Abstract
The effect of a null mutation for the metallothionein (MT)-I and -II isoforms in mice on the immunosuppressive action of ultraviolet B (UVB; 280–320 nm) radiation has been examined. Mice were exposed to a series of increasing daily UVB doses, each dose administered to the dorsum on 3 consecutive days. Erythema was assessed, and measured as its oedema component by the post-irradiation dorsal skinfold thickness, but there was no effect of the null mutation (MT–/–) observed after 3 × 3·4 kJ/m2 of UVB radiation. Immune function was assessed by the contact hypersensitivity (CHS) response, which was initiated by sensitization on unirradiated abdominal skin, and thus demonstrated the systemic effects of dorsal treatments. In comparison with the wild-type MT+/+ mouse, the MT–/– mouse was significantly more immunosuppressed by moderate daily UVB doses (1·75–5·9 kJ/m2). When topically applied cis-urocanic acid (cis-UCA) replaced UVB radiation as the immunosuppressive agent, contact hypersensitivity in MT–/– mice was again markedly more suppressed than in MT+/+ mice, in a dose-responsive manner. The results infer that MT, which was shown immunohistochemically to be strongly induced in the epidermis of MT+/+ mice, but to be absent in MT–/– epidermis, has the potential to protect from photoimmunosuppression, and that the mechanism of action may be via the inactivation of the epidermal UVB-photoproduct, cis-UCA.
Introduction
The metallothioneins (MTs) are a class of small cysteine-rich, heavy-metal-binding proteins produced in response to a variety of stresses, inflammation, and as components of the acute-phase response. Consequently a number of apparently unrelated functions have been ascribed to MT. It has been suggested that MT may play a major role in the prevention of tissue damage, and MT has been shown to be an efficient free radical scavenger. 1 MT also regulates essential trace metal homeostasis and plays a protective role in heavy metal detoxification reactions. However, the essential physiological role of MT remains unclear and may not have been identified to date. 2 At least four isoforms of MT exist in the mouse, with MT-I and -II having been localized immunohistochemically to a number of organs including the skin. MT-III appears to be localized in the brain, and MT-IV has been identified in some squamous epithelia and tumour tissue, but not, to date, in skin.
Irradiation of the skin with ultraviolet B (UVB) results in a range of pathological lesions, some directly induced by the radiation, some indirectly induced by secondary reactions involving oxidative processes and the release of reactive oxidative species such as hydroxyl and superoxide radicals. In vitro, cell lines which express higher than normal levels of MT have been demonstrated to be resistant to killing by UV radiation. 3, 4 Furthermore, the in vivo induction of cutaneous MT has been observed in response to a variety of oxidative or DNA-damaging agents, such as cadmium, 5 UVB radiation, 6, 7 1,25-dihydroxyvitamin D3 8 and cold stress, 9 and has been correlated with conferred protection from damage resulting from subsequent UV irradiation, measured as a reduction in sunburn cell formation.5, 8, 9 This photoprotective effect of MT, also described in cultured human and rodent cells, appears to be associated with its potential to reduce superoxide and hydroxyl radicals, in support of its suggested role as an endogenous antioxidant. 6
Exposure to UVB radiation not only causes critical damage to epidermal DNA, most abundantly seen as the formation of cyclobutyl pyrimidine dimers, but simultaneously selectively suppresses T-cell-mediated immune function such that initiated tumour cells are permitted to survive and proliferate. 10 It is not clear whether UV-induced oxidative damage comprises a contributing factor for photoimmunosuppression, but evidence is accumulating to suggest that antioxidant compounds may reduce the immunosuppression. 11–17 It has therefore been of interest to assess in vivo the role of MT in protection from other possible oxidant-dependent pathologies of UV irradiation besides sunburn cell formation, in particular, photoimmunological impairment. We have utilized the recently developed mouse strain bearing a null mutation for the MT-I and -II isoforms, to study effects on the systemic immune suppression induced by exposure to UVB radiation, or by its putative immunosuppressive epidermal mediator, cis-urocanic acid (cis-UCA), using the contact hypersensitivity (CHS) assay as the measure of the relevant T-lymphocyte immune function.
Materials and methods
Mice
The transgenic mice deficient in the MT-I and MT-II genes were created by introducing the mutations into embryonic stem cells by homologous recombination to produce chimeric mice of the 129Ola × C57BL/6J cross. 18 Lines of homozygous MT–/– and the wild-type MT+/+ were derived from this common progenitor stock and are therefore congenic. The two lines have been maintained by random breeding of successive homozygous generations. Six pairs of the two lines were obtained from the Murdoch Institute, and the experimental animals used in this study were produced by continuing random homozygous breeding in the Department of Veterinary Anatomy and Pathology under conventional animal house conditions. Coat colour phenotype may vary from black to light brown, and the animals appear to have no special husbandry requirements, to be fertile, and to have a normal healthy lifespan. The study was conducted with the ethical approval of the University Committee on Animal Care.
The mice were bred and maintained in wire-topped plastic boxes, on inert vermiculite bedding (Boral, Camellia, NSW), with an ambient temperature of 25° under gold lighting (GEC F40GO tubes, Davis Ultra Violet Pty Ltd, Marayong, NSW) which does not emit UVB radiation, on a 12-hr on/off cycle. They were fed stock laboratory mouse cubes (Norco Stockfeeds, Lismore, NSW) and tap water ad libitum. For the study, female mice aged 8–12 weeks were housed together in groups of six. Hair was clipped from the mice using Oster clippers 24 hr before either UV irradiation or UCA lotion application (dorsum) or sensitization (abdomen), and mice were selected for inclusion in the study if apparently in the resting phase of hair growth (pink skin). Coat colour had no measurable effect on either the erythema/oedema response or the suppression of CHS by UVB exposure, so black- and brown-haired mice were combined in experimental groups.
UVB radiation
A single unfiltered 120-cm fluorescent UVB tube (Oliphant FL40SE, NSW Ultraviolet, Maryong, NSW; equivalent to Westinghouse FS40 UVB tube) housed in a reflective batten provided the radiation. Irradiance was measured using an International Light IL1500 radiometer with two detectors (SEE 015/UVA and SEE 240/UVB; International Light Inc., Newburyport, MA) which had been calibrated to the spectral irradiance of the source (F. Wilkinson, CSIRO National Standards Laboratory, Bradfield Park, NSW), and was found to comprise 2·5 × 10−4 W/cm2 UVA (320–400 nm) and 4·1 × 10−4 W/cm2 UVB (280–320 nm).
Erythema is difficult to quantify in the mouse and therefore was assayed by the oedema component of this inflammatory response by measuring mid-dorsal skinfold thickness daily following UV irradiation, using a spring micrometer (Mercer, St Albans, UK). The minimal erythemal dose (MED) was found to be approximately 2·1 kJ/m2 UVA and 3·4 kJ/m2 UVB for both MT+/+ and MT–/– mice, administered in 14 min. Mice were exposed dorsally, unrestrained, with the wire cage tops removed, to UVB daily doses varying from 0·5 × MED (1·7 kJ/m2) to 1·74 × MED (5·9 kJ/m2) on 3 consecutive days. Ears were protected with topical application of a UVB-absorber (2-ethylhexyl-p-methoxycinnamate) solution in ethanol (5% v/v).
Immunohistochemical staining
Dorsal skin samples were collected before and 72 hr after UV irradiation and were fixed in Histochoice (Amresco, Parkway, OH) followed by embedding with paraffin. Deparaffinized 5-μm tissue sections were subjected to immunohistochemical staining for MT with rabbit polyclonal antibody against rat MT-1, prepared as described elsewhere. 19 The bound primary antibody was then visualized with the avidin–biotin peroxidase complex (ABC) immunostaining method (PK-4000,Vector Lab., Burlingame, CA) as previously described. 19
Urocanic acid lotions
UCA was purchased (Sigma Chemical Co., St. Louis, MO) as the trans isomer, and photoisomerized in dimethyl sulphoxide solution to a photostationary mixture of 52% trans and 48% cis isomers as previously described. 14 Lotions containing 0·2% (w/v) of trans-UCA, or between 0·02% and 0·5% (w/v) of UV-irradiated UCA, referred to here as cis-UCA, were prepared in an innocuous cosmetic oil-in-water emulsion and stored in the dark at 4°. The base lotion was identical in composition, without added UCA. 14 Aliquots of 0·1 ml (20–500 μg UCA) were spread evenly over the mouse dorsum and left to be absorbed for 30 min. The lotions were applied daily for 3 consecutive days.
Induction of contact hypersensitivity
CHS was induced in groups of six mice, on day 8 and day 9 following the first treatment with either UVB radiation or with UCA lotions, as previously described. 20 The sensitizer, 0·05 ml 0·15% (v/v) 2,4-dinitrofluorobenzene (DNFB; Sigma) freshly prepared in acetone, was applied to the unirradiated abdominal skin in order to measure systemic effects. The mice were challenged on day 15 with 5 μl 0·15% DNFB applied to each surface of both pinnae. Average ear swelling was calculated at the peak of the response, usually at 18–20 hr, by the difference between the group average pre- and post-challenge ear thicknesses measured with a spring micrometer (Mercer). The percentage suppression of CHS was calculated in comparison with the CHS responses of control mice which were not irradiated with UVB, or which received topical base lotion application (no UCA) only. Statistical significance of differences between treatments was assessed by a paired two-tailed t-test.
Results
Erythema/oedema
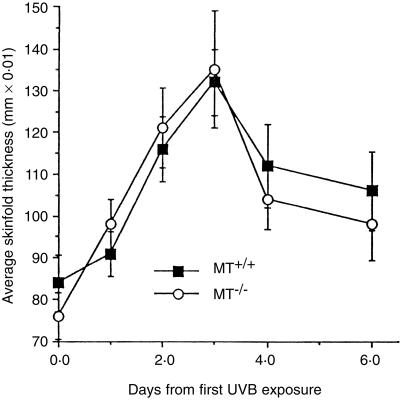
There was no significant difference between the oedema generated post-irradiation in MT+/+ and MT–/– mice, as shown in Fig. 1. Therefore, MT-I and -II do not appear to be important in regulating this response to UVB exposure.
Figure 1.
Progressive erythema/oedema, measured by mid-dorsal skinfold thickness, following exposure of MT+/+ and MT–/– mice (n = 10) to 3·4 kJ/m2 daily on 3 consecutive days. Bars =SD.
MT expression in skin
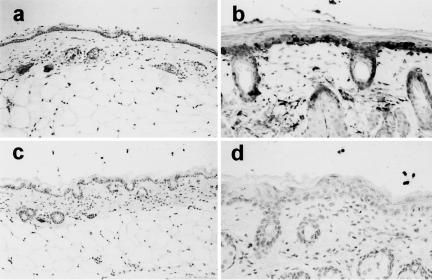
Constitutive immunopositive MT expression could be detected in the epidermal layer of untreated MT+/+ skin, but there was only very faint non-specific staining in the MT–/– skin (Fig. 2). At 72 hr following irradiation (3 × 1 MED) there was strong induction of immunopositive staining in MT+/+ skin, mainly localized in the basal cell layer of the epidermis and in the cells lining the hair follicles. There were also discrete immunopositive cells in the dermal layer, possibly fibroblasts. No positive staining was found in the epidermal basal cells or follicles or in the dermis in the MT–/– skin following UVB irradiation. The epidermal hyperplastic response contributing to erythema can be seen by the counterstain to be well established by 72 hr, the time-point at which the mid-dorsal skinfold thickness was maximal (Fig. 1), and to be present to a similar degree in both MT+/+ and MT–/– skin. Some diffuse positive staining for MT was also seen in the upper layers of the irradiated epidermis of both mouse strains, but this diffuse staining was very weak in the upper epidermis of MT–/– mice.
Figure 2.
Immunostaining for MT in the skin of MT+/+ mice (a and b) and MT–/– mice (c and d). Untreated skin (a and c, ×50 magnification) shows weak staining in MT+/+ epidermis, non-specific background staining in MT–/– epidermis. At 72 hr post-UVB irradiation (b and d, ×100 magnification) epidermal hyperplasia is evident in both mouse strains; strong MT expression has been induced in MT+/+ epidermis (b), but there is only background staining for MT in MT–/– epidermis (d).
CHS reaction
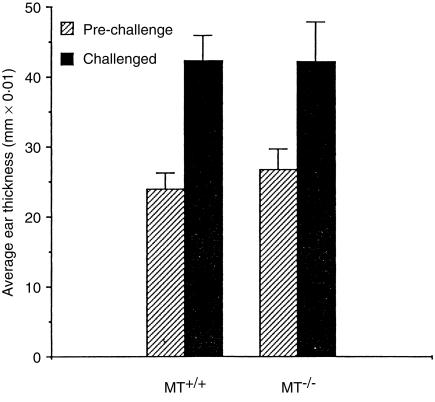
Both MT+/+ and MT–/– mice raised effective reactions to DNFB (Fig. 3), with ear thickness increasing approximately two-fold following the challenge treatment, responses of the magnitude of those observed in other mouse strains.13, 14, 20 There was no significant difference between the responsiveness of MT+/+ or MT–/– mice, indicating that MT-I and -II are not essential for this form of T-cell-mediated immune reaction.
Figure 3.
The CHS reaction in MT+/+ and MT–/– mice to DNFB, indicated by the pre- and post-challenge ear thickness measurements (n = 10 or 12; bars =SD).
Suppression of CHS by UVB radiation
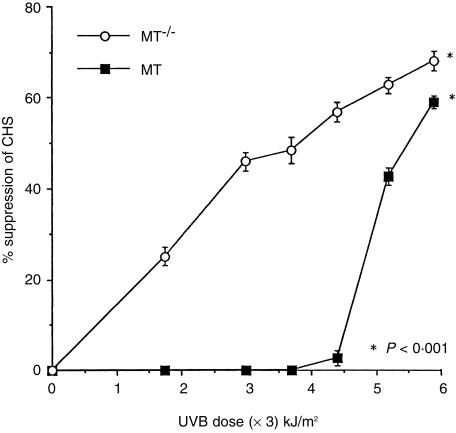
When MT+/+ mice were exposed to increasing daily doses of UVB radiation, CHS to DNFB became increasingly suppressed (Fig. 4). In these mice, the lowest daily doses (1·75–3·7 kJ/m2) of UVB were not immunosuppressive. Higher doses (4·4–5·9 kJ/m2) increased the suppression dose-responsively from 3% to 59% suppression.
Figure 4.
Per cent suppression of CHS to DNFB induced by increasing daily exposures to UVB radiation in MT+/+ and MT–/– mice, relative to the response in non-irradiated mice (n = 12; bars =SEM).
MT–/– mice were more readily immunosuppressed by UVB exposure (Fig. 4) than MT+/+ mice. At the lowest daily UVB dose of 1·75 kJ/m2 the response was suppressed by 25%, and there was a progressive UVB dose-related increase in the degree of suppression to 68% at 5·9 kJ/m2. At this dose, the responses of the two mouse strains were less disparate, but the suppression in MT–/– mice was still highly significantly greater (P < 0·001) compared with that in MT+/+ mice. If the relationship between UVB radiation dose and the degree of suppression of CHS was examined, it could be calculated that the 50% immunosuppressive UVB dose was approximately 5·5 kJ/m2 for MT+/+ mice, and approximately 3·8 kJ/m2 for MT–/– mice.
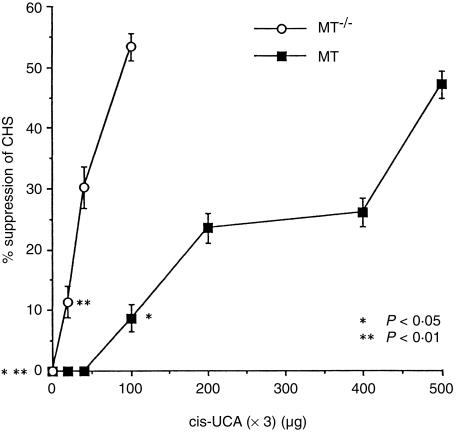
Suppression of CHS by cis-UCA
Application of trans-UCA (3 × 200 μg) resulted in 6·9 ± 3·0% suppression of CHS in MT+/+ mice, and 3·9 ± 3·5% suppression of CHS in MT–/– mice, neither response being significantly different from base lotion-treated mice. When MT+/+ mice were treated with increasing concentrations of cis-UCA, dose-responsively suppressed CHS became apparent (Fig. 5). Whereas 20–40 μg cis-UCA daily was not immunosuppressive, there was a small but significant 9% suppression of CHS following a daily dose of 100 μg cis-UCA (P < 0·05), and the suppression increased progressively with increasing cis-UCA concentration to 47% suppression at 500 μg cis-UCA.
Figure 5.
Per cent suppression of CHS to DNFB induced by increasing concentration of cis-UCA-containing lotions applied daily topically to MT+/+ and MT–/– mice, relative to the response in mice treated topically with base lotion, (n = 12; bars =SEM).
In contrast, MT–/– mice revealed a much greater sensitivity to cis-UCA, and 20 μg cis-UCA daily resulted in 11% immunosuppression (P < 0·01), with increasing suppression until 53% suppression following 100 μg cis-UCA (Fig. 5). Thus the relationship between cis-UCA concentration and the degree of suppression of CHS can be seen to differ markedly between MT+/+ and MT–/– mice, with approximately 50% suppression achieved in MT+/+ mice by 500 μg cis-UCA daily, and a similar level of suppression in MT–/– mice apparent after only 100 μg cis-UCA daily.
Discussion
This study has demonstrated that the systemic suppression of T-cell-mediated immunity by UVB radiation is exacerbated in mice bearing a null mutation for the MT-I and -II genes, and infers that one function of MT, which has not previously been described, is to provide a pathway of photoimmunoprotection. The observation is supported by currently available data showing that induced MT production in cells and experimental animals confers resistance to other forms of UV photodamage such as cell death, 3 cell proliferation, 4 sunburn cell formation 5 and the formation of superoxide and hydroxyl radicals. 6 We have demonstrated that exposure to UVB radiation induces strong epidermal MT expression in normal mice, whereas this is absent in the mutant MT–/– mouse.
A broad variety of studies have provided substantial evidence for the involvement of MT in essential trace metal homeostasis, in heavy metal detoxification and in antioxidant reactions in a number of tissues. However, there are sparse data indicating that MT might have an immune regulating function. Two studies have indicated that extracellular MT could modulate humoral immune responses in a zinc- or cadmium-dependent environment, 21 and, of more relevance here, that MT could induce lymphoproliferation, 22 although the possible mechanisms were not clarified. It is therefore of interest that MT is now known to be induced by a number of immunological mediators and cytokines such as interleukins −1 and -6 and tumour necrosis factor-α, 23, 24 mediators known to be released from UV radiation-damaged skin, and also to be associated with various aspects of the photoimmunosuppressed state. 25–28
We did not find that there was MT-dependence of the erythema/oedema response to UVB, in contrast to a recent study in which histological evaluation of the post-UVB epidermal hyperplasia revealed marked inhibition in the MT–/– mouse. 29 In that study, erythema was induced by a single UVB exposure of approximately 2 MED and resulted in a five-fold increase in epidermal thickness. In our study, a total exposure of 3 MED was administered in three separate daily irradiations of 1 MED and resulted in less than a two-fold increase in skinfold thickness at the maximum time-point. The UVB dosages and exposure regimes were thus different, as well as the assessment of epidermal thickness, and suggest that MT-dependence of erythema may be obvious only with more injurious UV doses than we have used. This would be consistent with the reported association of MT with proliferating cells, 30 since keratinocyte proliferation would be more marked in the post-irradiation repair period. Alternatively, since our data suggest that the MT+/+ strain is less immunologically sensitive to both UVB radiation and exogenous cis-UCA than we have found hairless Skh:HR or conventional C57BL mice to be, 14, 20, 25 but the MT–/– epidermal hyperplasia was compared in the histological study, not to its wild-type MT+/+ mouse, but to the sensitive C57BL mouse, it is possible that unmatched mouse strain genetics may explain the reported inhibition of hyperplasia. It is also relevant that immune function has been shown to be more sensitive to UV irradiation than erythema, 31 so that MT immune protection could be expected to be detectable at smaller UV doses than MT protection from the hyperplasia and oedema of the erythema response. We observed that the increasing susceptibility of the MT–/– mouse to photoimmunosuppression by increasing UVB exposure was most marked at the lower UVB doses we administered. This would be consistent with a photoimmunoprotective function of MT at moderate UVB exposures, but with protection from epidermal hyperplasia perhaps predominating at higher exposures. It is probable therefore that the mechanism by which MT inhibits photoimmunosuppression differs for the MT inhibition of erythema described by others.
Our results indicate that the immunoprotective role of MT may be less effective against UVB irradiation than cis-UCA treatment. This suggests that MT protection may not be effective against the numerous other mediators of photoimmunosuppression which have been described, 32–34 and which are likely to interact in different ways to result in the elicitation of photoimmunosuppression.
There is indirect evidence of an alignment between the antioxidant and photoimmunoprotective properties of MT, from studies with aloe extract in mice. These have demonstrated a concurrent capacity of aloe extract for the scavenging of radiation-produced reactive oxygen intermediates, maintenance of the cellular antioxidant enzymes glutathione transferase and superoxide dismutase, and induction of MT in the skin, 35 while other studies have demonstrated that aloe extract protects mice from photoimmunosuppression. 36 It is thus possible that the MT immune protective effect is mediated via its capacity for inactivation of oxidant photoproducts. Recent evidence indicates that cis-UCA-induced immunosuppression may be potentiated by reactive oxygen species, since a number of antioxidant treatments have been shown to inhibit immune suppression induced by both UV radiation and cis-UCA. 11–17 Our results indicate that MT is photoimmunoprotective by virtue of its antagonism of cis-UCA, and therefore would be consistent with the previously demonstrated oxidative radical scavenging function. 6 However, the nature of subsequent oxidant-dependent reactions of cis-UCA, or relevant cutaneous cytokine derangements that could be assigned to cis-UCA and which might culminate in immunosuppression, remains unclear.
It will be informative to compare the effects of both UVB exposure and cis-UCA treatment on cutaneous cytokine patterns in MT+/+ and MT–/– mice, since it is now apparent that photoimmunosuppression results from a preponderance of T helper type 2 (Th2)-associated cytokines over Th1 cytokines. 37 The mechanism by which MT might contribute to the endogenous photoprotective defences of the skin in an immunologically effective way might thus be revealed. In addition, since this study has compared only normal levels of MT in the MT+/+ mice with an MT deficiency in MT–/– mice, confirmation of the immunoprotective role of up-regulated expression of MT is currently being sought.
Acknowledgments
These studies were supported by the University of Sydney Cancer Research Fund. We thank Ms D. Domanski for excellent research assistance, and Ms L. Blyth, Ms C. Abeywardana and Mr J. Fisher for careful mouse husbandry.
Abbreviations
- CHS
contact hypersensitivity
- MED
minimal erythemal dose
- MT
metallothionein
- UCA
urocanic acid
- UVA
ultraviolet light at 320–400 nm wave length
- UVB
ultraviolet light at 280–320 nm wave length
References
- 1.Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Rad Biol & Med. 1993;14:325. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 2.Vallee BL. The function of metallothionein. Neurochem Int. 1995;27:23. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- 3.Dudek EJ, Peak JG, Roth RM, Peak MP. Isolation of V79 fibroblast cell lines containing elevated metallothionein levels that have increased resistance to the cytotoxic effects of ultraviolet-A radiation. Photochem Photobiol. 1993;58:836. doi: 10.1111/j.1751-1097.1993.tb04980.x. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi S, Hirota Y, Sayato-Suzuki J, et al. Possible role of metallothionein in the cellular defense mechanism against UVB irradiation in neonatal human skin fibroblasts. Photochem Photobiol. 1994;59:650. doi: 10.1111/j.1751-1097.1994.tb09671.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanada K, Gange RW, Siebert E, Hasan T. Protective effects of cadmium chloride against UVB injury in mouse skin and in cultured human cells:S a possible role of cadmium-induced metallothionein. Photodermatol Photoimmunol Photomed. 1991;8:111. [PubMed] [Google Scholar]
- 6.Hanada K, Baba T, Hashimoto I, Fukui R, Watanabe S. Possible role of cutaneous metallothionein in protection against photo-oxidative stress – epidermal localization and scavenging for superoxide and hydroxy radicals. Photoderm Photoimmunol Photomed. 1993;9:209. [PubMed] [Google Scholar]
- 7.Anstey A, Marks R, Long C, et al. In vivo photoinduction of metallothionein in human skin by ultraviolet irradiation. J Path. 1996;178:84. doi: 10.1002/(SICI)1096-9896(199601)178:1<84::AID-PATH430>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Hanada K, Sawamura D, Nakano H, Hashimoto I. Possible role of 1,25-dihydroxyvitamin D-3-induced metallothionein in photoprotection against UVB injury in mouse skin and cultured rat keratinocytes. J Dermatol Sci. 1995;9:203. doi: 10.1016/0923-1811(94)00378-r. [DOI] [PubMed] [Google Scholar]
- 9.Ota T, Hanada K, Hashimoto I. The effect of cold stress on UVB injury in mouse skin and cultured keratinocytes. Photochem Photobiol. 1996;64:984. doi: 10.1111/j.1751-1097.1996.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in UV irradiated mice. Science. 1982;216:1133. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 11.Yuen KS, Halliday GM. Alpha-tocopherol, an inhibitor of epidermal lipid peroxidation, prevents ultraviolet radiation from suppressing the skin immune system. Photochem Photobiol. 1997;65:587. doi: 10.1111/j.1751-1097.1997.tb08610.x. [DOI] [PubMed] [Google Scholar]
- 12.Katiyar SK, Elmets CA, Agarwal R, Mukhtar H. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. Photochem Photobiol. 1995;62:855. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 13.Reeve VE, Bosnic M, Rozinova E, Boehm-Wilcox C. A garlic extract protects from ultraviolet B (280 nm) radiation-induced suppression of contact hypersensitivity. Photochem Photobiol. 1993;58:813. doi: 10.1111/j.1751-1097.1993.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 14.Reeve VE, Bosnic M, Rozinova E. Carnosine (β-alanylhistidine) protects from the suppression of contact hypersensitivity by ultraviolet B (280 nm) radiation or by cis-urocanic acid. Immunology. 1993;78:99. [PMC free article] [PubMed] [Google Scholar]
- 15.Steenvoorden D, Vanhenegouwen G. Glutathione ethylester protects against local and systemic suppression of contact hypersensitivity induced by ultraviolet B radiation in mice. Radiation Res. 1998;150:292. [PubMed] [Google Scholar]
- 16.Nakamura T, Pinnell SR, Darr D, et al. Vitamin C abrogates the deleterious effects of UVB radiation on cutaneous immunity by a mechanism that does not depend on TNF-α. J Invest Dermatol. 1997;109:20. doi: 10.1111/1523-1747.ep12276349. [DOI] [PubMed] [Google Scholar]
- 17.Reeve VE, Tyrrell RM. Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proc Natl Acad Sci USA. 1999;96:9317. doi: 10.1073/pnas.96.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mice. Proc Natl Acad Sci USA. 1993;90:8088. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura H, Nishimura N, Kobayashi S, Tohyama C. Immunohistochemical localization of metallothionein in the eye of rats. Histochem. 1991;95:535. doi: 10.1007/BF00266738. [DOI] [PubMed] [Google Scholar]
- 20.Reeve VE, Boehm-Wilcox C, Bosnic M, Cope R, Ley RD. Lack of correlation between suppression of contact hypersensitivity by UV radiation and photoisomerization of epidermal urocanic acid in the hairless mouse. Photochem Photobiol. 1994;60:268. doi: 10.1111/j.1751-1097.1994.tb05103.x. [DOI] [PubMed] [Google Scholar]
- 21.Lynes MA, Borghesi LA, Youn J, Olson EA. Immunomodulatory activities of extracellular metallothionein. 1. Metallothionein effects on antibody production. Toxicology. 1993;85:161. doi: 10.1016/0300-483x(93)90040-y. [DOI] [PubMed] [Google Scholar]
- 22.Lynes MA, Garvey JS, Lawrence DA. Extracellular metallothionein effects on lymphocyte activities. Molec Immunol. 1990;27:211. doi: 10.1016/0161-5890(90)90132-j. [DOI] [PubMed] [Google Scholar]
- 23.Kaji T, Yamamoto C, Tsubaki S, et al. Metallothionein induction by cadmium, cytokines, thrombin and endothelin-1 in cultured vascular endothelial cells. Life Sci. 1993;53:1185. doi: 10.1016/0024-3205(93)90536-c. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Sasaki M, Hojo H. Antioxidative roles of metallothionein and manganese superoxide dismutase induced by tumor necrosis factor-alpha and interleukin-6. Arch Biochem Biophys. 1995;316:738. doi: 10.1006/abbi.1995.1098. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura N, Tohyama C, Satoh M, Nishimura H, Reeve VE. Defective immune response and severe skin damage following UVB-irradiation in interleukin-6-deficient mice. Immunology. 1999;97:77. doi: 10.1046/j.1365-2567.1999.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laihia JK, Jansen CT, Uksila J, et al. Effects of cis- and trans-urocanic acids on the secretion of interleukin-1β and tumor necrosis factor-α by human peripheral blood monocytes. Acta Dermatol Venereol. 1994;74:266. doi: 10.2340/0001555574266268. [DOI] [PubMed] [Google Scholar]
- 27.Urbanski A, Schwarz T, Neuner P, et al. Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990;94:808. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- 28.Streilein JW. Sunlight and skin-associated lymphoid tissue (SALT): if UVB is the trigger and TNF-α is its mediator, what is the message? J Invest Dermatol. 1993;100:47S. doi: 10.1111/1523-1747.ep12355578. [DOI] [PubMed] [Google Scholar]
- 29.Hanada K, Sawamura D, Hashimoto I, Kida K, Naganuma A. Epidermal proliferation of the skin in metallothionein-null mice. J Invest Dermatol. 1998;110:259. doi: 10.1046/j.1523-1747.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 30.Karasawa M, Nishimura N, Nishimura H, Tohyama C, Hashiba H, Kuroki T. Localization of metallothionein in hair follicles or normal skin and the basal cell layer of hyperplastic epidermis: possible association with cell proliferation. J Invest Dermatol. 1991;97:97. doi: 10.1111/1523-1747.ep12478393. [DOI] [PubMed] [Google Scholar]
- 31.Wolf P, Yarosh DB, Kripke ML. Effects of sunscreens and a DNA repair enzyme on ultraviolet radiation-induced inflammation, immune suppression, and cyclobutane pyrimidine dimer formation in mice. J Invest Dermatol. 1993;101:523. doi: 10.1111/1523-1747.ep12365902. [DOI] [PubMed] [Google Scholar]
- 32.Kripke ML, Fox PA, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung H-T, Burnham DK, Robertson B, Roberts LK, Daynes RA. Involvement of prostaglandins in the immune alterations caused by the exposure of mice to ultraviolet radiation. J Immunol. 1986;137:2478. [PubMed] [Google Scholar]
- 34.Jaksic A, Finlay-Jones JJ, Watson CJ, Spencer LK, Santucci I, Hart PH. Cis-urocanic acid synergizes with histamine for increased PGE2 production by human keratinocytes: Link to indomethacin-inhibitable UVB-induced immunosuppression. Photochem Photobiol. 1995;61:303. doi: 10.1111/j.1751-1097.1995.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, Ohta S, Shinoda M. Studies on chemical protectors against radiation: XXXI Protection effects of Aloe arborescens on skin injury induced by X-irradiation. J Pharmaceut Sci Jap. 1990;110:876. doi: 10.1248/yakushi1947.110.11_876. [DOI] [PubMed] [Google Scholar]
- 36.Strickland F, Pelley RP, Kripke ML. Prevention of UV radiation-induced suppression of contact and delayed type hypersensitivity by Aloe barbadensis gel extract. J Invest Dermatol. 1994;102:197. doi: 10.1111/1523-1747.ep12371762. [DOI] [PubMed] [Google Scholar]
- 37.Ullrich S. Does exposure to UV radiation induce a shift to a TH-2-like immune reaction? Photochem Photobiol. 1996;64:254. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]