from COL piglets (Fig. 5d) were most probably owing to lower VHB usage rather than merely mutation since only one VHB clone (3% of all VH usage) was detected by random sampling (Fig. 6a). A small proportion of hybrid genes, like those previously reported,28 were also detected (data not shown). Major differences between GF and COL piglets in the frequency of point mutation were observed (Fig. 6b,c). Seldom were transcripts found with more than two mutations in any CDR recovered from a GF piglet (Fig. 6b) whereas transcripts with more than two mutations in any CDR were common in COL piglets, including a few with more than six in CDR2 (Fig. 6).
IgA and IgM transcripts are equally diversified in mucosal tissues
Since colonization-dependent diversification of the VH repertoire was nearly restricted to mucosal tissues, we wished to determine if the VH repertoire encoding the initial B-cell receptor (IgM) was diversified to the same extent as the VH repertoire expressed by isotype-switched cells, e.g. IgA. This question is relevant since only the relative transcription of IgA in mucosal tissue was increased, not that of IgM (see above). When DH and VH usage by IgM and IgA transcripts were tested by DPPH (Fig. 7), those from COL piglets gave significantly lower values than GF piglets in the same manner, as was observed for undefined transcripts (Fig. 5). In GF piglets there was substantial hybridization with IgA transcripts that in general paralleled those with IgM transcripts, suggesting that both were derived from a common precursor clone. DPPH values for VHB were strikingly reduced for both IgM and IgA transcripts (Fig. 7a) in the same manner as had been observed for undefined transcripts from COL piglets (Fig. 5d) or in conventional piglets (Fig. 2). There was no evidence that IgM transcripts remained in an undiversified configuration while only switched transcripts were diversified.
Isolator piglets do not respond to PC-dependent conjugates
When anti-PC activity was measured, all piglets had IgM anti-PC activity that was < 1% of that found in the serum of a reference adult sow, although in contrast to adult serum, no hapten-inhibitable IgG or IgA anti-PC could be detected in piglet sera. There were no differences in the IgM anti-PC responses of immunized and non-immunized piglets, regardless of treatment group (data not shown).
Discussion
Our observation that serum immunoglobulin levels in COL piglets were 10–100-fold higher than in their GF littermates (Fig. 1) is consistent with earlier studies comparing conventional and GF rodents and piglets.9,41,42 The increase in serum immunoglobulin levels (Fig. 1) and increase in IgA transcription (data not shown) have generally been regarded as a consequence of the neonate's immune response to bacterial antigens41,43 or perhaps due to polyclonal activation by lipopolysaccharide (LPS) and other microbial mitogens.19 LPS from normal flora also appears to enhance resistance to infection44,45 and also makes mice susceptible to oral tolerance induction,46 perhaps by generating regulatory T cells.14
The unexpected paradox of our failure to observe diversification of VH and DH gene segments in PBBs and only subtle diversification in splenic B cells from COL piglets (Figs 2,3,4), while observing a very significant increase in the levels of serum immunoglobulins (Fig. 1) combined with a two-fold increase in IgA transcripts (data not shown), at first suggested that mitogen-driven expansion of the preimmune repertoire, without diversification, could explain our results. However, examination of transcripts from mucosal lymphoid tissues in COL piglets showed a significant reduction in their ability to hybridize with especially VHB- and DH-specific probes (Figs 5 and 7; an indication of diversification of the repertoire, see the Materials and Methods). Furthermore, VDJ transcripts from COL piglets had a much higher frequency of point mutations than those from GF piglets (Fig. 6b,c). The effect that colonization had on total VH diversification in mucosal cDNA (Fig. 5c) and in the low usage of VHB (Figs 5, 6) paralleled what we had observed in the PBBs of conventional piglets (Fig. 2) and in adults (Fig. 4a). It is noteworthy, that VHB is the first functional 3′ VH gene in swine28 and the developmentally dependent reduction in its usage in conventional piglets is reminiscent of the decline in usage of VH 81x in developing mouse pups.47 Since this reduction in VHB usage was not unique to Cα transcripts but was also observed with Cµ transcripts (Fig. 7b), it suggests that the Cµ transcripts came from B cells with diversified VDJs, not B cells displaying the preimmune germline repertoire (see below).
The reduced VHB probe hybridization observed in splenic cDNA may be due to IgM and IgA cells stimulated at inductive sites of the mucosal immune system, e.g. Peyers patches, that had taken up residence in the spleen while trafficking to, e.g. the parotid (Fig. 5). Splenic IgA at this time is very oligoclonal although clones with the same CDR3 length can be found among the highly polyclonal IgA transcripts in the IPP (unpublished data). Increases in 3′ N-nucleotide additions in splenic B cells (Fig. 4b) and a trend toward lower VHB and VHE usage but increased VHA usage (Fig. 4a) is reminiscent of the effects seen in mucosal transcripts (Fig. 6a).
Our finding of preferential diversification in mucosal lymphoid tissues, combined with the preferentially elevated level of serum IgA (Fig. 1d) and elevated IgA transcription, suggests that diversification might be correlated with antigen-driven switch from IgM to IgA. This is further supported by the reciprocal change in serum IgA and IgM concentrations during weeks 2–6 in colonized animals (Fig. 1). However, since switch recombination occurs in fetal life in piglets28,39 and fetal IgA transcripts are nearly as polyclonal as those for IgM (Butter et al., submitted) exposure to environmental antigen is not required. The tendency of DPPH values for VH and DH in IgA and IgM transcripts generally to parallel each other (Fig. 7), might therefore indicate that the IgA-producing clones were derived by switch recombination from diversified IgM-bearing precursors, or that IgA and IgM clones diversify in parallel. In any case, our findings are inconsistent with the notion that IgM-associated VDJs resemble germline VH genes whereas those associated with switched isotypes are diversified.48 Our finding of diversified IgM transcripts, suggest that the predominance of IgM plasma cells in the lamina propria of conventionally reared piglets in the first month of life49,50 is an antigen (or mitogen) selected element of the mucosal immune response in young piglets, not merely the consequence of an undiversified primary immune response that later switches to IgA. This is consistent with the reduction in VHB usage in IgM transcripts (Fig. 7) that appears to be a reliable indicator of repertoire diversification (Figs 2, 4b and 5).
The IPPs were collected for study since it has been suggested they may be equivalent in function to bursal elements in the chicken22 or the appendix in the rabbit.43 However, the abundance of IgA transcripts in the IPP of 6-week-old piglets, and even fetal piglets,36 together with the vigorous IgA transcription in this organ (data not shown) suggest that the IPP of 6-week-old piglets functions like a part of the mucosal immune system.
Our failure to observe anti-PC responses to parenterally administered PC-OVA may suggest that a typical T-dependent mechanism for responding to parenterally administered antigen is lacking in 2-week-old piglets. Others have reported that both neonatal and fetal piglets can respond to sheep red blood cells51 and we observed strong responses to PC delivered as R36A Streptococcus pneumoniae or conjugated to T-independent Brucella abortus.52 Since the carbohydrate epitopes of sheep red blood cells can be regarded as TI-2 antigens, not T-dependent as when PC is conjugated to OVA, perhaps this finding suggests poor development of helper T cells in 2-week-old animals colonized with a benign exclusion culture. However, two-thirds of T cells at birth are α/β and both their proportion and the proportion of CD4+ cells decrease during the first year.53,54 Since no apparent T-cell deficiency exists, a weakness in antigen presentation or co-stimulation during this period is implicated.
Although our report is based on only two animals per group, the differences in immunoglobulin levels (Fig. 1), DPPH data (Figs 5, 7) and VDJ sequences (Fig. 6) between COL and GF animals are highly significant and the differences between animals receiving the same treatment were not significant. Since the species is outbred, we have presented data for individual animals, not means of treatment groups (Figs, 1, 2, 3, 5 and 7). Since each DPPH assay samples > 30 clones (based on CDR3 spectrotype) in all tissue samples from each animal [parotid excepted; the parotid repertoire is very oligoclonal (< 10 clones) as judged by CDR3 spectrotyping, resulting in sampling bias that can also explain the statistical diffrences between DPPH values even for animals in the same treatment group (Fig. 5b–d)], each bar of a DPPH-based histogram represents data on > 120 clones. Furthermore, we have summarized data on > 100 sequences that either did not (Fig. 4) or did (Fig. 7) show major differences. Therefore we contend that the amount of data presented on each animal and the significant differences between treatments but not between animals, are sufficient to support our conclusions using just four animals. It is important to remember that these animals were reared in a highly controlled environment and as such, animal variation is seldom observed in such situations in comparison to conventionally reared animals. Regarding the latter, each animal can be influenced by different environmental factors.
The data presented in this report are the first to show that colonization of the intestinal tract of a neonatal mammal with a benign exclusion culture differs from ‘conventionalization’ (where conventionalization refers to rearing newborns with their mothers, thus exposing them to maternal regulatory and protective factors and allowing them to encounter pathogenic and non-pathogenic micro-organisms and parasites) and primarily results in diversification of the IgA and IgM preimmune repertoire associated with the mucosa even when measured 6 weeks after colonization. Determining what factors trigger repertoire development in newborn piglets and are responsible for the differences in repertoire development between isolator-colonized and conventional piglets may be of economic importance to the swine industry since mortality in piglets from birth to weaning is ≈ 10% of live births.55 Weaning marks the time at which protective immunity shifts from passive maternal immunity to the active, adaptive immunity of the piglet.
The results of this study may also be of general immunological interest since the piglet model of developmental immunology is not compromised by the passive transfer of maternal antibodies and other regulatory factors in utero. While GF mouse pups can indeed be reared on their GF dams, including immunoglobulin-deficient severe combine immunodeficiency dams,24 this arrangement does not prevent other immunoregulatory factors in milk56,57 from reaching the developing pups. On the contrary, piglets are precosial and can be reared in isolators from birth, so the influence of factors transferred postnatally in colostrum/milk can also be experimentally controlled. The isolator piglet model we describe here is a baseline to which other ingredients comprising the natural environment can be added.
Acknowledgments
The research was supported by NSF MCB 9723721 and National Pork Producers Council Grant 52871. The authors acknowledge Marcia Reeve for preparation of the typescript and Drs Gail Bishop and Marek Sinkora for critical reviews.
References
- 1.Kenworthy R, Crabb WE. Intestinal flora of young pigs, with reference to early weaning, Escherichia coli and scowers. J Comp Pathol. 1963;73:215. doi: 10.1016/s0368-1742(63)80025-9. [DOI] [PubMed] [Google Scholar]
- 2.Drasar BS, Barrow PA. Aspects of Microbiology 10: Intestinal Microbiology. Wokingham, UK: Van Nostrand Reinhold Ltd; 1985. [Google Scholar]
- 3.Smith HW, Jones JET. The effect of the addition of copper sulfate to the diet on the bacterial flora of the alimentary tract of the pig. J Appl Bact. 1963;26:262. [Google Scholar]
- 4.Tannock GW. The normal microflora: new concepts in health promotion. Microbiol Sci. 1988;5:4. [PubMed] [Google Scholar]
- 5.Schoeni JL, Wong AC. Inhibition of Campylobacter jejuni colonization in chicks by defined competitive exclusion bacteria. Appl Environ Microbiol. 1994;60:1191. doi: 10.1128/aem.60.4.1191-1197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorbecke GJ. Some histological functional aspects of lymphoid tissue in germfree animals. I. Morphological studies. Ann NY Acad Sci. 1959;78:237.. doi: 10.1111/j.1749-6632.1959.tb53106.x. [DOI] [PubMed] [Google Scholar]
- 7.Berg RD. Host immune response to antigens of the indigenous intestinal flora. In: Hentges DJ, editor. Human Intestinal Microflora in Health Disease. New York: Academic Press; 1983. p. 101. [Google Scholar]
- 8.Ohwaki M, Yasutake N, Yasui H, Ogura R. Comparative study on humoral immune responses in germfree and conventional mice. Immunology. 1976;32:43. [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen E, Friis CW. Influence of an intestinal flora on the development of the immunoglobulins IgG1, IgG2a, IgM and IgA in germ-free BALB/c mice. Acta path Microbiol Scand Sect C. 1980;88:121. doi: 10.1111/j.1699-0463.1980.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 10.Koopman JP, Mullink JAMA, Prins RA, Welling GW, Hectors MC. Association of germfree mice with intestinal microflora obtained from ‘normal’ mice. Lab Animal. 1982;16:59. doi: 10.1258/002367782780908724. [DOI] [PubMed] [Google Scholar]
- 11.Dobber R, Hertogh-hurjbregts A, Tozing J, Bottomly K, Nagelkerken L. The involvement of the intestinal microflora in the expansion of CD4+ T cells with a naive phenotype in the periphery. Dev Immunol. 1992;2:141. doi: 10.1155/1992/57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald TT, Carter PB. Requirement for a bacterial flora before mice generate cells capable of mediating DTH reactions to sheep red blood cells. J Immunol. 1979;122:2624. [PubMed] [Google Scholar]
- 13.Woolverton CJ, Holt LC, Mitchell D, Sartor RB. Identification and characterization of rat intestinal lamina propria cells: consequences of microbial colonization. Vet Immunol Immunopathol. 1992;34:127. doi: 10.1016/0165-2427(92)90156-k. [DOI] [PubMed] [Google Scholar]
- 14.Sudo N, Sawamura SA, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739. [PubMed] [Google Scholar]
- 15.Kweon M-N, Fujihashi K, Van Cott JL, et al. Lack of orally induced systemic unresponsiveness in IFNγ knockout mice. J Immunol. 1998;160:1687. [PubMed] [Google Scholar]
- 16.Stepankova R, Kovaru F, Kruml J. Lymphatic tissue of the intestinal tract of germ-free and conventional rabbits. Folia Microbiol. 1980;25:491. doi: 10.1007/BF02897215. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds JD, Morris B. The effect of antigen on the development of Peyer's patches in sheep. Eur J Immunol. 1984;14:1. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- 18.Smith MW, James PS, Tivey DR. M-cell numbers increase after transfer of SPF mice to a normal animal house environment. Am J Pathol. 1987;128:385. [PMC free article] [PubMed] [Google Scholar]
- 19.Mandel L, Trebichavsky I, Tlaskalova H. Development of immune responses in early pig ontogeny. Vet Immunol Immunopathol. 1992;43:135. doi: 10.1016/0165-2427(94)90129-5. (Cited from Tlaskalova-Hogenova et al 1994.) [DOI] [PubMed] [Google Scholar]
- 20.Ekino S. Role of environmental antigens in B cell proliferation in the bursa of Fabricius at neonatal stage. Eur J Immunol. 1993;23:772. doi: 10.1002/eji.1830230331. [DOI] [PubMed] [Google Scholar]
- 21.Crane MA, Kingzette M, Knight KL. Evidence for limited B-lymphopoiesis in adult rabbits. J Exp Med. 1996;183:2119. doi: 10.1084/jem.183.5.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynaud C-A, Garcia C, Hein WR, Weil J-C. Hypermutation generating the sheep immunoglobulin repetoire is an antigen-independent process. Cell. 1995;80:115. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 23.Van der Heijden P, Bianchi ATJ, Heidt PJ, Stok W, Bokhout BA. Background (spontaneous) immunoglobulin production in the murine small intestine before and after weaning. J Reprod Immunol. 1989;15:217. doi: 10.1016/0165-0378(89)90013-2. [DOI] [PubMed] [Google Scholar]
- 24.Kramer DR, Cebra JJ. Early appearance of natural mucosal IgA responses and germinal centers in suckling mice developing in the absence of maternal antibodies. J Immunol. 1995;154:2051. [PubMed] [Google Scholar]
- 25.Van der Waaij LA, Mesander G, Limburg PC, Van der Waaij D. Direct flow cytometry of anaerobic bacteria in human feces. Cytometry. 1994;16:270. doi: 10.1002/cyto.990160312. [DOI] [PubMed] [Google Scholar]
- 26.Kroese FGM, de Waard R, Bos NA. B-1 cells and their reactivity with the murine intestinal microflora. Seminars Immunol. 1996;8:11. doi: 10.1006/smim.1996.0003. [DOI] [PubMed] [Google Scholar]
- 27.Kroese FGM, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA-producing plasma cells in the murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1:75. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Butler JE. Molecular characterization of VDJ transcripts from a newborn piglet. Immunology. 1996;88:331. doi: 10.1046/j.1365-2567.1996.d01-676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Hayward C, Shinde R, Christensen R, Ford SP, Butler JE. Antibody repertoire development in fetal and neonatal piglets. I. Four VH genes account for 80% of VH usage during 84 days of fetal life. J Immunol. 1998;161:5070. [PubMed] [Google Scholar]
- 30.Butler JE, Sun J, Navarro P. The swine immunoglobulin heavy chain locus has a single JH and no identifiable IgD. Int Immunol. 1996;8:1897. doi: 10.1093/intimm/8.12.1897. [DOI] [PubMed] [Google Scholar]
- 31.Cukrowska B, Sinkora J, Rehakova Z, et al. Isotype and antibody specificity of spontaneously formed immunoglobulins in pig fetuses and germ-free piglets: production by CD5– B-cells. Immunology. 1996;88:611. doi: 10.1046/j.1365-2567.1996.d01-699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman ML, Tunyaplin C, Zhai SK, Knight KL. Neonatal VH, D and JH gene usage in rabbit B-lineage cells. J Immunol. 1994;152:632. [PubMed] [Google Scholar]
- 33.Sun J, Kacskovics I, Brown WR, Butler JE. Expressed swine VH genes belong to a small VH gene family homologous to human VHIII. J Immunol. 1994;153:5618. [PubMed] [Google Scholar]
- 34.Sun J, Shey M, Butler JE. Determination of gene usage by differential polymerase chain reaction product hybridization. Analyt Biochem. 1998;260:71. doi: 10.1006/abio.1998.2693. [DOI] [PubMed] [Google Scholar]
- 35.Miniats OP, Jol D. Gnotobiotic pigs-derivision and rearing. Can J Comp Med. 1978;42:428. [PMC free article] [PubMed] [Google Scholar]
- 36.Navaro P. The Univesity of Iowa: 1998. The allelic hinge variants of swine IgA. MSc Thesis, December. [Google Scholar]
- 37.Butler JE, Hamilton RG. Quantitation of specific antibodies: Methods of expression, standards, solid-phase considerations and specific application. In: Butler JE, editor. Immunochemistry of Solid-Phase Immunoassay. Boca Raton: CRC Press; 1991. p. 173. [Google Scholar]
- 38.Butler JE. The immunochemistry of sandwich ELISAs. In: Kemeny DM, Challacombe SJ, editors. Principles and Applications for the Quantitative Determination of Immunoglobulins. Chichester: John Wiley and Son, Ltd.; 1988. p. 155. [Google Scholar]
- 39.Cukrowska B, Sinkora J, Mandel L, et al. Thymic B cells in pig fetuses and germ-free pigs spontaneously produce IgM, IgG and IgA: detectioin by ELISPOT method. Immunology. 1996;87:487. doi: 10.1046/j.1365-2567.1996.499573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleeson M, Cripps AW, Clancy RL. Modifiers of the human mucosal immune system. Immunol Cell Biol. 1995;73:397. doi: 10.1038/icb.1995.62. [DOI] [PubMed] [Google Scholar]
- 41.Porter P, Kenworthy R. Effects of Escherichia coli on germ-free and gnotobiotic pigs. II. Serum proteins and antibodies. J Comp Pathol. 1970;80:233. doi: 10.1016/0021-9975(70)90090-3. [DOI] [PubMed] [Google Scholar]
- 42.Benveniste J, Lespinants G, Adams C, Salomon J-C. Immunoglobulins in intact, immunized and contaminated axenic mice: Study of serum IgA. J Immunol. 1971;107:1647. [PubMed] [Google Scholar]
- 43.Knight KL, Crane MA. Generating the antibody repertoire in rabbit. Adv Immunol. 1994;56:179. doi: 10.1016/s0065-2776(08)60452-6. [DOI] [PubMed] [Google Scholar]
- 44.Dubois RJ, Lee C, Costello R. Lasting biologic effects of early environmental influences. J Exp Med. 1969;130:963. doi: 10.1084/jem.130.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tlaskalova-hogenova H, Mandel L, Blabac V, Prokesova L, Hofman J, Kovaru F. Pathophysiological and immunological effects of lipopolysaccharide in germfree and conventional piglets. In: Agarwal MK, editor. Bacterial Endotoxins and Host Response. Amsterdam: Elsevier/North Holland; 1980. p. 143. [Google Scholar]
- 46.Wannemuehler MJ, Kiyono H, Babb JL, Michalek SM, McGhee JR. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982;129:959. [PubMed] [Google Scholar]
- 47.Marshall AJ, Wu GE, Paige CJ. Frequency of VH81x usage during B-cell development: initial decline in usage is independent of Ig heavy chain surface expression. J Immunol. 1996;156:2077. [PubMed] [Google Scholar]
- 48.Huang C, Stollar BD. A majority of IgH chain cDNA of normal human adult blood lymphocytes resembles cDNA for fetal Ig and natural antibodies. J Immunol. 1993;151:52909. [PubMed] [Google Scholar]
- 49.Allen WD, Porter P. The relative frequencies and distribution of immunoglobulin-bearing cells in the intestinal mucosa of neonatal and weaned pigs and their significance in the development of secretory immunity. Immunology. 1976;32:819. [PMC free article] [PubMed] [Google Scholar]
- 50.Butler JE, Klobasa F, Werhahn E. The differential localization of IgA, IgM and IgG in the gut of suckled neonatal piglets. Vet Immunol Immunopathol. 1981;2:53. doi: 10.1016/0165-2427(81)90038-6. [DOI] [PubMed] [Google Scholar]
- 51.Tlaskalova-hogenova H, Sterzl J, Hajek P, et al. The development of antibody formation during embryonal and postnatal periods. In: Sterzl J, Riha I, editors. Developmental Aspects of Antibody Formation. Prague: Czech. Acad. Science, II; 1969. p. 767. [Google Scholar]
- 52.Butler JE, Cambier JC, Klobasa F. The characterization of a TEPC-15, hapten modifiable idiotype system in swine. Mol Immunol. 1985;22:1159. doi: 10.1016/0161-5890(85)90004-5. [DOI] [PubMed] [Google Scholar]
- 53.Sinkora M, Sinkora J, Rehakova Z, Splichal I, Wang H, Parkhouse RME. Prenatal ontogeny of lymphocyte subpopulations in pigs. Immunology. 1998;95:595. doi: 10.1046/j.1365-2567.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H, Parkhouse RME. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Animal Health Monitoring System. 1995. Center for Epidemiology and Animal Health. USDA, APHIS. U.S. Fort Collins, CO. Part 1, October, 1995.
- 56.Koldovsky O, Goldman AS. Growth factors and cytokines in milk. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. San Diego: Academic Press; 1998. p. 1523. [Google Scholar]
- 57.Butler JE. Immunoglobulins and immune cells in animal milks. In: Ogra PL, Mestecky J, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. San Diego: Academic Press; 1998. p. 1531. Chapt 98. [Google Scholar]