INTRODUCTION
T-cell activation is a critical event in the organization of effective cellular and humoral immune responses. Activated T cells are essential for provision of T-cell help, promoting the development of high-affinity antibody production and the generation of cytotoxic T-cell responses. Accordingly, defects in proteins required for T-cell activation give rise to significant infectious pathology and malignancies. However, the decision to allow T-cell activation also has potentially dangerous consequences for the host and must therefore also be tightly controlled. Defects in proteins involved in regulating activated T-cell behaviour therefore tend to lead to autoimmunity. Thus, the major challenge faced in regulating T-cell responses is how to maintain a sufficiently large immune repertoire capable of recognizing all possible foreign antigens, whilst at the same time maintaining T cells in an unresponsive state towards self-antigens.
In recent years significant progress has been made in our understanding of the mechanisms of self-tolerance. In the thymus it is clear that large numbers of potentially ‘self-reactive’ T cells are eliminated during negative selection in a process termed central tolerance. However, paradoxically, the process of positive selection that permits the expansion of T cells with low avidity for self–major histocompatibility complex (MHC) interactions must also lead to a degree of self-reactivity which is presumably tolerable in peripheral T cells. The question is how such T cells (albeit weakly self-reactive) can be ensured to remain non-reactive amongst a different array of self-antigens in the periphery. In the last few years a number of proteins have been identified that may serve the function of ‘quality controlling’ peripheral T-cell activation. This review focuses on two proteins, CD28 and cytotoxic T lymphocyte antigen-4 (CTLA-4), and explores how their interactions with their natural ligands may regulate the outcome of T-cell receptor engagement amongst peripheral T cells.
TAKE YOUR PARTNERS: CD28, CTLA-4 AND THEIR LIGANDS
CD28 and CTLA-4 (CD152) are transmembrane protein members of the immunoglobulin gene superfamily containing a single extracellular ‘V-like’ domain.1–3 Both proteins are predominantly expressed by T cells and whilst CD28 is found in substantial amounts on the cell surface of the majority of resting T cells, in contrast CTLA-4 surface expression is much more limited.4 The levels of CTLA-4 expression in most resting T cells are extremely low (or probably absent), and CTLA-4 predominantly appears following T-cell activation. However, despite maximal expression being reported at 48–72 hr post-activation, remarkably little stable surface CTLA-4 is found, although mRNA is equivalent to that of CD28.5,6 This lower level of cell-surface expression results from a motif in the cytoplasmic domain of CTLA-4 that facilitates its interaction with the clathrin pit adaptor complex (AP-50) causing its rapid internalization from the cell surface.7–9 Consequently the majority of CTLA-4 is found in intracellular vesicles that may be then targeted to the cell surface at the site of T-cell receptor (TCR) contact.10 It has been suggested that phosphorylation of the CTLA-4 cytoplasmic domain results in disengagement from the AP-50 internalization system and therefore stabilizes cell-surface expression.8
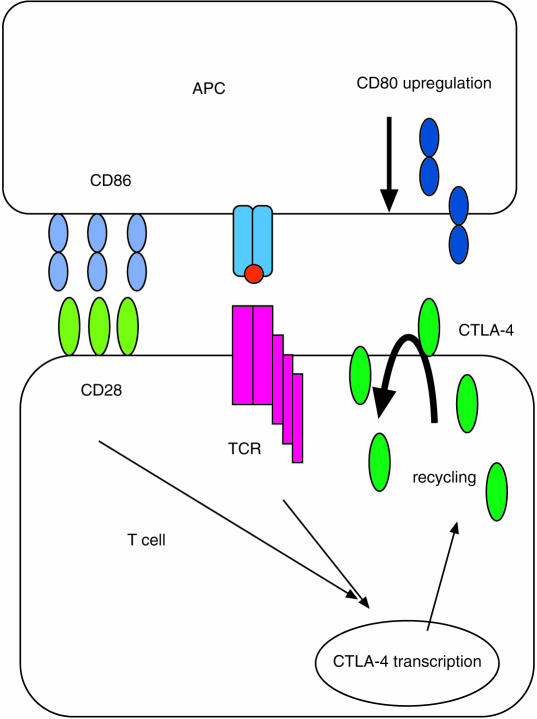
The complexity of the CD28/CTLA-4 receptor interactions stems from the fact that there are two natural ligands CD80 (B7-1) and CD86 (B7-2) for these receptors.11–17 Whilst these ligands can both interact with either receptor, they are only approximately 25% identical in sequence and it has therefore been attractive to speculate that they may serve different functions. Predictably, for co-stimulatory ligands, CD80 and CD86 are found on professional antigen-presenting cells such as dendritic cells, monocytes and activated B cells, although they can be induced on other cell types including T cells.13,17–21 In general CD86 is the more abundant in terms of expression, and is increased more rapidly upon activation. In contrast CD80 is not generally found on resting antigen-presenting cells (APCs) and is induced more slowly upon cellular activation. A large variety of stimuli have been investigated in the control of CD80 and CD86 expression. Most of these, such as CD40, interferon-γ (IFN-γ), interferon-α (IFN-α), granulocyte–macrophage colony-stimulating factor (GM-CSF) and lipopolysaccharide (LPS) appear to result in increased expression18,22–27 whereas others such as interlekin-10 (IL-10) and interleukin-4 (IL-4) may inhibit expression.28–30 These expression studies, together with findings in CD80 and CD86 KO mice,31–33 tend to indicate that CD86 is probably the major initial ligand for CD28 during T-cell activation, based mainly on its more rapid and abundant expression on APCs. However, functional data indicate that CD80 is probably the more potent CD28 ligand in terms of activation,34,35 which is consistent with affinity measurements. Whilst affinity estimates have varied, the interaction of CD80 with both CD28 and CTLA-4 (4 µm and 0·4 µm, respectively) appears substantially better than that of CD86 (approx. 15–40 µm and 4 µm, respectively), although overall these interactions are still relatively weak.36,37 An additional factor in these studies may relate to the fact that structural data indicate that CD80 is expressed as a dimer.38 Thus in summary, CD28 can be considered a highly expressed but low-affinity receptor, whereas CTLA-4 is a low abundance but higher-affinity receptor where both receptors interact with CD80 and CD86. A diagram depicting these interactions in general is shown in Fig. 1.
Figure 1.
A schematic diagram of CD28 and CTLA-4 interactions with their ligands is depicted. CD86 is generally expressed at higher levels on antigen-presenting cells and is found more widely than CD80. CD80 is induced upon activation with a number of stimuli (see text) and is generally expressed at lower levels with later kinetics. Consequently, CD86 is the more likely primary ligand for CD28. On T cells CD28 expression is constitutive whereas CTLA-4 is not expressed by resting T cells. Both T-cell receptor stimulation and CD28 co-stimulation synergize to up-regulate CTLA-4, although CD28 stimulation is not essential. CTLA-4 expression is thought to be transient at the cell surface and rapidly re-internalized by a clathrin pit mechanism. It should be noted that both ligands can interact with both receptors.
CD28: AN ENHANCER FOR T-CELL ACTIVATION
Current interest in the CD28 molecule stems from the concept that efficient activation of T cells requires signals from both the TCR and an additional co-stimulatory receptor. In the absence of this second signal, T cells either remain unresponsive or become actively tolerant to antigens. This concept was stimulated experimentally in a series of experiments by Jenkins et al., which involved the use of chemically modified peptide-pulsed APCs.39,40 These APCs were highly impaired in antigen presentation and T cells subsequently became unresponsive (T-cell anergy) to the same antigen. Anergy could also be prevented by provision of a co-stimulatory signal.41,42 Similar conclusions were reached in studies of transgenic expression of MHC molecules on non-APCs.43,44 Thus the concept of a ‘co-stimulatory signal’, which could rescue from anergy if provided at the same time as TCR engagement, began to emerge and was consistent with the functions of the newly identified CD28- ligand, CD80.12,25 In particular CD28 was shown to be important in enhancement of proliferation and cytokine production by T cells, as well as in the preventing T-cell anergy, thus identifying it as a key second signal for T-cell activation.45–47
Whilst this two-signal model is likely to be an oversimplification (there are an increasing array of alternative co-stimulatory molecules) these studies provided impetus for the concept that T-cell tolerance in the periphery might be maintained by restricting the provision of co-stimulatory signals. This has resulted in the use of a recombinant molecule (CTLA-4–Ig) which acts as a high-affinity antagonist of both CD80 and CD86 co-stimulatory ligands. Results using this protein have demonstrated considerable potential for blocking CD28/CTLA-4 interaction with their ligands.48–52 However, the mechanism by which CTLA-4–Ig works is not entirely clear, as both preventing T-cell activation and engineering tolerance are possible. In this regard several studies have indicated that CTLA-4 engagement may actually be necessary for tolerance induction (see below).53–55 At first sight this appears to be at odds with data from CTLA-4–Ig treatment, which should theoretically remove the ability of CTLA-4 to interact with its only known ligands, CD80 and CD86. One possibility, is that the doses of CTLA-4–Ig used do not entirely blockade CD80/CD86 interactions, but selectively promotes CTLA-4–ligand interactions by restricting the amount of available ligand. Overall, in vivo studies have yielded impressive results in transplantation models and early results in human trials look encouraging.56 Whatever the mechanisms, these studies have provided support for the view that one way to maintain peripheral tolerance is to limit the provision of co-stimulatory signals through CD80 and CD86.
Despite considerable evidence that CD28 is critical in T-cell regulation, it is not entirely clear how its effects are mediated. The signalling pathways emerging from CD28 ligation have been studied in some detail, and have been reviewed elsewhere.57 However, the absolute requirement for CD28 in vivo for T-cell proliferation has been brought into question by CD28KO mice.58–60 Here, the response of T cells to antigen is not as severely impaired as might have been predicted. Nonetheless, there are substantial defects in the maintenance of responses and particularly in T-cell survival, which along with other studies supports a role for CD28 in maintaining T-cell responses.61–64 Perhaps the most striking defect in vivo in CD28 KO mice is the lack of germinal centres, suggesting a gross defect in the ability of T cells to interact with B cells. This feature may well relate to the ability of T cells to express the chemokine receptor CXCR5 which is strongly influenced by CD28.65 Thus CD28-deficient T cells may fail to migrate to appropriate sites of interaction with B cells.
Mechanistically, there is evidence that CD28 may exert its co-stimulatory effects by lowering the threshold for T-cell activation, consistent with the presence of CD28 in lipid ‘rafts’ that are rich in signalling proteins.66,67 In addition, CD28 is also thought to exert effects on the cytoskeleton and promote its reorganization to the TCR contact site.68,69 One of the more interesting recent observations has been the suggestion that CD28 may be involved in the control of a population of CD25+ regulatory T cells.70 Here, both CD28KO and CD80–CD86 double knockout (KO) mice crossed on to a non-obese diabetic (NOD) background demonstrated exacerbated diabetes that may be attributed to the lack of regulatory T cells. This study would suggest that CD28 co-stimulation may well be required for the proliferation and survival of this important T-cell subset.
Overall, there is now an overwhelming body of data implicating CD28 as a critical molecule in the T-cell activation process and inhibition of CD28 functions can prevent or substantially decrease T-cell activation. However, some of these strategies are complicated by the fact that the same ligands also control the functions of CTLA-4, which appears to have opposite functions to CD28.
CTLA-4: AN INHIBITOR OF T-CELL ACTIVATION
Whilst studies on CD28 have demonstrated a co-stimulatory role in T-cell activation, the role of CTLA-4 has been more difficult to elucidate. It is now generally accepted that CTLA-4 plays a role in the inhibition of T-cell activation;71–73 although there are some more controversial suggestions of a stimulatory role.74 Nonetheless, several laboratories have shown that blocking CTLA-4 enhances T-cell proliferation whereas ligating CTLA-4 with agonistic antibodies suppresses T-cell proliferation, consistent with the function of a negative regulator.75 However, the most compelling evidence for a regulatory function for CTLA-4 has come from CTLA-4 knockout mice that develop fatal lymphoproliferative disease at 3–4 weeks of age, suggesting a critical role for CTLA-4 in maintaining self-tolerance.76,77 This phenotype results from polyclonal activation of peripheral T cells that then infiltrate and cause multiorgan destruction. This disease can be effectively cured by preventing CD28 co-stimulation either using CD80 and CD86 double KO mice, CTLA-4–Ig78,79 or by crossing on to single TCR transgenic mice.80–82 In addition the lymphoproliferation has been suggested to be CD4+ dependent.83 Very recently, CTLA-4 blockade in normal mice has been shown to give rise to spontaneous autoimmunity.84 Collectively, these studies suggest that one possible function of CTLA-4 may be to ‘threshold out’ weak TCR engagements that may exist for large numbers of potentially autoreactive circulating T cells. Thus in the absence of CTLA-4, B7 ligands provide unopposed stimulatory signals through CD28 that permit weakly self-reactive T cells to become fully activated.
Whilst the importance of CTLA-4 is unquestioned, the nature of this inhibitory pathway is as yet poorly understood. Data from two laboratories indicate that CTLA-4 blocks T-cell function at a relatively early stage (within 24 hr), preventing up-regulation of activation markers, entry into cell cycle, and the generation of IL-2.72,73 However, most strikingly these functional effects are seen when surface levels of CTLA-4 are undetectable. Our own analysis of CTLA-4 expression in humans (unpublished data) support the view that resting T cells express little or no CTLA-4 but that CTLA-4 transcription can rapidly up-regulate the protein within 6 hr of activation. However, whether this up-regulation is sufficiently rapid to prevent activation, or whether expression is actually a reflection of activation, is not yet clear. Interestingly, where CTLA-4 protein is detected at later timepoints (24–72 hr) after activation, it is exclusively confined to activated, proliferating T cells which, by definition, are not those inhibited through CTLA-4. This poses the question as to whether CTLA-4 is highly efficient in extremely low amounts or whether there are alternative explanations for this early inhibitory function (see below and Fig. 2). When interpreting data using CTLA-4 monoclonal antibodies it is important to consider that anti-CTLA-4 antibodies do not experience competition with CD28, in contrast with the natural ligands, and this may lead to inhibitory responses at significantly lower levels of CTLA-4 expression.
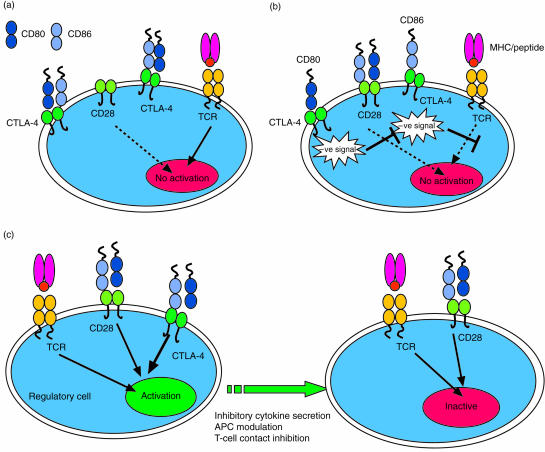
Figure 2.
Three models of CTLA-4 function are shown. (a) The ligand competition model is shown. This model requires that CTLA-4 is expressed at sufficient levels to sequester ligands away from CD28 thus preventing co-stimulatory signals from being received. This model does not require a CTLA-4 signalling component and relies on the higher affinity of CTLA-4 for both ligands compared with CD28. In (b) a CTLA-4 signalling model is shown. Here CTLA-4 ligation results in signals that most likely inhibit T-cell receptor signalling resulting in lack of activation signals. In the third (regulatory cell) model (c), a CTLA-4-expressing regulatory cell is stimulated via CTLA-4 to exert suppression over other T cells. This mode of suppression may be either by cell contact or inhibitory cytokines. It should be noted that none of these mechanisms is mutually exclusive of the others.
Whilst it is clear that natural ligands stimulate CTLA-4 function in vivo, the circumstances under which CTLA-4 function predominates have yet to be clearly established. For example, does CTLA-4 regulate all types of T-cell stimulation or are its effects confined to certain ‘qualities’ of TCR stimulation? So far, the majority of T-cell experiments using transfected ligands in vitro have indicated that engagement of CD80/CD86 in the presence of anti-CD3 effectively delivers proliferative signals via CD28 with relatively little evidence for CTLA-4-dominated functions under these circumstances.
STOP AND GO: THE BALANCE BETWEEN CTLA-4 and CD28
The data discussed above provide a working model in which CD28 enhances and CTLA-4 inhibits T-cell responses yet both interact with the same ligands. This raises the obvious question of ‘How do T cells choose between using CD28 and CTLA-4?’ The answer to this question depends to a large extent on which models of CTLA-4 function are being considered. Several possibilities are outlined below; however, it should be noted that none of the models is mutually exclusive and all of these may operate under defined circumstances.
LIGAND COMPETITION
One of the most frequently cited models of CTLA-4 regulation is the concept that CTLA-4 may act as a competitive inhibitor for the ligands required for CD28 activation (Fig. 2a). Thus, by virtue of its higher affinity, CTLA-4 should be capable of ‘out-competing’ CD28 for ligand binding. An extension of this hypothesis is that CTLA−4 interactions would be favoured where the levels of ligands are low (for example on resting APCs). However the concept that low levels of ligand are relevant to control of CTLA-4 has yet to be convincingly demonstrated. Our own experiments aimed at testing this hypothesis (C. Ellwood et al., submitted) do not generally support this model. Nonetheless, the competition hypothesis is almost certainly correct given the relative affinities of CD28 and CTLA-4 for their ligands. There are, however, some caveats to this hypothesis. Competition is unlikely to have a significant effect in regulating the activation of resting T cells as these express undetectable levels of CTLA-4, making competition highly unlikely at this initial stage. In contrast, activated cells express more CTLA-4 and therefore ligand competition becomes possible during secondary stimulation of T cells with the directed expression of intracellular CTLA-4 at the ‘immunological synapse’.10 This concept is also supported by several studies that indicate more impressive CTLA-4 effects on secondary responses.80,85 In addition, further support for ligand competition comes from studies of CTLA-4 KO mice that have been made transgenic for a CTLA-4 protein lacking a cytoplasmic domain.86 Here, some but not all of the features of CTLA-4 KO mice were prevented, suggesting that competition is a distinct mechanism that accounts for only some of the features of CTLA-4 regulation. Whilst the temporal control of CTLA-4 expression is lost in this model, it nonetheless indicates that competition for ligand can be an effective mode of CTLA-4 operation. The most obvious problem with the competition model is the fact that agonistic antibodies to CTLA-4 act as potent inhibitors of T-cell proliferation.75 Clearly as there are no ligands present in this context, competition alone cannot be the sole mode of CTLA-4 action. This observation provides support for a CTLA-4 signalling mechanism.
CTLA-4 SIGNALLING
As mentioned above, support for a CTLA-4 signalling model (Fig. 2b) comes most clearly from studies with agonistic monoclonal antibodies. Whilst signalling studies have been hampered by the low level of expression of this protein, generally these indicate that CTLA-4 can interfere with TCR-derived signals and block early signalling events.87–90 The fact that CTLA-4 function can be observed in the absence of CD28 expression also supports the view that CTLA-4 may regulate TCR signals.91 One possibility is that CTLA-4 recruits a tyrosine phosphatase SHP-2 (SYP, PTP-1D) through a phosphorylated YVKM motif in the cytoplasmic domain. This interaction is then thought to be involved in de-phosphorylating the TCR signalling machinery, thereby blocking early activation signals. However, in contrast to the models where phosphorylated tyrosine residues in the CTLA-4 cytoplasmic domain are required for recruitment of signalling molecules,88,92 several recent studies have also shown that tyrosine residues are not essential for CTLA-4 function.88,93,94 Thus at present the nature of CTLA-4 inhibitory signalling mechanisms are still unclear. Whilst it is not strictly a part of the signalling model, the most common interpretation of the signalling hypothesis is that each individual T cell undergoes a ‘fate’ decision based on the relative in dominance of CD28 versus CTLA-4 signals (i.e. where CTLA-4 signals are dominant a given T cell will not be activated due to inhibition of TCR and CD28 activation signals). However, this is not the only possible interpretation of CTLA-4 signalling as discussed below.
REGULATORY CELL/CYTOKINE HYPOTHESES
One possible explanation of the biological effects of CTLA-4 observed when CTLA-4 expression levels are very low, is that these effects are actually mediated by a small number of regulatory cells that already express CTLA-4 (Fig. 2c). This possibility is supported by a number of recent findings and overcomes some of the problems discussed above. First, a recent study by Bachmann,95 demonstrated that CTLA-4 functions were not necessarily T-cell autonomous. Here, CTLA-4 KO bone marrow was transferred into RAG2−/− mice either alone or mixed with wild-type CTLA-4 expressing bone marrow. Whilst CTLA-4-deficient bone marrow caused fatal T-cell infiltration of multiple organs, wild-type bone marrow could suppress disease, clearly demonstrating an ability of CTLA-4-positive cells to regulate CTLA-4 negative cells. Other studies have also suggested a link between CTLA-4 and the cytokine transforming growth factor-β (TGF-β).96 This is consistent with similarities between TGF-β KO mice and CTLA-4 KO mice, although this link is still somewhat controversial at present. Consistent with this overall concept is the relatively recent re-emergence of regulatory T cells that cause the development of autoimmunity when removed97,98 (for review see reefs 99,100). Two recent studies, and our own observations, suggest that that a relatively small number of CD4+ CD25+ T cells may also express CTLA-4 (C.Ellwood, unpublished observations).84,101 Thus, the concept that important CTLA-4 functions may be mediated by a rare subset of regulatory T cell that then influences the function of the majority of other T cells is now a distinct possibility.
CD80 AND CD86: WHAT'S THE DIFFERENCE?
Given that CD28 and CTLA-4 have such contrasting functions it is therefore somewhat perplexing that they should share ligands. The most obvious and attractive hypothesis is that these ligands have clearly separate and discrete functions, yet to date this has been extremely difficult to demonstrate convincingly. Nonetheless there are numerous studies that indicate differences between these ligands. Initial studies using transfectants revealed no obvious differences and were consistent with the inescapable conclusion that both ligands are indeed capable of co-stimulation via CD28.102 However, in general, CD80 appears to be the more potent stimulatory ligand.34 Other studies that have attempted to address the differences between ligands have exploited antibody-blocking approaches; however, this has yielded conflicting results. In disease studies, blocking either molecule can exacerbate disease or inhibit disease depending on the model studied.103–105 These and other studies also provide evidence that CD80 or CD86 can bias towards T helper 1 (Th1) or T helper 2 (Th2) phenotypes.106 However, whilst there is no overall consistency, there are sufficient reports to suggest that these pathways do play a role in T-cell differentiation.107,108 One of the major problems in interpreting these studies is that each uses a precise and highly variable set of conditions, e.g. mouse strain, type of antigen, adjuvent use, route of immunization, timing of observation, etc., making any attempts at generalization extremely difficult. It is therefore likely that in many cases, fundamentally different immunological processes are being investigated, which then compounds the problem of defining the specific functions of CD80 and CD86. Given the different models outlined above for CTLA-4 function, then any or all of these mechanisms may be differentially regulated by CD80 or CD86, and it is therefore necessary to know precisely which mechanism is being studied in any given model.
Further attempts to clarify the role of CD80 and CD86 have been made using genetic approaches and in particular KO mice.31–33 Here, the roles of CD80 and CD86 have been confirmed as co-stimulators and the more dominant initial functions of CD86 have been substantiated. These studies also rule out the concept that either CD80 or CD86 is strictly required for Th1 or Th2 cytokine production. However, a different problem emerges with KO approaches for CD28/CTLA-4 ligands in that by removing a given ligand, both the stimulatory and inhibitory functions of that ligand are eliminated. It is therefore theoretically possible that these two opposing effects may compensate for each other and not truly reveal the functions of a given ligand. For example, based on affinity data, CD80 is both the best ligand for CD28 and CTLA-4; therefore, it is conceivable that CD80 knockouts do not display a CTLA-4 KO-like phenotype because the proliferative drive via CD28–CD80 interactions has also been eliminated. Thus, true differences between CD80 and CD86 are an integrated function of their interactions with both CD28 and CTLA-4, and therefore requires study of both pathways together.
Whilst it is difficult to speculate, clear differences have been observed that could support a model where CD80 might be considered a preferential (but not exclusive) inhibitory ligand for CTLA-4. First, the predominant early expression of CD86 on APCs would generally promote the initial establishment of T-cell responses, whereas the later and generally more restricted expression of an inhibitory CD80 molecule could then potentially regulate responses subsequently. Second, blocking antibodies to CD80 clearly exacerbate diabetes in NOD mice.103 This model of disease is also known to be controlled by CTLA-4 regulation at an early stage and T-cell infiltration is exacerbated by CTLA-4 blockade.109 Third, in a transgenic model of diabetes, CD86 expression promoted T-cell infiltration whilst CD80 did not. In this model, if one accepts that CD80 is a more potent co-stimulator, this would argue for an additional role for CD80 in T-cell regulation that is not seen in the CD86 trangenic. Fourth, in transplant models, CD80 has been indicated to be the preferential CTLA-4 ligand controlling graft survival.51 An interesting feature that links these studies is that CTLA-4 function is being studied in the context of spontaneous reactivity to auto- or alloantigens and that T-cell responses are not the result of an immunization protocol in the presence of adjuvant as in some models. One possibility is that these studies may all involve a consistent and distinct ‘mode’ of CTLA-4 function that may contrast with its role in the presence of ‘danger signals’ or during activation of the innate immune system. Fifth, structural data suggest that CD80 is organized as a dimer, which has not been shown for CD86, a factor that may influence its ability to differentially signal via CTLA-4.38 Sixth, transgenic expression of CD80 and CD86 on T cells results in hyperproliferation only in the CD86 transgenic, but not in those expressing CD80.110 Again, this would be consistent with a lack of regulatory ability within CD86. Consistent with this idea, transgenic expression of CD80 has previously been shown to provide a negative regulatory function.111 Finally, our own recent data (C. Ellwood et al., submitted) directly comparing CD80 and CD86 transfectants, only reveal CTLA-4 inhibition when it is ligated to CD80 and not CD86. Whilst these are clearly highly selected arguments, and numerous alternative interpretations are possible, they serve to illustrate that there is still little data that conclusively argue against one ligand performing preferential functions in relation to CTLA-4. Whilst it is clear that both ligands can and do interact with both receptors, their mechanisms of action are thus not necessarily similar. Given the likelihood that several discrete modes of CTLA-4 function exist, it seems that without models where the mode of T regulation by CD28 or CTLA-4 is very clearly defined, it will continue to be difficult to effect comparisons between CD80 and CD86.
The CD28/CTLA-4 pathway offers important opportunities as targets for immunomodulation and insights into our ability to tolerate self-antigens. Whilst our understanding of these molecules has advanced considerably in the last few years, significant questions still remain regarding why two ligands share receptors that have opposing functions. The still unresolved confusion in this area suggests we are in need of more precise models of the functions of each of the participants in order to resolve this important paradox.
REFERENCES
- 1.Brunet JF, Denziot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the Immunoglobulin superfamily-CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 2.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573–7. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barclay AN, Brown MH, Law SKA, McKnight AJ, Tomlinson MG, van der Merwe PA. The Leucocyte Antigen Facts Book. 2. London: Academic Press; 1997. [Google Scholar]
- 4.Alegre M-L, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA-4 on mouse T cells. J Immunol. 1996;157:4762–70. [PubMed] [Google Scholar]
- 5.Linsley P, Ledbetter J. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 6.Freeman GJ, Lombard DB, Gimmi CD, et al. CTLA-4 and CD28 mRNA are coexpressed in most T cells after activation. J Immunol. 1992;149:3795–801. [PubMed] [Google Scholar]
- 7.Chuang E, Alegre ML, Duckett CS, Noel PJ, VanderHeiden MG, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159:144–51. [PubMed] [Google Scholar]
- 8.Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–9. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Allison JP. Interaction of CTLA-4 with AP-50, a clathrin-coated pit adaptor protein. Proc Natl Acad Sci USA. 1997;94:9273–8. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localisation towards sites of TCR engagement. Immunity. 1996;4:535–43. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 11.Linsley PS, Clark EA, Ledbetter JA. T cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB1. Proc Natl Acad Sci USA. 1990;87:5031–5. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azuma M, Ito D, Yagita H, Okumura K, Phillips J, Lanier L, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143:2714–20. [PubMed] [Google Scholar]
- 15.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Lombard LA, Gray GS, Nadler LM. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–12. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, Brady W, Urnes M, Grosmaire L, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262:905–7. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994;152:5208–19. [PubMed] [Google Scholar]
- 19.Sansom DM, Hall ND. B7/BB1, the ligand for CD28 is expressed on repeatedly activated human T cells in vitro. Eur J Immunol. 1993;23:295–8. doi: 10.1002/eji.1830230148. [DOI] [PubMed] [Google Scholar]
- 20.Razi-Wolf Z, Freeman GJ, Galvin F, Benacerraf B, Nadler L, Reiser H. Expression and function of the murine B7 antigen, the major costimulatoty molecule expressed on peritoneal exudate cells. Proc Natl Acad Sci USA. 1992;89:4210–4. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Witmerpack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice – abundant expression on dendritic cells in-situ and during maturation in-vitro. J Exp Med. 1994;180:1849–60. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu MF, Li JS, Weng TH, Lei HY. Differential expression and modulation of costimulatory molecules CD80 and CD86 on monocytes from patients with systemic lupus erythematosus. Scand J Immunol. 1999;49(1):82–7. doi: 10.1046/j.1365-3083.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 23.Radvanyi LG, Banerjee A, Weir M, Messner H. Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 1999;50(5):499–509. doi: 10.1046/j.1365-3083.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 24.Stack RM, Lenschow DJ, Gray GS, Bluestone JA, Fitch FW. IL-4 treatment of small splenic B cells induces costimulatory molecules B7-1 and B7-2. J Immunol. 1994;152(12):5723–33. [PubMed] [Google Scholar]
- 25.Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci USA. 1991;88:6575–9. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman AS, Freeman GJ, Rhynhart K, Nadler LM. Selective induction of B7/BB1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991;137:429–40. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 27.Liu YJ, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2(3):239–48. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 28.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151(3):1224–34. [PubMed] [Google Scholar]
- 29.Buelens C, Willems F, Delvaux A, Pierard G, Delville JP, Velu T, Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25(9):2668–72. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 30.Schweitzer AN, Sharpe AH. Mutual regulation between B7-1 (CD80) expressed on T cells and IL-4. J Immunol. 1999;163(9):4819–25. [PubMed] [Google Scholar]
- 31.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161(6):2762–71. [PubMed] [Google Scholar]
- 32.Schweitzer AN, Borriello F, Wong RCK, Abbas AK, Sharpe AH. Role of costimulators in T cell differentiation – studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–22. [PubMed] [Google Scholar]
- 33.Borriello F, Sethna MP, Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 34.Fields PE, Finch RJ, Gray GS, et al. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J Immunol. 1998;161(10):5268–75. [PubMed] [Google Scholar]
- 35.Olsson C, Michaelsson E, Parra E, Pettersson U, Lando PA, Dohlsten M. Biased dependency of CD80 versus CD86 in the induction of transcription factors regulating the human IL-2 promoter. Int Immunol. 1998;10(4):499–506. doi: 10.1093/intimm/10.4.499. [DOI] [PubMed] [Google Scholar]
- 36.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linsley PS, Greene JL, Bradey W, Bajorth J, Ledbetter JA, Peach R. Human B7-1 (CD80) and 1984, B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 38.Ikemizu S, Gilbert RJ, Fennelly JA, Collins AV, Harlos K, Jones EY, Stuart DI, Davis SJ. Structure and dimerization of a soluble form of B7-1. Immunity. 2000;12(1):51–60. doi: 10.1016/s1074-7613(00)80158-2. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins MK, Pardoll DM, Mizuguchi J, Chused TM, Schwartz RH. Molecular events in the induction of a non responsive state in interleukin 2 producing helper T lymphocyte clones. Proc Natl Acad Sci USA. 1987;84:5409–13. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins MK, Aswell JD, Schwartz RH. Allogeneic non- T spleen cells restore the responsivenes of normal T cell clones stimulated with antigen and chemically modified antigen presenting cells. J Immunol. 1988;140:3324–30. [PubMed] [Google Scholar]
- 42.Schwartz RH. A cell culture model for T lymphocte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 43.Lo D, Burkly LC, Cowing C, Flavell RA, Palmiter RD, Brinster RL. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988;53:159–68. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- 44.Lo D, Burkly LC, Flavell RA, Palmiter RD, Brinster RL. Tolerance in transgenic mice expressing class II major histocompatibility complex on pancreatic acinar cells. J Exp Med. 1989;170:87–104. doi: 10.1084/jem.170.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiser H, Freeman GJ, Razi-Wolf Z, Gimmi C, Benacerraf B, Nadler L. Murine B7 antigen provides an efficient costimulatory signal for activation of murine lymphocytes via the T cell receptor/CD3 complex. Proc Natl Acad Sci USA. 1992;89:271–5. doi: 10.1073/pnas.89.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen specific IL-2 production by human T cells. J Immunol. 1991;147:2461–6. [PubMed] [Google Scholar]
- 47.Harding F, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents the induction of anergy in T cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 48.Lenschow DJ, Zeng Y, Thistlewaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA-4Ig. Science. 1992;257:789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 49.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 50.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6(4):464–9. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 51.Judge TA, Wu Z, Zheng XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999;162(4):1947–51. [PubMed] [Google Scholar]
- 52.Sayegh MH, Zheng XG, Magee C, Hancock WW, Turka LA. Donor antigen is necessary for the prevention of chronic rejection in CTLA4Ig-treated murine cardiac allograft recipients. Transplantation. 1997;64(12):1646–50. doi: 10.1097/00007890-199712270-00003. [DOI] [PubMed] [Google Scholar]
- 53.Lane P, Haller C, McConnell F. Evidence that induction of tolerance in vivo involves active signaling via a B7 ligand-dependent mechanism: CTLA4-Ig protects Vβ8+ T cells from tolerance induction by the superantigen staphylococcal enterotoxin B. Eur J Immunol. 1996;26(4):858–62. doi: 10.1002/eji.1830260420. [DOI] [PubMed] [Google Scholar]
- 54.Perez VL, VanParijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 55.Zheng XX, Markees TG, Hancock WW, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162(8):4983–90. [PubMed] [Google Scholar]
- 56.Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103(9):1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward SG. CD28: a signalling perspective. Biochem J. 1996;318(2):361–77. doi: 10.1042/bj3180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD28 deficient mice. Science. 1993;261:609–12. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 59.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–8. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 60.Mittrucker H, Shahinian A, Bouchard D, Kundig TM, Mak TW. Induction of unresponsiveness and impaired T cell expansion by staphylococcal enterotoxin B in CD28-deficient mice. J Exp Med. 1996;183:2481–8. doi: 10.1084/jem.183.6.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 62.McLeod JD, Walker LSK, Ellwood C, Patel YI, Boulougouris G, Sansom DM. Activation of human T cells with superantigen and CD28 confers resistance to apoptosis by CD95. J Immunol. 1998;160:2072–9. [PubMed] [Google Scholar]
- 63.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–17. [PubMed] [Google Scholar]
- 64.Dahl AM, Klein C, Andres PG, London CA, Lodge MP, Mulligan RC, Abbas AK. Expression of bcl-X (L) restores cell survival, but not proliferation and effector differentiation, in CD28-deficient T lymphocytes. J Exp Med. 2000;191(12):2031–8. doi: 10.1084/jem.191.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker LS, Gulbranson-Judge A, Flynn S, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190(8):1115–22. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–6. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 67.Lezzi G, Karljalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 68.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282(5397):2266–9. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 69.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283(5402):680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 70.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 71.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 72.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y. is CTLA-4 a negative regulator for T cell activation? Immunol Today. 1997;18:569–72. doi: 10.1016/s0167-5699(97)01170-5. [DOI] [PubMed] [Google Scholar]
- 75.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 77.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 78.Tivol EA, Boyd SD, McKeon S, Borriello F, Nickerson P, Strom TB, Sharpe AH. CTLA-4–Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J Immunol. 1997;158:5091–4. [PubMed] [Google Scholar]
- 79.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–40. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur J Immunol. 1998;28(10):3137–43. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 81.Waterhouse P, Bachmann MF, Penninger JM, Ohashi PS, Mak TW. Normal thymic selection, normal viability and decreased lymphoproliferation in T cell receptor-transgenic CTLA-4-deficient mice. Eur J Immunol. 1997;27(8):1887–92. doi: 10.1002/eji.1830270811. [DOI] [PubMed] [Google Scholar]
- 82.Bachmann MF, Waterhouse P, Speiser DE, McKall-Faienza K, Mak TW, Ohashi PS. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J Immunol. 1998;160(1):95–100. [PubMed] [Google Scholar]
- 83.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ cells. Immunity. 1997;7:885–95. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak T, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ regulatroy T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–9. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chambers CA, Kuhns MS, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4 (+) T cell responses. Proc Natl Acad Sci USA. 1999;96(15):8603–8. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164(10):5319–27. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 87.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162(10):5813–20. [PubMed] [Google Scholar]
- 88.Marengere LEM, Waterhouse P, Duncan G, Mittrucker H, Feng G, Mak TW. Regulation of T cell receptor signalling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–3. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 89.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 90.Olsson C, Riebeck K, Dohlsten M, Michaelsson E. CTLA-4 ligation suppresses CD28-induced NF-kappaB and AP-1 activity in mouse T cell blasts. J Biol Chem. 1999;274:14400–5. doi: 10.1074/jbc.274.20.14400. [DOI] [PubMed] [Google Scholar]
- 91.Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med. 1998;188(1):205–10. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider H, Prasad VS, Shoelson SE, Rudd CE. CTLA-4 binding to lipid linkase phosphatidyl-3-kinase in T cells. J Exp Med. 1995;181:351–6. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baroja ML, Luxenberg D, Chau T, Ling V, Strathdee CA, Carreno BM, Madrenas J. The inhibitory function of CTLA-4 does not require its tyrosine phosphorylation. J Immunol. 2000;164(1):49–55. doi: 10.4049/jimmunol.164.1.49. [DOI] [PubMed] [Google Scholar]
- 94.Nakaseko C, Miyatake S, Iida T, Hara S, Abe R, Ohno H, Saito Y, Saito T. Cytotoxic T lymphocyte antigen 4 (CTLA-4) engagement delivers an inhibitory signal through the membrane-proximal region in the absence of the tyrosine motif in the cytoplasmic tail. J Exp Med. 1999;190(6):765–74. doi: 10.1084/jem.190.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163(3):1128–31. [PubMed] [Google Scholar]
- 96.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-β) production by murine CD4 (+) T cells. J Exp Med. 1998;188(10):1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. [PubMed] [Google Scholar]
- 98.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162(9):5317–26. [PubMed] [Google Scholar]
- 99.Groux H, Powrie F. Regulatory T cells and inflammatory bowel disease. Immunol Today. 1999;20(10):442–5. doi: 10.1016/s0167-5699(99)01510-8. [DOI] [PubMed] [Google Scholar]
- 100.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 101.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lanier L, O'fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 103.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th-1 and Th-2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–93. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 105.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 106.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 107.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 response. J Immunol. 1999;162:5784–91. [PubMed] [Google Scholar]
- 108.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 109.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–32. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu X, Fournier S, Allison JP, Sharpe AH, Hodes RJ. The role of B7 costimulation in CD4/CD8 T cell homeostasis. J Immunol. 2000;164(7):3543–53. doi: 10.4049/jimmunol.164.7.3543. [DOI] [PubMed] [Google Scholar]
- 111.Sethna MP, Van Parijs L, Sharpe AH, Abbas AK, Freeman GJ. A negative regulatory function of B7 revaled in B7-1 transgenic mice. Immunity. 1994;1:415–21. doi: 10.1016/1074-7613(94)90072-8. [DOI] [PubMed] [Google Scholar]