Abstract
Compared with young mice, old mice infected with influenza virus have significantly higher pulmonary viral titres, although these can be reduced significantly with dietary vitamin E supplementation. T helper 1 (Th1) cytokines, especially interferon-γ (IFN-γ), play an important role in defending against influenza infection. However, there is an age-associated loss of Th1 cytokine production. Prostaglandin E2 (PGE2) production, which increases with age, can modulate the T helper cell function by suppressing Th1 cytokine production. To investigate the mechanism of vitamin E supplementation on reduction of influenza severity in old mice, we studied the cytokine production by splenocytes, and PGE2 production by macrophages (Mφ), in young and old C57BL mice fed semipurified diets containing 30 (control) or 500 parts per million (ppm) (supplemented) vitamin E for 8 weeks, and then infected with influenza A/PC/1/73 (H3N2). Old mice fed the control diet had significantly higher viral titres than young mice; old mice fed the vitamin E-supplemented diet had significantly lower pulmonary viral titres than those fed the control diet (P = 0·02 and 0·001 for overall age and diet effect, respectively). Following influenza infection, interleukin (IL)-2 and IFN-γ production was significantly lower in old mice than in young mice. Vitamin E supplementation increased production of IL-2 and IFN-γ in old mice; higher IFN-γ production was associated with lower pulmonary viral titre. Old mice fed the control diet showed significantly higher lipopolysaccharide (LPS)-stimulated Mφ PGE2 production than old mice fed the vitamin E diet or young mice fed either diet. There was no significant age difference in IL-6, IL-1β, or tumour necrosis factor-α (TNF-α) production by splenocytes. Young mice fed the vitamin E-supplemented diet had significantly lower IL-1β (day 7) and TNF-α production (day 5) compared with those fed the control diet. Old mice fed the vitamin E-supplemented diet had significantly lower TNF-α production (day 2) than those fed the control diet. Our results indicate that the vitamin E-induced decrease in influenza viral titre is mediated through enhancement of Th1 cytokines, which may be the result of reduced PGE2 production caused by vitamin E.
Introduction
Ageing is associated with decreased immune function, which contributes to increased susceptibility to some infections in the elderly. Respiratory infections are particularly common in older persons, and influenza and pneumonia are major causes of morbidity and mortality.1,2
Immunological factors involved in the control of influenza virus include cytotoxic T lymphocytes (CTL), CD4+ T cells, natural killer (NK) cells, production of interferon (IFN)-α, -β, or -γ, and antibody (Ab) production by B cells. Most of these factors have been shown to be affected by ageing. CD8+ CTL have been shown to be important for virus clearance in mice infected with influenza virus. In aged mice, delayed and/or decreased splenic and pulmonary CTL activity occurs following influenza infection, which correlates with their prolonged pulmonary viral shedding.3
CD4+ T cells can mediate virus clearance by promoting an influenza-specific B-cell response or by secreting cytokines. Taylor et al.4 reported age-related loss of CD4+ T-cell functions in influenza-infected mice, including decreased anti-influenza class II-restricted CTL activity, pulmonary IFN-γ production and serum-neutralizing Ab levels. Among the CD4+ T-lymphocyte population, dysregulation of T helper 1 (Th1) and T helper 2 (Th2) functions occur and this contributes to the immunological changes observed with ageing. Production of the Th1 cytokines, interleukin (IL)-2 and IFN-γ, decreases with ageing while production of IL-4 (a Th2 cytokine) is similar or increases.5,6 Aged mice produce less IL-2 following influenza infection than do young animals.7 In humans, T cells from older donors produce significantly less IFN-γ than those from young donors following in vitro stimulation with influenza virus.8 These changes in the Th1/Th2 balance can contribute to the delayed clearance and recovery from influenza infection. Th1 clones are cytolytic in vitro against influenza-infected target cells, and adoptive transfer of a Th1 clone protects against lethal challenge with influenza virus in vivo.9,10 However, Th2 clones did not show cytolytic activity and failed to promote recovery from lethal infection after adoptive transfer.10 Furthermore, in vivo treatment with IL-4 suppressed the CTL response and IFN-γ production, and delayed viral clearance.11
IL-1β, IL-6 and tumour necrosis factor-α (TNF-α) levels increase in the early stages of influenza infection and are involved in the initiation of the immune response as well as in the inflammatory response. IL-6 is involved in T-cell activation and represents an essential competence factor that synergizes with IL-1 to control the initial steps of T-cell activation, including induction of IL-2 and enhancement of responsiveness to IL-2.12 TNF-α was reported to have an antiviral effect against influenza virus13 and to regulate the synthesis of IL-6 and IL-1.14
Vitamin E supplementation enhances immune functions of mice and humans. Immunostimulatory effects of vitamin E include increased an antibody titre to hepatitis B and tetanus vaccine, a delayed-type hypersensitivity (DTH) skin response, production of IL-2 and a mitogenic response to concanavalin A (Con A).15,16 The immunostimulatory effects of vitamin E are mediated, in part, by reduced prostaglandin E2 (PGE2) synthesis.15 PGE2 was shown to have a direct inhibitory effect on an early stage of T-cell activation, resulting in decreased IL-2 production, decreased IL-2 receptor expression, decreased responsiveness to exogenous IL-2 and decreased proliferation.17 In addition, PGE2 suppresses IL-12 production by monocytes and reduces IL-12 receptor expression in peripheral blood mononuclear cells (PBMC) and purified T cells, resulting in inhibition of Th1 cell differentiation and reduced production of Th1 cytokines.18,19
Previously, we showed that influenza-infected old mice fed a diet high in vitamin E (500 parts per million [ppm]) had a significantly lower lung viral titre than those fed a diet containing an adequate level of vitamin E (30 ppm).20 The effect of vitamin E is not mediated through enhancing CTL activity, as evidenced by the lack of effect of vitamin E supplementation on primary pulmonary CTL activity.20 NK activity was significantly higher in old mice fed a vitamin E-supplemented diet than old mice fed a diet containing an adequate level of vitamin E. However, the magnitude of decrease in viral titre (25-fold) could not be solely explained by the increased NK activity (threefold). In this study we investigated the effect of vitamin E on Th1 and Th2 cytokine production by splenocytes following influenza infection in order to delineate further the mechanism of how vitamin E lowers the influenza viral titre in aged mice.
Materials and methods
Animals and infection
Seventy specific pathogen-free young (4 months old) male and 70 specific pathogen-free aged (22 months old) male C57BL/6NCrlBR mice bred in our colony were individually housed in filtered cages in an environmentally controlled atmosphere (temperature 23°; 45% relative humidity) with a 12-hr light 12-hr dark cycle. All conditions and handling of the animals were approved by the Animal Care and Use Committee at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University and conducted according to the National Institutes if Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
Mice were fed semipurified diets containing either 30 ppm. (control diet) or 500 ppm (supplemented diet) of dl-α-tocopherol acetate (vitamin E) for 8 weeks. The composition of the diet was the same as that previously described.21 After the 8-week dietary period, mice were anaesthetized with an intraperitoneal (i.p.) injection of a combination of ketamine and acepromazine (0·5 mg ketamine and 0·02 mg acepromazine for young mice and 0·75 mg ketamine and 0·03 mg acepromazine for old mice) and infected intranasally with 40 µl of influenza A/Port Chalmers/1/73 (H3N2; 50% tissue culture infective dose [TCID50] 106·75/10 µl).22 Mice (six to nine mice/group) were then killed via CO2 asphyxiation on days 0, 2, 5, or 7 after infection. Lungs and spleens were harvested on days 0, 2, 5 and 7 and peritoneal macrophages (Mφ) were collected on day 0.
Tissue preparation
Lungs were aseptically removed and processed as previously described.23 Lungs were cut into small pieces and incubated at 37° for 1 hr in 6 ml of Iscove's medium (Gibco, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (FBS; Gibco) and 40 U/ml of collagenase (Sigma, St. Louis, MO). Lung pieces were ground and washed through a 60-mesh stainless steel screen. After centrifugation at 400 g for 5 min, supernatant was removed and frozen for virus quantification.
Spleens were aseptically removed and placed in sterile endotoxin-free RPMI-1640 (BioWhittaker, Walkerville, MD) media supplemented with 25 mm HEPES (Gibco), 2 mm glutamine (Gibco), 100 U/ml of penicillin and 100 µg/ml of streptomycin (Gibco) (complete ETRPMI). Single-cell suspensions were prepared by gently disrupting spleens between two sterile frosted glass slides. Splenocytes were isolated by centrifugation at 400 g and red blood cells were lysed using Gey's reagent. Splenocytes were washed twice with complete ETRPMI and viability was determined by Trypan Blue exclusion. Splenocytes were suspended in complete ETRPMI (containing 10% heat-inactivated FBS) at appropriate concentrations for different cultures.
Peritoneal exudate cells (PEC) were obtained by peritoneal lavage with cold Ca2+- and Mg2+-free Hanks' balanced salt solution (Gibco). PEC were enriched for Mφ using the method of Kumagai et al.24 Cells were plated at 5 × 105 cells/well on 24-well plates in complete ETRPMI medium with 5% FBS. The cells were allowed to adhere for 2 hr at 37° in 5% CO2, at which time non-adherent cells were removed by washing. Peritoneal Mφ prepared in this manner were at least 90% Mφ, as assessed by Mac-1 and F4/80 cell-surface antibodies. The percentage of Mφ that adhered to the plates did not differ among the different age and diet groups (data not shown).
Determination of lung virus titre
Lung virus titre was measured as previously described.22 Lung supernatant was added to round-bottom 96-well plates, in triplicate, in serial 10-fold dilutions prepared in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 2·5 µg/ml of amphotericin B (AmpB, Sigma), 5 µl/ml of gentamicin solution (Sigma) and 10% FBS, and then Madin Darby canine kidney (MDCK) cells (3 × 104 cells/well) were added. The plates were incubated at 35° for 24 hr and the media was replaced with DMEM (Sigma) containing AmpB, gentamicin and 0·0002% trypsin (Worthington, Freehol, NJ). After 3 days of incubation, 50 µl of 0·5% fresh chicken red blood cell suspension was added and tested for haemagglutination. The TCID50 was calculated using the method of Reed & Muench.25
Cytokine production and measurement
For IL-2, IFN-γ and IL-4 production, splenocytes were cultured at 5 × 106 cells/well in the presence of Con A (5 µg/ml; Sigma), in 24-well culture plates (Becton Dickinson) for 24 hr. For IL-1β, IL-6 and TNF-α production, splenocytes were cultured at 5 × 106 cells/well in the presence of lipopolysaccharide (LPS) (10 µg/ml; Sigma), in 24-well culture plates for 24 hr. Cell-free supernatants were collected and stored at −70° for later analysis. The concentrations of IL-2, IFN-γ, IL-4, IL-6, TNF-α and IL-1β were measured using enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions, with rat anti-mouse IL-2, IFN-γ, IL-4, IL-6 or TNF-α monoclonal antibodies (mAbs) (PharMingen, San Diego, CA) or rat anti-mouse IL-1β polyclonal antibody (Endogen, Cambridge, MA) and biotinylated rat anti-mouse IL-2, IFN-γ, IL-4, IL-6, TNF-α or IL-1β.
PGE2 production and measurement
Peritoneal Mφ were isolated and plated (5 × 105 cells/well) as described previously.21 One millilitre of ETRPMI containing 5% FBS, with either 0 or 5 µg/ml of LPS, was added to each well. Plates were incubated at 37° in 5% CO2 for 48 hr, after which the supernatant was collected and stored at −70° for analysis. PGE2 production was measured by radioimmunoassay, as previously described.26
Statistical analysis
Data were analysed using analysis of variance (anova) for overall effect of age, infection and diet, and then by Fisher's least significant difference post hoc test for individual comparisons, using the systat (1992) statistical package (SYSTAT, Evanston, IL). Pearson correlation was used to determine association between lung viral titres and levels of IFN-γ production by splenocytes. Data are reported as mean + SEM. Significance was set at P < 0·05.
Results
Effects of age and vitamin E supplementation on pulmonary influenza viral titre
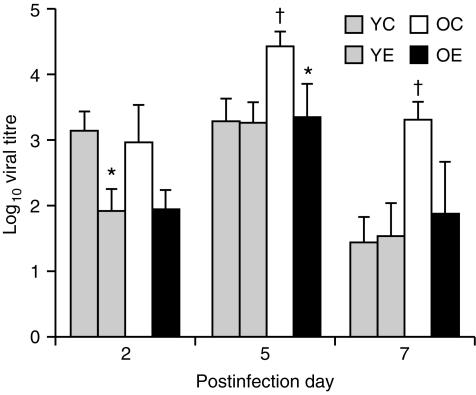
There were significant effects of age (P < 0·05) and diet (P < 0·001) on pulmonary influenza viral titre. On days 5 and 7 after infection, old mice fed the control diet had significantly higher viral titres than young mice fed the control diet (P < 0·05). Old mice fed the vitamin E diet had a significantly lower viral titre on day 5 (P < 0·05) and tended to have a lower viral titre on days 2 and 7 (P < 0·1) compared with those fed the control diet (Fig. 1). Young mice fed the vitamin E diet had a lower viral titre than young mice fed the control diet on day 2 only (P < 0·05).
Figure 1.
Pulmonary viral titres following infection with H3N2 influenza virus in young (6 months) and old (24 months) C57BL/6NCrlBR mice fed diets containing 30 parts per million (ppm) (control) or 500 ppm (supplemented) of dl-α-tocopheryl acetate (vitamin E) for 8 weeks. Values are expressed as mean + SEM, n = 6–9. †Significantly higher than young mice fed the control diet, at P < 0·05. *Significantly different from mice of the same age fed the control diet, as determined by Fisher's least significant test at P < 0·05. YC, young mice fed the control diet; YE, young mice fed the vitamin E-supplemented diet; OC, old mice fed the control diet; OE, old mice fed the vitamin E-supplemented diet.
Effects of age and vitamin E supplementation on IL-2, IFN-γ and IL-4 production by splenocytes following influenza infection
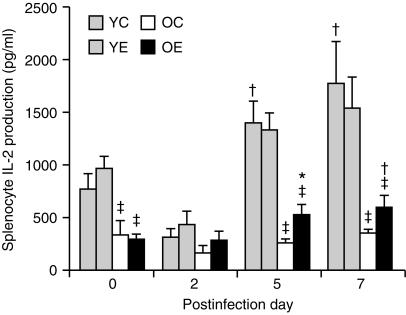
There were significant effects of age (P < 0·001) and infection (P < 0·001) on IL-2 production by splenocytes. There was also a significant age and infection interaction (P < 0·001). Old mice produced a significantly lower amount of IL-2 than young mice on days 0, 5 and 7 (P < 0·05). The level of IL-2 production by old mice on day 0 was ≈ 30% of that of the young mice. In old mice, there was a significant effect of infection (P < 0·05) and diet (P < 0·05) on IL-2 production (Fig. 2); in old mice fed the control diet, IL-2 production did not increase with progression of infection, whereas old mice fed the vitamin E diet had a higher IL-2 production on day 5 (530 + 100 pg/ml, P < 0·1) and day 7 (599 + 110 pg/ml, P < 0·05) than on day 0 (290 + 57 pg/ml). Old mice fed the vitamin E diet had a significantly higher IL-2 production on day 5 (P < 0·05) than old mice fed the control diet. In young mice, there was a significant effect of infection (P < 0·001), but not of diet, on IL-2 production.
Figure 2.
Interleukin (IL)-2 production (following infection with influenza) by splenocytes from young and old mice fed diets containing 30 parts per million (ppm) (control) or 500 ppm (supplemented) vitamin E for 8 weeks. Splenocytes (5 × 106 cells/well) were stimulated with concanavalin A (Con A) (5 µg/ml) for 24 hr. Values are expressed as mean + SEM, n = 4–9. *Significantly different from mice fed the control diet, as determined by Fisher's least significant test at P < 0·05. †Significantly different from day 0 of the same age and diet group, as determined by Fisher's least significant test at P < 0·05. ‡Significantly lower than young mice of the same diet and postday at P < 0·05. YC, young mice fed the control diet; YE, young mice fed the vitamin E-supplemented diet; OC, old mice fed the control diet; OE, old mice fed the vitamin E-supplemented diet.
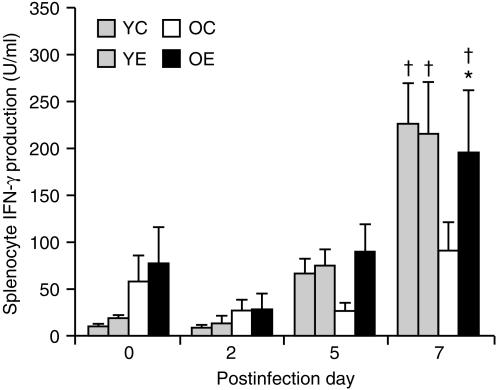
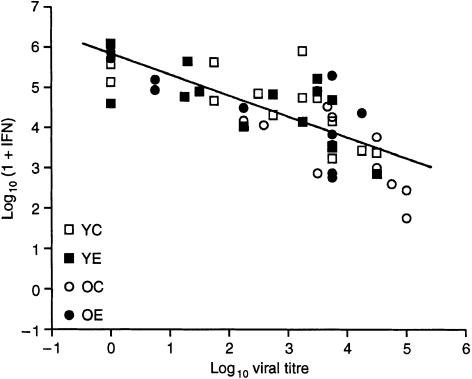
There were significant effects of infection (P < 0·001) and diet (P = 0·06), but not age, on IFN-γ production by splenocytes. There was also a significant age and infection interaction (P < 0·001). In young mice, there was a significant overall effect of infection (P < 0·001), but not diet, on IFN-γ production. Following infection with influenza, IFN-γ production increased in young mice, with higher levels observed on day 7 (P < 0·001) than on day 0. Overall, there was a significant effect of vitamin E supplementation (P < 0·05) and infection (P < 0·001) on IFN-γ production in old mice. In old mice fed the control diet, there was no significant increase of IFN-γ production following infection. However, old mice fed the vitamin E diet had a significantly higher IFN-γ production on day 7 than on day 0 (197 + 67 U/ml on day 7 versus 78 + 38 U/ml on day 0, P < 0·05). The level of IFN-γ produced on day 7 by splenocytes from old mice fed the vitamin E diet was significantly higher (P < 0·05) than that from old mice fed the control diet (Fig. 3). On days 5 and 7, a significant, inverse correlation was observed between IFN-γ levels and viral titres (r = − 0·721, P < 0·001, n = 49) (Fig. 4) indicating that higher IFN-γ production is associated with lower pulmonary viral titre. There was also a weaker (r = 0·39), but significant (P < 0·05), correlation between IL-2 levels and viral titres. There was a positive correlation between IL-2 and IFN-γ levels (r = 0·558, P < 0·05).
Figure 3.
Interferon-γ (IFN-γ) production (following infection with influenza) by splenocytes from young and old mice fed diets containing 30 parts per million (ppm) (control) or 500 ppm (supplemented) vitamin E for 8 weeks. Splenocytes (5 × 106 cells/well) were stimulated with concanavalin A (Con A) (5 µg/ml) for 24 hr. Values are expressed as mean + SEM, n = 4–9. *Significantly different from mice of the same age fed the control diet, as determined by Fisher's least significant test at P < 0·05. †Significantly different from day 0 of the same age and diet group, as determined by Fisher's least significant test at P < 0·05. YC, young mice fed the control diet; YE, young mice fed the vitamin E-supplemented diet; OC, old mice fed the control diet; OE, old mice fed the vitamin E-supplemented diet.
Figure 4.
Correlation between interferon-γ (IFN-γ) levels and pulmonary viral titres on days 5 and 7. A significant, inverse correlation was observed between IFN-γ levels and viral titres (r = − 0·721, P < 0·001, n = 49), as determined by Pearson correlation.
There was no significant overall effect of age, diet, or infection on IL-4 production by splenocytes (data not shown).
Effects of age and vitamin E supplementation on IL-6, IL-1β and TNF-α production by splenocytes following influenza infection
Overall, there was no significant effect of age, infection or diet on IL-6 production by splenocytes (data not shown).
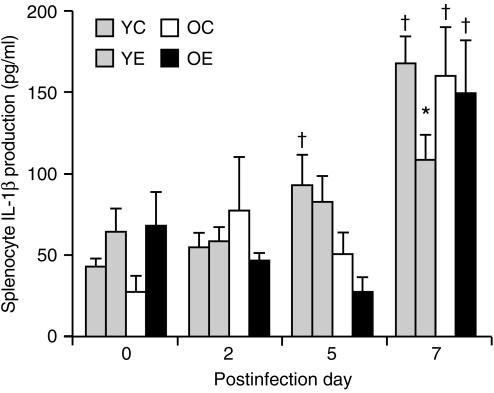
There were significant effects of infection (P < 0·001) and a significant age and infection interaction (P < 0·05) in IL-1β production by splenocytes. There was no significant age difference in IL-1β production by splenocytes. In young mice, there was a significant effect of infection (P < 0·001), but not of diet. IL-1β production increased significantly on days 5 and 7 (P < 0·05) after infection in young mice fed the control diet but not in those fed the vitamin E diet. On day 7, young mice fed the vitamin E diet had a significantly lower production of IL-1β than those fed the control diet (P = 0·009). IL-1β production had increased significantly by day 7 following infection in old mice fed either diet. There was no effect of vitamin E supplementation on IL-1β production in old mice (Fig. 5).
Figure 5.
Interleukin-1β (IL-1β) production (following infection with influenza) by splenocytes from young and old mice fed diets containing 30 parts per million (ppm) (control) or 500 ppm (supplemented) vitamin E for 8 weeks. Splenocytes (5 × 106 cells/well) were stimulated with lipopolysaccharide (LPS) (10 µg/ml) for 24 hr. Values are expressed as mean + SEM, n = 4–9. *Significantly different from mice fed the control diet, as determined by Fisher's least significant test at P < 0·05. †Significantly different from day 0 of the same age and diet group, as determined by Fisher's least significant test at P < 0·05. YC, young mice fed the control diet; YE, young mice fed the vitamin E-supplemented diet; OC, old mice fed the control diet; OE, old mice fed the vitamin E-supplemented diet.
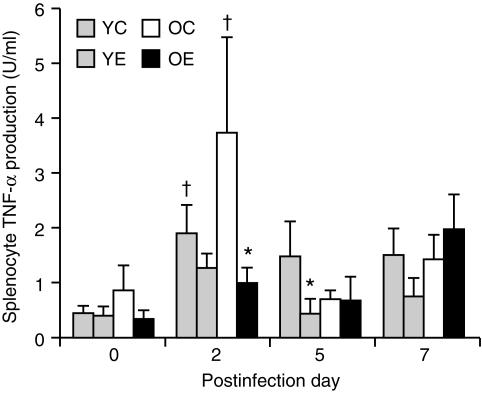
There was no age difference in TNF-α production by splenocytes. However, there were significant effects of infection (P < 0·001) and diet (P < 0·05) on the production of TNF-α levels. In young mice fed the control diet, TNF-α production was significantly higher on day 2 (P < 0·05) than on day 0. However, in young mice fed the vitamin E diet, production of TNF-α (on any day following infection) was not significantly higher than on day 0. Young mice fed the vitamin E diet had a significantly lower production of TNF-α on day 5 than those fed the control diet (P < 0·05). In old mice fed the control diet, levels of TNF-α production on day 2 were significantly higher than on day 0 (P < 0·01). However, in old mice fed the vitamin E diet, the level of TNF-α production following influenza infection was not significantly higher than on day 0. On day 2, old mice fed the vitamin E diet had significantly lower TNF-α production than those fed the control diet (P < 0·01) (Fig. 6).
Figure 6.
Tumour necrosis factor-α (TNF-α) production (following infection with influenza) by splenocytes from young and old mice fed diets containing 30 parts per million (ppm) (control) or 500 ppm (supplemented) vitamin E for 8 weeks. Splenocytes (5 × 106 cells/well) were stimulated with lipopolysaccharide (LPS) (10 µg/ml) for 24 hr. Values are expressed as mean + SEM, n = 4–9. *Significantly different from mice fed the control diet, as determined by Fisher's least significant test at P < 0·05. †Significantly different from day 0 of the same age and diet group, as determined by Fisher's least significant test at P < 0·05. YC, young mice fed the control diet; YE, young mice fed the vitamin E-supplemented diet; OC, old mice fed the control diet; OE, old mice fed the vitamin E-supplemented diet.
Effects of age and vitamin E supplementation on PGE2 production by Mφ
As shown in Table 1, there were significant overall effects of age and diet (P < 0·005), as well as a significant age and diet interaction (P < 0·001), on LPS-stimulated Mφ PGE2 production. Old mice fed the control diet had significantly higher levels of PGE2 production than young mice fed the control or vitamin E diet and old mice fed the vitamin E diet (P < 0·001). There was a significant effect of vitamin E supplementation on PGE2 production in old mice but not in young mice. Mφ from old mice fed the vitamin E diet produced significantly less PGE2 than those from old mice fed the control diet (P < 0·001).
Table 1.
Effect of vitamin E supplementation on prostaglandin E2 (PGE2) production (ng/ml) by macrophages (Mφ) from uninfected (day 0) young and old C57BL/6NCrlBR mice
| Young | Old | |||
|---|---|---|---|---|
| Control | Vitamin E | Control | Vitamin E | |
| Medium | 2·09 + 0·48 | 1·80 + 0·21 | 2·61 + 0·08 | 2·00 + 0·09 |
| LPS stimulated | 12·55 + 1·08 | 14·94 + 1·16 | 21·91 + 1·43† | 12·69 + 0·52* |
Values are expressed as mean + SEM, n = 8.
Significantly different from old mice fed the control diet, as determined by Fisher's least significant test (P < 0·05).
Significantly different from young mice, as determined by Fisher's least significant test (P < 0·001).
LPS, lipopolysaccharide.
Discussion
To determine the mechanism of vitamin E-induced reduction in influenza viral titre of old mice, we investigated the effect of vitamin E supplementation on splenocyte Th1 and Th2 cytokine production following influenza infection. Our results confirm that there is a loss of Th1 function with ageing. Most importantly, vitamin E supplementation can increase the production of Th1 cytokines following influenza infection; the increase in IFN-γ is also associated with lower pulmonary viral titres.
Although CD8+ CTL activity is important for the clearance of influenza virus, it is not the sole mechanism responsible for the control of influenza infection. Recently, CD4+ T cells, especially Th1 cells, were shown to play an important role in the protection against and recovery from influenza infection. For example, Scherle et al.27 showed that adoptive transfer of virus-specific major histocompatibility complex (MHC) class II-restricted Th cell clones can reduce the mortality and lung viral titres in nude mice infected with influenza virus. Tamura et al.28 showed that nasal Th1 cells, capable of producing IFN-γ and mediating DTH, are involved in the type-specific acceleration of recovery from influenza after challenge in mice immunized intranasally with adjuvant-combined recombinant nucleoprotein (NP). In addition, injection of DNA encoding influenza virus NP induced lymphoproliferation of CD4+ T cells and Th1 cytokine secretion in mice. Transfer of CD4+ T cells from NP DNA-vaccinated mice resulted in complete protection from death.29 While induction of a Th1 cytokine profile is thought to mediate protective function against influenza infection, the Th2 subset of CD4+ T cells may not play a primary role in virus clearance and recovery.10 Rather, treatment of mice with IL-4, a Th2 cytokine, resulted in a significant delay in influenza virus clearance.11
Dysregulation of CD4+ T-cell function is observed with ageing. While decreased production of and response to IL-2 seems to be a common phenomenon in ageing,5 IFN-γ production in the aged was reported to be lower,30 show no difference,31 or be higher6 than in the young. However, aged mice have a decreased ability to produce IFN-γ following infection with Legionella pneumophila32 or influenza,4,33 whereas IL-4 production seems to be the same or higher5,6 in aged mice than in young mice.
In this study, we observed production of significantly lower IL-2 levels (30% of the young) and similar levels of IFN-γ or IL-4 in uninfected aged mice compared with young mice. Production of lower levels of IL-2 and IFN-γ on day 2 in both young and old mice suggests a reduced ability to defend against viral infection. Lower lymphocyte proliferation on day 2 (S. N. Han et al., unpublished) also suggests an immunosuppressive effect of viral infection. There was a clear age-related difference in IL-2 and IFN-γ production following influenza infection, with significantly lower levels in aged compared with young mice. While IL-2 and IFN-γ production in young mice had increased significantly by days 5 and 7, old mice fed the control diet failed to produce significantly higher levels of these cytokines. Overall, our results indicate that old mice fed the control diet were unable to induce an efficient Th1 response following influenza infection. With vitamin E supplementation, IL-2 and IFN-γ production increased significantly following influenza infection in old mice.
Although significantly affected by vitamin E, IL-2 production in old mice fed the vitamin E diet was still significantly lower than that of young mice. In contrast, there was no significant difference in the level of IFN-γ produced by old mice fed the vitamin E diet and that of young mice (≈ 100% higher than that of old mice fed the control diet). In addition, there was a significant inverse correlation between viral titre and IFN-γ production. IFN-γ plays an important role in regulating influenza infection via direct antiviral activities, activation of alveolar Mφ and NK cells, up-regulation of MHC expression and control of antibody class switching.34 Thus, the vitamin E-induced increase in IFN-γ may play an important role in its beneficial effect on reducing viral titre in old mice.
Previously, we showed that vitamin E supplementation can inhibit cyclooxygenase activity and decrease PGE2 production in old mice.21,35 Consistently, in the current study, peritoneal Mφ isolated from uninfected old mice fed the vitamin E diet produced a significantly lower level of PGE2 than those isolated from uninfected old mice fed the control diet. PGE2 has a key role in down-regulating the Th1 response36 and PGE2 production has been shown to be increased with ageing.37 Our data therefore suggest that the increased Th1 response observed in this study with vitamin E supplementation may be caused by a decreased production of PGE2.
Vitamin E supplementation had some effect on production of IL-1β and TNF-α by splenocytes in both young and old mice. Young mice fed the vitamin E diet had a significantly lower IL-1β production on day 7 and significantly lower TNF-α production on day 5 than did young mice fed the control diet. Old mice fed the vitamin E diet had a significantly lower TNF-α production on day 2 compared with old mice fed the control diet. TNF-α production reached its maximum level on day 2 while the highest IL-1β production was observed on day 7. Increased production of these cytokines reflects the inflammatory response to the virus but their effect on influenza virus replication is not clear. It seems that neither IL-1β nor TNF-α plays an important role in the lowered viral titre observed with vitamin E supplementation because their production decreased with vitamin E supplementation.
This study demonstrates that old mice are unable to produce a strong Th1 response upon challenge with influenza virus. Vitamin E supplementation enhances the Th1 response in old mice which, at least in part, explains its beneficial effect in reducing viral titres of old mice infected with influenza virus. This effect of vitamin E is probably mediated through a reduction in PGE2 production.
Acknowledgments
This material is based upon work supported by the U.S. Department of Agriculture, under agreement no. 58-1950-9-001. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. The authors thank Dr Hong Wang for technical assistance and Ms Joanne Meegan for preparation of this manuscript.
References
- 1.Pinner RW, Teutsch SM, Simonsen L, et al. Trends in infectious diseases mortality in the United States. JAMA. 1996;275:189. [PubMed] [Google Scholar]
- 2.CDC. Pneumonia and influenza death rates-United States. MMWR. 1979;1995:535. [PubMed] [Google Scholar]
- 3.Bender BS, Johnson MP, Small PA., Jr Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology. 1991;72:514. [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor SF, Cottey RJ, Zander DS, Bender BS. Influenza infection of β2-microglobulin-deficient (β2m–/–) mice reveals a loss of CD4+ T cell functions with aging. J Immunol. 1997;159:3453. [PubMed] [Google Scholar]
- 5.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs MV, Weigle WO, Noonan DJ, et al. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602. [PubMed] [Google Scholar]
- 7.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983;81:298. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- 8.Mbawuike I, Acuna CL, Walz KC, Atmar RL, Greenberg SB, Couch RB. Cytokines and impaired CD8+ CTL activity among elderly persons and the enhancing effect of IL-12. Mech Ageing Dev. 1997;94:25. doi: 10.1016/s0047-6374(96)01855-6. [DOI] [PubMed] [Google Scholar]
- 9.Lukacher A, Morrison L, Braciale T. T lymphocyte function in recovery from experimental viral infection: the influenza model. In: Steinman R, North R, editors. Mechanisms of Host Resistance to Infectious Agents, Tumors, and Allografts. New York: Rockefeller University Press; 1986. pp. 233–254. [Google Scholar]
- 10.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran TM, Isobe H, Fernandez-Sesma A, Schulman JL. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996;70:5230. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 13.Van Campen H. Influenza A virus replication is inhibited by tumor necrosis factor-alpha in vitro. Arch Virol. 1994;136:439. doi: 10.1007/BF01321073. [DOI] [PubMed] [Google Scholar]
- 14.Beyaert R, Fiers W. In: Tumor Necrosis Factor and Lymphotoxin Cytokines. Mire-Sluis AR, Thrope R, editors. San Diego: Academic Press; 1998. p. 335. [Google Scholar]
- 15.Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandine E2 synthesis and enhances the immune response of aged mice. Mech Ageing Dev. 1986;34:191. doi: 10.1016/0047-6374(86)90034-5. [DOI] [PubMed] [Google Scholar]
- 16.Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy subjects. JAMA. 1997;277:1380. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 17.Vercammen C, Ceuppens JL. Prostaglandine E2 inhibits T-cell proliferation after crosslinking of the CD3-Ti complex by directly affecting T cells at an early step of the activation process. Cell Immunol. 1987;104:24. doi: 10.1016/0008-8749(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 18.van der Pouw Kraan TCTM, Boeije LCM, Smeenk RJT, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C-Y, Wang K, McDyer JF, Sedar RA. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol. 1998;161:2723. [PubMed] [Google Scholar]
- 20.Hayek MG, Taylor SF, Bender BS, et al. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis. 1997;176:273. doi: 10.1086/517265. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Mura C, Beharka AA, et al. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol. 1998;275:C661. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- 22.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender BS, Taylor SF, Zander DS, Cottey R. Pulmonary immune response of young and aged mice after influenza challenge. J Lab Clin Med. 1995;126:169. [PubMed] [Google Scholar]
- 24.Kumagai K, Itoh K, Hinuma S, Tada M. Pretreatment of plastic petri dishes with fetal calf serum: a simple method for macrophage isolation. J Immunol Methods. 1979;29:17. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- 25.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1937;27:493. [Google Scholar]
- 26.Meydani SN, Dupont J. Effect of zinc deficiency on prostaglandin synthesis in different organs of the rat. J Nutr. 1982;112:1098. doi: 10.1093/jn/112.6.1098. [DOI] [PubMed] [Google Scholar]
- 27.Scherle PA, Palladino G, Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J Immunol. 1992;148:212. [PubMed] [Google Scholar]
- 28.Tamura S-I, Miyata K, Matsuo K, et al. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892. [PubMed] [Google Scholar]
- 29.Ulmer JB, Fu T-M, Deck RR, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abb J, Abb H, Deinhardt F. Age-related decline of human interferon alpha and interferon gamma production. Blut. 1984;48:285. doi: 10.1007/BF00320399. [DOI] [PubMed] [Google Scholar]
- 31.Sindermann J, Kruse A, Frercks H-J, Schutz RM, Kirchner H. Investigation of the lymphokine system in elderly individuals. Mech Ageing Dev. 1993;70:149. doi: 10.1016/0047-6374(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 32.Fujio H, Kawamura I, Miyamoto H, Mitsuyama M, Yoshida S-I. Decreased capacity of aged mice to produce interferon-gamma in Legionella pneumophila infection. Mech Ageing Dev. 1995;81:97. doi: 10.1016/0047-6374(95)01588-q. [DOI] [PubMed] [Google Scholar]
- 33.Mbawuike IN, Acuna C, Caballero D, et al. Reversal of age-related deficient influenza virus-specific CTL responses and IFN-gamma production by monophosphoryl lipid A. Cell Immunol. 1996;173:64. doi: 10.1006/cimm.1996.0252. 10.1006/cimm.1996.0252. [DOI] [PubMed] [Google Scholar]
- 34.Boeham U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 35.Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93:59. doi: 10.1016/s0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- 36.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108. [PubMed] [Google Scholar]
- 37.Hayek MG, Meydani SN, Meydani M, Blumberg JB. Age differences in eicosanoid production of mouse splenocytes: effects on mitogen-induced T-cell proliferation. J Gerontol. 1994;49:B197. doi: 10.1093/geronj/49.5.b197. [DOI] [PubMed] [Google Scholar]