Abstract
Rearrangement of gene segments occurs in T lymphocytes during thymic development as the T-cell receptor (TCR) is first expressed, allowing T cells to become central regulators of antigen specificity in the acquired immune system. However, further development of T cells occurs after population of peripheral lymphoid tissues, which can result in T-cell expansion and differentiation into effectors of various immune function, or progression to memory T cells, anergic cells or death by apoptosis. This review focuses on more recent developments concerning the choices that peripheral T cells make between first encountering antigen through TCR recognition and death. These decisions are associated with a process of genetic reprogramming that alters the behaviour of cells so that immune responses are appropriately regulated.
INTERACTION OF T-CELL RECEPTOR and COSTIMULATORY MOLECULE SIGNALLING IN ACTIVATION OF NAIVE T CELLS
Naive T cells have a high threshold for activation in order to prevent T-cell responses to self-antigens, particularly those which are not expressed in the thymus. This generally requires the antigen to be presented by a dendritic cell (DC). This contrasts with effector/memory cells that have lower thresholds of activation owing to their need to activate less efficient antigen-presenting cells (APCs), such as B cells and macrophages, in order to effect a response. DCs express particularly high levels of major histocompatibility complex (MHC) molecules, which will increase the likelihood of full T-cell activation after interaction with a T cell. However, it is the high and constitutive expression of costimulatory molecules that makes DCs so critical for the primary immune response. These costimulatory signals include those delivered by CD28:CD80/CD86,1 CD2:CD58,2 CD40 ligand (CD40L):CD40,3 lymphocyte function-associated antigen-1 (LFA-1):intracellular adhesion molecule (ICAM),4 OX40:OX40L,5 4-1BB:4-1BBL6 and CD27:CD707 interaction. Some have been shown to amplify the T-cell receptor (TCR)-induced signal, thus making a T-cell response possible with a limited number of MHC–peptide complexes. It has been proposed that CD28 costimulation reduces the number of TCR molecules that need to be engaged on a single T cell in order to induce activation, and amplifies TCR phosphorylation and consumption of Lck.8 However, it also appears that CD28 and other costimulators, such as LFA-1, can transduce signals not induced by the TCR such as phosphatidyl inositol-3 (PI-3) kinase and C-JUN NH2-terminal (JNK) mitogen-activated protein (MAP) kinase activation.4,9,10
The full complexity of the molecular events surrounding the engagement of the TCR with MHC–peptide complexes is only now becoming apparent. A host of costimulatory and signal-transducing molecules are recruited to membrane microdomains of TCR–MHC interaction known as immune synapses.11–13 These areas involve rearrangement of the cytoskeleton to result in tight physical contacts;14 signalling molecules then migrate in and out of the area in a highly co-ordinated fashion to deliver the complex patterns of biochemical events that are required for full activation.15 The complexity of the process gives ample opportunity for regulation of signals, which may alter subsequent development of the T cell. Once the initial stimulus has been received, proliferation of the cells is the first response to be evoked. This allows the antigen-specific cells to be expanded so that there are sufficient numbers to deal with the situation. This expansion is the first stage of peripheral development and involves expression of cell-surface molecules such as CD25 and CD69 and utilization of interleukin (IL)-2 as an autocrine growth factor. These activated cells have been termed p T helper (pTh) cells as they are activated but have not differentiated to express full effector function, normally defined by their ability to produce high levels of effector cytokines such as interferon-γ (IFN-γ) and IL-4. Instead their cytokine-secreting potential is limited to IL-2 (required for proliferation) and possibly IL-3. The cells also become much more responsive to signals that will direct their future differentiation; for example they can respond to IL-4 alone by proliferating.16 Activation of the JNK MAP kinase signalling pathway now becomes necessary for further T-cell development, although it is redundant for IL-2 secretion and proliferation.17
Development of an effective primary T-cell response may require co-operation via cytokines between multiple responding T cells. This requirement could contribute effectively to peripheral tolerance as self-reactive naive T cells will encounter antigen individually as soon as they exit the thymus and become anergised or die as a result of lack of cytokine costimulation. In contrast, T cells responsive to foreign antigens will accumulate in the periphery and be activated together when antigen enters the body, resulting in an effective response.18 Another mechanism of tolerance involves engagement of the cytotoxic T-lymphocyte antigen-4 (CTLA-4) (CD152) receptor instead of CD28 by CD80/CD86 on APCs.19 CTLA-4 transduces an important signal that prevents tyrosine phosphorylation of TCR components20 and its absence results in lymphoproliferative disease.21 As well as blocking T-cell activation and IL-2 synthesis, it directly induces the secretion of transforming growth factor-β (TGF-β) (a potent immunosuppressive cytokine) by the T cell.22 Unlike CD28, CTLA-4 expression is not constitutive and regulation of its expression may therefore be critical to tolerance induction.
SIGNALLING and CYTOKINES IN THE GENERATION OF TYPE 1 VERSUS TYPE 2 EFFECTOR T CELLS
After T cells have received the full complement of activation signals, the decision between two major pathways of effector development is made. Differentiation into the type 1 and type 2 subsets of effectors expressing IFN-γ- or IL-4-associated cytokines, respectively, controls the balance of cell-mediated and humoral immunity, thus ensuring appropriate responses to pathogens. This occurs in both CD4 and CD8 subsets,23,24 although important differences exist in the regulation of cytokine profiles between the two subsets,25 and a fundamental functional dichotomy in the T cytotoxic (Tc) 1 and Tc2 subsets has yet to be demonstrated. Regulation of the development of type 1 versus type 2 CD4 and CD8 effector cells has traditionally been thought to be under the control of cytokines, primarily IL-4, IL-12 and IFN-γ,26 derived from T cells or DCs/APCs, respectively.27,28 These cytokine signals are transmitted through signalling via signal transducer and activator of transcription-4 (STAT-4),29 (IL-12) and STAT-630 (IL-4). In humans, STAT-4 is also triggered by the IFN-α receptor so IFN-α can replace IL-12 in directing Th1 responses.31 However, the primary stimulus that the cell receives is via the TCR and it is now apparent that differential signalling via the immune synapse can have a profound influence on type 1/type 2 development.32 The intensity and duration of TCR triggering can clearly influence subsequent differentiation. This can be demonstrated by altering the dose of antigen used to trigger T cells. Low doses favour Th2 development while higher doses favour Th1 development.33,34 As well as the number of TCRs triggered by peptide–MHC complexes, the affinity of the interaction also plays a critical role. Peptides that bind with high affinity to the TCR induce a predominant Th1 phenotype in subsequent effectors, while low-affinity peptides induce Th2 cells.35–38 Affinity can be affected by altering the sequence of the peptide or the contact region of the TCR39 and an analogous effect has been demonstrated in CD8 T cells.40 The participation of the MHC in the interaction is also important as different MHC alleles can influence the Th1/Th2 balance because they affect affinity.41 It has also been proposed that kinetic off-rates for dissociation of TCR–MHC complexes, rather than binding affinity itself, are more important for differential activation.42 The duration of TCR engagement must also be considered because Th2 development requires a longer period of TCR engagement.43 Together the data clearly indicate that the nature of the antigenic complex itself can determine the class of immune response initiated. This phenomenon is not restricted to models using altered peptide ligands – immunization of mice with different protein antigens can induce Tc1 or Tc0/Tc2 phenotypes in cytotoxic T lymphocytes (CTLs) in vivo.44
To explain how the affinity of cognate interactions and duration of signalling can alter development, a model is required in which qualitatively as well as quantitatively distinct signals can be transduced through the TCR complex.45 Current data suggest that the balance of the three major TCR-transduced intracellular signals – protein kinase C (PKC), calcineurin and Ras/MAP kinase activation – can be regulated and result in altered type 1/type 2 differentiation. High levels of PKC activity combined with low calcium signals favour Th2 and Tc2 development, while a predominance of calcium signalling or MAP kinase activity favour type 1 development (ref. 40; A. Noble, unpublished). Peptides with weaker affinity for the TCR induce less phosphorylation of TCRζ chains46 and greatly reduced calcium flux47,48 while PKC signals appear to be relatively unchanged,40 explaining their type 2-promoting effect. They are also more dependent on participation of the CD4 co-receptor to induce effective signalling.49 Differences in the kinetics of signalling and association/dissociation of binding might result in net alterations in the balance of signals received after different time-periods of TCR ligation. Furthermore, interaction of TCR with the peptide–MHC complex may involve conformational changes that affect subsequent signalling events.50 These findings extend the potential roles of altered peptides to every stage in T-cell differentiation because they are also able to induce positive selection during thymic development51,52 and inactivation of differentiated effector T cells.53 The immune synapse can therefore be thought of as the ‘brains’ of the T cell, controlling activities throughout life and able to transmit complex information from the outside world rather than simply acting as an ‘on/off’ switch. The major downstream targets of PKC, calcium and MAP kinase signalling are the nuclear factor (NF)-κB, nuclear factor of activated T cells (NFAT) and activator protein-1 (AP-1) transcription factors that initiate expression of various genes involved in T-cell activation. NFAT and AP-1 bind co-operatively to the IL-4 promoter region, and different NFAT family members can differentially regulate IL-4 expression.54
The mechanism of TCR control over effector differentiation may be mediated via altered cytokine synthesis.55 Type 2-promoting TCR stimuli may increase the sensitivity of cells to the differentiative effects of IL-4 (A. Noble, unpublished) and the Th2-promoting effect of CD28 ligation is associated with increased phosphorylation of IL-4 receptor-α (IL-4Rα) and STAT-6 in the presence of IL-4, thus increasing IL-4 sensitivity without altering receptor expression.56 Early secretion of IL-4 by activated T cells could down-regulate IL-12Rβ2 expression, inducing intermediate cells unable to enter the Th1 pathway.57 Cytokines could regulate early IL-4 secretion; for example, IL-18 has recently been reported to enhance IL-4-dependent Th2 development.58 Alternatively, primary stimuli could directly induce the expression of transcription factors that are selectively expressed in Th1 or Th2 cells and which induce type or type 2 differentiation independently. These include the guanosine triphosphatase Rac2, which is selectively expressed in Th1 cells and activates the IFN-γ promoter via NF-κB and p38 MAP kinase.59 T-bet is another Th1-specific factor that potently induces IFN-γ production, even in fully differentiated Th2 or Tc2 cell lines, and is key to initiating the Th1 pathway.60 The Th1-specific factor ERM can co-operate in IFN-γ gene induction.61 Such Th1 transcription factors are generally induced by IL-12 signalling via STAT-4, e.g. IFN regulating factor-1 (IRF-1).62 Another transcription factor, GATA-3, appears to be a critical control point where Th2 cell development is initiated, as its expression in STAT-6-deficient cells can fully restore Th2 development.27,63 As well as initiating Th2 cytokine expression, it suppresses Th1 development directly without the need for IL-4.64 GATA-3 has a repressor, repressor of GATA (ROG), which is also up-regulated by T-cell activation.65 Additional transcription factors c-maf, JunB and nuclear factor of activated T cells c1 (NFATc1) are involved in switching on the Th2 programme. 66–68 GATA-3 induces IL-4 gene expression via c-maf, but directly targets the IL-5 promoter.69
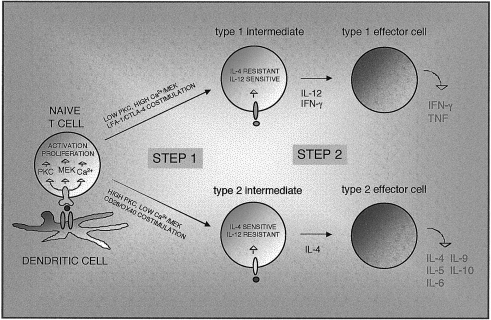
The above data suggest that effector cell development is effectively a two-stage process in which the TCR-transduced stimulus can induce activated cells with a propensity for type 1 or type 2 development; these are then matured by the action of the cytokines IL-4 or IL-12 to become fully active effectors with the capacity for high-level cytokine production. During the initial stage of differentiation, lasting ≈ 2 days in vitro, both IL-4 and IFN-γ mRNAs are transcribed, and further development involves termination of the expression of one of the genes.70 While IL-4 is usually required for generation of a Th2 response, in some models Th2 cells can be generated in IL-4/IL-4R or STAT-6 knockout mice.71 This seems to confirm that IL-4 acts at a later stage of differentiation to stabilize Th2 development. Similarly, a STAT-4/IL-12-independent pathway for Th1 development has been demonstrated using knockout mice.72 A hypothetical two-stage model for T-effector differentiation is shown in Fig. 1.
Figure 1.
A two-step model of type 1/type 2 effector T-cell differentiation. Naive T cells are stimulated through immune synapses capable of transducing differential signalling patterns. The balance of T-cell receptor (TCR)-induced and costimulatory signals received activates cells into an intermediate stage in the type 1 or type 2 pathway. Sensitivity to critical cytokines interleukin (IL)-4 and IL-12 is modulated via changes in receptor expression or signals downstream of the receptors. Differentiation to mature type 1 and type 2 effectors then proceeds in response to cytokines. Note that CD8 T cells do not require IL-12 for type 1 development. CTLA-4, cytotoxic T-lymphocyte antigen-4; IFN-γ, interferon-γ; LFA-1, lymphocyte function-associated antigen-1; MAP kinase kinase (MEK); PKC, protein kinase C; TNF, tumour necrosis factor.
Costimulatory signals are widely reported to modulate type 1/type 2 immunity. CD28 ligation can dramatically favour the development of Th2 cells, perhaps by enhancing IL-4 production73 or by direct activation of STAT-6.74 An absence of CD28 signalling results in a defect of the Th2 responses,75 and CD28 also induces Tc2-cell generation.73 CD40–CD40L interaction selectively induces Th1 cells but this is a result of the production of IL-12 from APCs.75 OX40–OX40L interaction favours Th2 responses76,77 as do CD30–CD30L78 and CD4 signals.79 In contrast, LFA-1 signals appear to potently switch cells towards the Th1 phenotype,80 and CTLA-4 engagement favours Th1 differentiation as well as suppressing the T-cell response.81 The roles of costimulatory signals in type 1/type 2 development appear secondary to the TCR-transduced stimulus, as CD28 fails to induce Th2 cells in the presence of a high-affinity peptide ligand38 and can also result from differential cytokine production. However, it is possible that signals unique to costimulatory receptors independently control differentiation. In this regard it is interesting to note that LFA-1 costimulation induced JNK activation, which might favour Th1 responses.4,82
In addition to their role as specialized naive T-cell activators, DCs can influence type 1 versus type 2 differentiation. DC phenotypes that elicit Th1 or Th2 development have been described as DC1 and DC2, respectively. Distinct subsets of DCs (distinguishable by their expression of CD8α) have been described in the mouse, which selectively induce Th1 or Th2 responses as a result of their differential secretion ofIL-12.83 A functional dichotomy has also been proposed for human DCs – lymphoid DCs expressing high levels of IL-3Rα and defective IL-12 secretion promote Th2 development via an IL-4-independent mechanism.84 However, DCs that selectively prime Th1 or Th2 responses may not always originate from distinct lineages as IL-12 and IL-10 secretion can be directly regulated by T cells via signalling through the osteopontin (Eta-1) molecule on T cells, which binds an integrin receptor and CD44 to promote IL-12 and suppress IL-10 production, respectively.85 CD40 ligation is also crucial to DC maturation and IL-12 synthesis,86 so T-cell–DC interaction involves bidirectional dialogue mediated by both surface molecules and cytokines.
PLASTICITY and STABILITY OF TYPE 1 and TYPE 2 EFFECTORS
The differentiation of effector cells into the Th1 and Th2 phenotypes has been shown to involve permanent changes in chromatin structure in genetic loci of Th1 and Th2 cytokine genes. This gene remodelling process involves demethylation of DNA sequences in the IL-4 gene locus in Th2 cells.87 Such permanent rearrangement of DNA to ensure terminally differentiated cells with stable phenotypes probably requires multiple (up to 13) cell divisions.88 In contrast, emergence of cells secreting IL-4 or IFN-γ requires around seven divisions.89 These observations establish type 1 and type 2 as bona fide differentiative pathways rather than phenotypes that are modulated by the environment of a cell. This is further confirmed by the existence of stable cell-surface markers characteristic of Th1 or Th2 cells. The ST2L molecule is permanently expressed on Th2 cells, associating closely with IL-4-secreting cells.90 Furthermore, knockout mice indicate that this molecule is functionally important for Th2-induced eosinophil recruitment and may be important for Th2 development.91 The signalling component of the IL-12 receptor is a marker for Th1 cells and is of obvious functional significance. The expression of stable cell-surface markers is therefore another key step in T-cell differentiation, which reinforces functional divergence of subsets. Th2 cells rapidly down-regulate expression of the IL-12Rβ2, reinforcing their polarized phenotype.57 However, Th2 cells cannot be converted to a Th1 phenotype, even when expressing transgenic IL-12Rβ2.92 Even fully differentiated effectors, analogous to in vitro-generated long-term clones, can be induced to alter their behaviour by cytokines, presumably to allow a degree of flexibility in cytokine production so that even chronic responses can be regulated if necessary. For example, IL-12 induces IFN-γ production in Tc2 or Th2 clones,93,94 and IL-4 suppresses IFN-γ production from fully differentiated Th1 cells.95 TCR signalling may also play a role in lineage commitment. The patterns of TCR signalling that induce selective development of type 1 or type 2 cells apparently become imprinted on the fully differentiated effector cells, as both Th2 and Tc2 clones exhibit much weaker calcium signals than their type 1 counterparts when stimulated identically by anti-CD3.40,96,97 Th1 cells, but not Th2 cells, also signal via JNK2 MAP kinase.98 This phenomenon may also contribute to the stability of type 1 and type 2 phenotypes as altering the antigenic peptide might fail to modulate TCR signalling in differentiated cells provided that sufficient engagement is achieved.
DIFFERENCES IN THE DEVELOPMENT OF CD4, CD8 and γδ T-CELLS
Initial activation of CD4 and CD8 T cells differs in that different costimulatory signals appear to be critical for each subset. CD40L triggering is more effective at costimulating CD4 cells,99 while LFA-1–ICAM interaction is a superior, and perhaps critical, signal for CD8 cells.100,101 CD28 can costimulate both subsets, but in CD8 cells this signal can be replaced by those from IL-6 and TNF-α;102 CD28 signalling also seems to be unable to prevent anergy in CD8 cells in the same way as it does for CD4 cells.103 4-1BB has also been proposed as a selective activator of CD8 cells.104 It has been suggested that CD8 cells can develop into fully active effectors in the complete absence of costimulation if the primary stimulus is sufficient.105 This lack of dependence on costimulation is more apparent once effector cells are developed, when CD8 cytotoxic effectors need to attack antigen-bearing non-professional APCs with the highest possible efficiency.
CD8 T cells display a clear bias towards the type 1 cytokine phenotype; so much so that Tc2-type cells were initially described more than 6 years after Th1/Th2.106 A molecular explanation for this bias has now been put forward, in that CD8 cells have no requirement for STAT-4 signalling via IL-12 in order to develop into Tc1 effectors,107 and IL-12 serves only to enhance IFN-γ production.23 Regulation of the Tc1/Tc2 phenotype is also regulated by TGF-β in that IL-4 can act to promote Tc1 development and cytotoxicity in the presence of TGF-β.108 IL-2 is also distinctly regulated in CD8 cells, while generally associating with the Th1 CD4 phenotype. In CD8 cells, IL-2 producers, generated at a much lower frequency than in the CD4 subset, are strongly suppressed by IL-4, so that IL-4 induces a state of anergy in CD8 cells.25,109 The tight regulation of IL-2 production explains why CD8 responses are often dependent on CD4 T-cell help.110 This help is dependent on CD4 cells activating APCs via CD40 so that they can effectively stimulate CD8 cells,111 as well as providing cytokines.112 In some cases, however, CD8 responses do not require such help.113 The differences that can emerge between CD4 and CD8 cytokine profiles allow bidirectional regulation between the two subsets. Th2 cell-derived IL-4 probably assists the development of Tc2 cells in allergic states.114 Conversely, CD8 T-cell responses often regulate concomitant CD4 responses, suppressing Th2 immunity and enhancing Th1 responses. This is associated with the relative propensity of CD8 cells to secrete high levels of IFN-γ.115,116
γδ T cells mediating immunity to unconventional antigens are capable of differentiating into subsets comparable to αβ Th1 and Th2 cells in both cytokine profile and function.117 Like CD8 cells, they are biased towards the type 1 pathway compared with their CD4 αβ counterparts. Unlike CD8 cells, however, this bias is the result of persistent expression and function of the IL-12 receptor, which ensures continued IFN-γ production in the presence of IL-4.118
TYPE 1/TYPE 2 AS A POPULATION PHENOMENON
The integrity of the Th1/Th2 paradigm has been challenged by observations that acquisition of expression of any particular cytokine during CD4 T-cell development is stochastic.119 This means that the probability of a T cell expressing IL-4 and IFN-γ simultaneously is no less than predicted by random association. CD8 T-cell cloning also results in clones expressing virtually all possible combinations of cytokines,93 and intracellular cytokine analysis reveals heterogeneity of cytokine profiles in newly generated effector cells.120 The emergence of Th1 or Th2 phenotypes therefore depends on increased probabilities of each Th1 or Th2 cytokine being expressed in the population as a whole, rather than co-ordinate regulation of cytokines in individual cells. This process occurs gradually during an immune response in vivo, despite rapid Th1/Th2 polarization being achievable in vitro. This presumably allows more sophisticated regulation of an emerging immune response with a greater variety of cytokines being available. Many cells may remain uncommitted to either type 1 or type 2 pathways except after chronic exposure to antigen in vivo. CD4 T cells cultured in non-polarizing conditions in vitro display a mixed Th1/Th2 profile, as demonstrated by intracellular cytokine staining.40 Interestingly, isolation of viable cytokine-positive cells suggests that once an individual cell starts to secrete IL-4, it becomes committed to the type 2 pathway.27 Cells simultaneously producing IL-4 and IFN-γ are rare in most systems and the Th0 phenotype is therefore largely a population phenomenon.120 It has also been suggested that Th1 and Th2 cells in vivo may arise from different precursors, which would mean that regulation of individual cell fates was less important in immune regulation.121
T-CELL SUBSETS OTHER THAN TYPE 1 and TYPE 2
A number of cytokine profiles have been described that are distinct from the classical type 1 and type 2 phenotypes. High-level secretion of TGF-β, along with varying quantities of IL-4 and IL-10, has been termed the Th3 phenotype and correlates with a suppressor phenotype that is induced during oral tolerance.122 In addition to such peripheral differentiation, regulatory cells expressing a particular cell surface phenotype (CD25+, CD4+, CTLA-4+) appear to emerge from the thymus as a discrete subset and act to suppress autoimmune responses via production of TGF-β, IL-4 or IL-10.123 However, these cells require exposure to antigen in the periphery before they differentiate into active suppressor cells.124,125 A similar regulatory cell develops in response to gut antigens and prevents intestinal inflammation.126,127 It appears that both CD4 and CD8 regulatory subsets producing similar cytokines can contribute to oral tolerance.128 Differentiation into Th3-like cells may require IL-4.129 Another suppressor cell subset, known as T regulatory-1 (Tr1), is characterized by high-level IL-10 and IL-5 secretion and is a slow-growing cell type induced by culture in the presence of IL-10.130 Differentiation of CD4 T cells into regulatory phenotypes may be induced by the interaction of two receptors – Serrate-1 (expressed by APC) and Notch-1 (expressed on T cells) – that also determine cell fates during thymic T-cell differentiation.131 It is not entirely clear whether regulatory cells are a result of differentiation into separate lineages or caused by differential signalling. However, it is evident that IL-10 production can be regulated independently from classical type 1 and type 2 cytokines in both CD4 and CD8 subsets, can be induced by both IL-4 and IL-12, and that this is an important aspect of immune regulation.132,133 It should be noted that regulatory cells may not selectively secrete suppressive cytokines but mediate their effects via induction of apoptosis in reactive cells via CD95 ligation.134 Such cells have recently been described in allograft rejection and display a novel CD4– CD8– phenotype.135
Generation of memory cells
Another potential result of naive T-cell activation is the generation of memory cells. These differ from effector cells in that they are smaller quiescent cells that require priming and restimulation before exerting effector function. Their ability to mediate immunological memory is a result of their increased clone size and their ability to proliferate and differentiate very rapidly. However, they have a lower threshold of activation than naive cells and express different adhesion molecules and chemokine receptors that affect their recirculation and homing properties so that they are ready to respond to further presence of antigen in tissues. However, recent evidence clearly indicates that memory cells are derived directly from effector cells rather than as a separate lineage from naive T cells.136,137 Some memory cells express lymph node homing receptors that allow them to populate lymph nodes for extended periods of time. These ‘central memory’ cells can differentiate rapidly into ‘effector memory’ cells after re-encountering antigen, and then express receptors for migration into inflamed tissues.138 Effector cells therefore choose between death and the memory pathway once formed; the signals that control this choice are not entirely clear but ligation of OX40 along with other APC-derived factors can greatly amplify the memory pool.139 Memory cells also express increased levels of the survival factor bcl-2.140 IL-15 can selectively promote survival of CD8 memory cells,141 and death of activated T cells can be prevented by all the cytokines that signal through the IL-2R common γ-chain (IL-2, -4, -7 and -15) and this could contribute to maintaining a memory pool.16 Memory cells are able to recall the pattern of cytokine production they previously acquired in addition to their antigen specificity.137
UNRESPONSIVE PHENOTYPES and ACTIVATION-INDUCED CELL DEATH
Induction of anergic or unresponsive T cells is classically attributed to TCR-mediated stimulation in the absence of costimulatory signals, leading to a block in IL-2 secretion.142 Lack of CD28 or 4-1BB143 signals can result in anergy, butIL-10 has more recently been demonstrated to interfere with T-cell activation and induce an anergic T-cell phenotype.144 CD28 signalling prevents anergy by up-regulating IL-2 secretion.142 In addition, activation of T cells leads to up-regulation of CTLA-4 and, if this is ligated, an anergic phenotype can develop.145 Anergy can be induced by altered peptides by inducing reduced levels of TCRζ phosphorylation, transducing a distinct signal through the TCR which blocks IL-2 production,146 and anergic cells retain defective TCR signalling which results in faulty induction of the IL-2 gene.147 In some models anergy cannot be reversed by addition of IL-2 or other γ-chain cytokines and this has been associated with defective signalling via Jak-3 and STAT-5 through the common γ-chain itself.148 In CD8 cells, lack of CD4 help in the form of IL-2 or other signals may lead to CD8-cell anergy.110 It has been speculated that a lack of secondary stimulation after initial T-cell differentiation, for example by B cells, could prevent full effector function, leading to anergy or death because CTLA-4 is not down-regulated.18 The link between CTLA-4 signalling and TGF-β may be critical here. TGF-β seems to be crucial for the maintenance of an unresponsive state towards self-antigens as abrogation of TGF-β receptor signalling results in autoimmunity.149 Paradoxically, TGF-β in combination with IL-2 prevents apoptosis of Th2-effector cells, allowing rapid expansion in effector-cell numbers.150 DCs undoubtedly play a role in maintaining T-cell tolerance by processing tissue self-antigens, probably from apoptotic cells,151,152 and in the absence of inflammatory/danger signals,153 presenting them to both CD4 and CD8 cells in a tolerogenic fashion.154,155 This involves transfer of antigen from migratory to resident lymph node DCs.156
Another important aspect of T-cell differentiation is the development of altered susceptibility to regulation by induction of apoptosis. Activation of T cells and their subsequent exposure to IL-2 programmes them for activation-induced cell death (AICD).157,158 This process ensures that on secondary stimulation of effectors, a proportion of the cells undergo apoptosis, limiting the expansion of cells and providing an important mechanism of tolerance.159 This phenomenon most commonly involves CD95–CD95L interaction and occurs much more readily in Th1 cells, Th2 cells being resistant, perhaps owing to differential TCR signalling or enhanced expression of the protective Fas-associated protein-1 (FAP-1) molecule.160 Costimulatory signals may prevent AICD, and up-regulation of additional costimulators (such as 4-1BB) after activation could help prevent premature apoptosis.161 AICD is distinguished from another form of T-cell apoptosis that occurs when T cells are starved of the cytokines and other signals they need for continued turnover or survival. Unlike AICD, this type of death can be prevented by IL-2 or other cytokines that signal via the common γ-chain. IL-15 can prevent apoptosis but appears to direct the cells into an anergic phenotype,162 while IFN-β reverts activated cells to a resting state with prolonged survival.163 IFN-γ can enhance AICD by inducing nitric oxide production from APCs.164 Eventually, in old age, the number of T cells with defective signalling165 and reduced proliferative capacity166 increases. These cells often exhibit an anergic phenotype.167 Thus, the final stage of differentiation in the long life of some T cells may be the development of senescence associated with chromosomal telomere shortening.168 This phenomenon might result in defective immune responses in extreme old age.
Conclusions
Multiple steps in differentiation occur after engagement of the TCR complex of peripheral T cells by an antigenic peptide–MHC complex. Complex information can be transmitted into the T-cell cytoplasm, influencing the critical choice between effector response or tolerance, and modulating subsequent responses to key immunoregulatory cytokines. Divergent differentiation, enforced by genetic reprogramming, ensures that an appropriate class of immunity is generated, while differential regulation of apoptosis controls the expansion of effector cells and helps to develop an appropriately sized pool of memory cells ready for secondary responses. Manipulation of the multiple intracellular signalling pathways involved in these regulatory processes may allow much more effective control of immunopathologies than has previously been possible.
Acknowledgments
I am grateful for the support of the Medical Research Council and the Biotechnology and Biological Sciences Research Council.
References
- 1.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–8. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 2.Bierer BE, Peterson A, Gorga JC, Herrmann SH, Burakoff SJ. Synergistic T cell activation via the physiological ligands for CD2 and the T cell receptor. J Exp Med. 1988;168:1145–56. doi: 10.1084/jem.168.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–72. doi: 10.1073/pnas.93.24.13967. 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni HT, Deeths MJ, Li W, Mueller DL, Mescher MF. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–9. [PubMed] [Google Scholar]
- 5.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurtado JC, Kim SH, Pollok KE, Lee ZH, Kwon BS. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J Immunol. 1995;155:3360–7. [PubMed] [Google Scholar]
- 7.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154:2612–23. [PubMed] [Google Scholar]
- 8.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 9.Bischof A, Hara T, Lin CH, Beyers AD, Hunig T. Autonomous induction of proliferation, JNK and NF-alphaB activation in primary resting T cells by mobilized CD28. Eur J Immunol. 2000;30:876–82. doi: 10.1002/1521-4141(200003)30:3<876::AID-IMMU876>3.0.CO;2-M. 10.1002/(SICI)1521-4141(200003)30:03<876::AID-IMMU876>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Weiss L, Whitmarsh AJ, Yang DD, Rincon M, Davis RJ, Flavell RA. Regulation of c-Jun NH(2)-terminal kinase (Jnk) gene expression during T cell activation. J Exp Med. 2000;191:139–46. doi: 10.1084/jem.191.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–9. doi: 10.1126/science.282.5397.2266. 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 12.Xavier R, Seed B. Membrane compartmentation and the response to antigen. Curr Opin Immunol. 1999;11:265–9. doi: 10.1016/s0952-7915(99)80043-0. 10.1016/S0952-7915(99)80043-0. [DOI] [PubMed] [Google Scholar]
- 13.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–32. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 14.Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–84. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- 15.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 16.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–5. doi: 10.1073/pnas.95.7.3810. 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–4. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 18.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA. 1999;96:185–90. doi: 10.1073/pnas.96.1.185. 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 20.Griffin MD, Hong DK, Holman PO, et al. Blockade of T cell activation using a surface-linked single-chain antibody to CTLA-4 (CD152) J Immunol. 2000;164:4433–42. doi: 10.4049/jimmunol.164.9.4433. [DOI] [PubMed] [Google Scholar]
- 21.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4+ T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 25.Noble A, Macary PA, Kemeny DM. IFN-γ and IL-4 regulate the growth and differentiation of CD8+ T cells into subpopulations with distinct cytokine profiles. J Immunol. 1995;155:2928–37. [PubMed] [Google Scholar]
- 26.Swain SL. T-cell subsets. Who does the polarizing? Curr Biol. 1995;5:849–51. doi: 10.1016/s0960-9822(95)00170-9. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 28.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–7. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 30.Shimoda K, van-Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–3. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 31.Farrar JD, Smith JD, Murphy TL, Murphy KM. Recruitment of stat4 to the human interferon-alpha/beta receptor requires activated stat2. J Biol Chem. 2000;275:2693–7. doi: 10.1074/jbc.275.4.2693. [DOI] [PubMed] [Google Scholar]
- 32.Constant SL, Bottomly K. Induction of Th1 and Th2, CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 33.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–74. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc Natl Acad Sci USA. 1995;92:9510–4. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158:4237–44. [PubMed] [Google Scholar]
- 38.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2, CD4+ T cell differentiation. J Immunol. 1997;159:5956–63. [PubMed] [Google Scholar]
- 39.Blander JM, Sant'angelo DB, Bottomly K, Janeway CA., Jr Alteration at a single amino acid residue in the T cell receptor alpha chain complementarity determining region 2 changes the differentiation of naive CD4 T cells in response to antigen from T helper cell type 1 (Th1) to Th2. J Exp Med. 2000;191:2065–74. doi: 10.1084/jem.191.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble A, Truman JP, Vyas B, Vukmanovic-Stejic M, Hirst WJ, Kemeny DM. The balance of protein kinase C and calcium signaling directs T cell subset development. J Immunol. 2000;164:1807–13. doi: 10.4049/jimmunol.164.4.1807. [DOI] [PubMed] [Google Scholar]
- 41.Murray JS, Pfeiffer C, Madri J, Bottomly K. Major histocompatibility complex (MHC) control of CD4 T cell subset activation. II. A single peptide induces either humoral or cell-mediated responses in mice of distinct MHC genotype. Eur J Immunol. 1992;22:559–65. doi: 10.1002/eji.1830220239. [DOI] [PubMed] [Google Scholar]
- 42.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–26. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 43.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 44.Ma H, Kapp JA. Antigenic epitopes regulate the phenotype of CD8+ CTL primed by exogenous antigens. J Immunol. 2000;164:5698–703. doi: 10.4049/jimmunol.164.11.5698. [DOI] [PubMed] [Google Scholar]
- 45.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Kersh EN, Shaw AS, Allen PM. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998;281:572–5. doi: 10.1126/science.281.5376.572. 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 47.Wulfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–25. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boutin Y, Leitenberg D, Tao X, Bottomly K. Distinct biochemical signals characterize agonist- and altered peptide ligand-induced differentiation of naive CD4+ T cells into Th1 and Th2 subsets. J Immunol. 1997;159:5802–9. [PubMed] [Google Scholar]
- 49.Leitenberg D, Boutin Y, Constant S, Bottomly K. CD4 regulation of TCR signaling and T cell differentiation following stimulation with peptides of different affinities for the TCR. J Immunol. 1998;161:1194–203. [PubMed] [Google Scholar]
- 50.Boniface JJ, Reich Z, Lyons DS, Davis MM. Thermodynamics of T cell receptor binding to peptide–MHC: evidence for a general mechanism of molecular scanning. Proc Natl Acad Sci USA. 1999;96:11446–51. doi: 10.1073/pnas.96.20.11446. 10.1073/pnas.96.20.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu BL, Evavold BD, Allen PM. Modulation of T cell development by an endogenous altered peptide ligand. J Exp Med. 1995;181:805–10. doi: 10.1084/jem.181.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams CB, Engle DL, Kersh GJ, Michael White J, Allen PM. A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor-ligand complex. J Exp Med. 1999;189:1531–44. doi: 10.1084/jem.189.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–22. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 54.Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–35. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- 55.Grakoui A, Donermeyer DL, Kanagawa O, Murphy KM, Allen PM. TCR-independent pathways mediate the effects of antigen dose and altered peptide ligands on Th cell polarization. J Immunol. 1999;162:1923–30. [PubMed] [Google Scholar]
- 56.Kubo M, Yamashita M, Abe R, Tada T, Okumura K, Ransom JT, Nakayama T. CD28 costimulation accelerates IL-4 receptor sensitivity and IL-4-mediated Th2 differentiation. J Immunol. 1999;163:2432–42. [PubMed] [Google Scholar]
- 57.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimoto T, Mizutani H, Tsutsui H, et al. IL-18 induction of IgE: dependence on CD4 T cells, IL-4 and STAT6. Nature Immunol. 2000;1:132–7. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Yu H, Zheng W, et al. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219–22. doi: 10.1126/science.288.5474.2219. 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 60.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 61.Ouyang W, Jacobson NG, Bhattacharya D, Gorham JD, Fenoglio D, Sha WC, Murphy TL, Murphy KM. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc Natl Acad Sci USA. 1999;96:3888–93. doi: 10.1073/pnas.96.7.3888. 10.1073/pnas.96.7.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galon J, Sudarshan C, Ito S, Finbloom D, O'shea JJ. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J Immunol. 1999;162:7256. [PubMed] [Google Scholar]
- 63.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 65.Miaw SC, Choi A, Yu E, Kishikawa H, Ho IC. ROG, repressor of GATA, regulates the expression of cytokine genes. Immunity. 2000;12:323–33. doi: 10.1016/s1074-7613(00)80185-5. [DOI] [PubMed] [Google Scholar]
- 66.Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188:1859–66. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18:420–32. doi: 10.1093/emboj/18.2.420. 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshida H, Nishina H, Takimoto H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–24. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhang DH, Yang L, Ray A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J Immunol. 1998;161:3817–21. [PubMed] [Google Scholar]
- 70.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-γ-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–94. [PubMed] [Google Scholar]
- 71.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–55. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 72.Kaplan MH, Wurster AL, Grusby MJ. A signal transducer and activator of transcription (Stat) 4-independent pathway for the development of T helper type 1 cells. J Exp Med. 1998;188:1191–6. doi: 10.1084/jem.188.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 74.Oki S, Otsuki N, Kohsaka T, Azuma M. Stat6 activation and Th2 cell proliferation driven by CD28 signals. Eur J Immunol. 2000;30:1416–24. doi: 10.1002/(SICI)1521-4141(200005)30:5<1416::AID-IMMU1416>3.0.CO;2-M. 10.1002/(SICI)1521-4141(200005)30:5<1416::AID-IMMU1416>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 75.Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–70. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 76.Akiba H, Miyahira Y, Atsuta M, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–80. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4+ T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 78.Del Prete G, De Carli M, D'elios MM, et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–61. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rogers PR, Croft M. CD28, Ox-40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J Immunol. 2000;164:2955–63. doi: 10.4049/jimmunol.164.6.2955. [DOI] [PubMed] [Google Scholar]
- 80.Salomon B, Bluestone JA. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–42. [PubMed] [Google Scholar]
- 81.Kato T, Nariuchi H. Polarization of naive CD4+ T cells toward the Th1 subset by CTLA-4 costimulation. J Immunol. 2000;164:3554–62. doi: 10.4049/jimmunol.164.7.3554. [DOI] [PubMed] [Google Scholar]
- 82.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 83.Maldonado-Lopez R, De Smedt T, et al. CD8α+ and CD8α– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 85.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 86.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–75. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 88.Kelso A, Groves P. A single peripheral CD8+ T cell can give rise to progeny expressing type 1 and/or type 2 cytokine genes and can retain its multipotentiality through many cell divisions. Proc Natl Acad Sci USA. 1997;94:8070–5. doi: 10.1073/pnas.94.15.8070. 10.1073/pnas.94.15.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA. 1998;95:9488–93. doi: 10.1073/pnas.95.16.9488. 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–94. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–76. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishikomori R, Ehrhardt RO, Strober W. T helper type 2 cell differentiation occurs in the presence of interleukin 12 receptor beta2 chain expression and signaling. J Exp Med. 2000;191:847–58. doi: 10.1084/jem.191.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vukmanovic-Stejic M, Vyas B, Gorak-Stolinska P, Noble A, Kemeny DM. Human Tc1 and Tc2/Tc0, CD8 T-cell clones display distinct cell surface and functional phenotypes. Blood. 2000;95:231–40. [PubMed] [Google Scholar]
- 94.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–13. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gajewski TF, Schell SR, Fitch FW. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990;144:4110–20. [PubMed] [Google Scholar]
- 97.Sloan Lancaster J, Steinberg TH, Allen PM. Selective loss of the calcium ion signaling pathway in T cells maturing toward a T helper 2 phenotype. J Immunol. 1997;159:1160–8. [PubMed] [Google Scholar]
- 98.Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ, Rincon M, Flavell RA. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–85. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 99.Cayabyab M, Phillips JH, Lanier LL. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J Immunol. 1994;152:1523–31. [PubMed] [Google Scholar]
- 100.Deeths MJ, Mescher MF. ICAM-1 and B7-1 provide similar but distinct costimulation for CD8+ T cells, while CD4+ T cells are poorly costimulated by ICAM-1. Eur J Immunol. 1999;29:45–53. doi: 10.1002/(SICI)1521-4141(199901)29:01<45::AID-IMMU45>3.0.CO;2-I. 10.1002/(SICI)1521-4141(199901)29:01<45::AID-IMMU45>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 101.Shier P, Ngo K, Fung-Leung WP. Defective CD8+ T cell activation and cytolytic function in the absence of LFA-1 cannot be restored by increased TCR signaling. J Immunol. 1999;163:4826–32. [PubMed] [Google Scholar]
- 102.Sepulveda H, Cerwenka A, Morgan T, Dutton RW. CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation. J Immunol. 1999;163:1133–42. [PubMed] [Google Scholar]
- 103.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163:102–10. [PubMed] [Google Scholar]
- 104.Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang B, Maile R, Greenwood R, Collins EJ, Frelinger JA. Naive CD8+ T cells do not require costimulation for proliferation and differentiation into cytotoxic effector cells. J Immunol. 2000;164:1216–22. doi: 10.4049/jimmunol.164.3.1216. [DOI] [PubMed] [Google Scholar]
- 106.Seder RA, Boulay JL, Finkelman F, Barbier S, Ben-Sasson SZ, Le-Gros G, Paul WE. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992;148:1652–6. [PubMed] [Google Scholar]
- 107.Carter LL, Murphy KM. Lineage-specific requirement for signal transducer and activator of transcription (Stat) 4 in interferon gamma production from CD4+ versus CD8+ T cells. J Exp Med. 1999;189:1355–60. doi: 10.1084/jem.189.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Erard F, Garcia-Sanz JA, Moriggl R, Wild MT. Presence or absence of TGF-beta determines IL-4-induced generation of type 1 or type 2, CD8 T cell subsets. J Immunol. 1999;162:209–14. [PubMed] [Google Scholar]
- 109.Sad S, Mosmann TR. Interleukin (IL)-4, in the absence of antigen stimulation, induces an anergy-like state in differentiated CD8+ TC1 cells: loss of IL-2 synthesis and autonomous proliferation but retention of cytotoxicity and synthesis of other cytokines. J Exp Med. 1995;182:1505–15. doi: 10.1084/jem.182.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–64. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 112.Lu Z, Yuan L, Zhou X, Sotomayor E, Levitsky HI, Pardoll DM. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J Exp Med. 2000;191:541–50. doi: 10.1084/jem.191.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ. CD40–CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–8. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 114.Ying S, Humbert M, Barkans J, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–44. [PubMed] [Google Scholar]
- 115.Noble A, Zhao Z, Cantor H. Suppression of immune responses by CD8 cells: 2. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998;160:566–71. [PubMed] [Google Scholar]
- 116.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–99. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wen L, Barber DF, Pao W, Wong FS, Owen MJ, Hayday A. Primary gamma delta cell clones can be defined phenotypically and functionally as Th1/Th2 cells and illustrate the association of CD4 with Th2 differentiation. J Immunol. 1998;160:1965–74. [PubMed] [Google Scholar]
- 118.Yin Z, Zhang DH, Welte T, et al. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-γ: failure to down-regulate IL-12 receptor beta 2-chain expression. J Immunol. 2000;164:3056–64. doi: 10.4049/jimmunol.164.6.3056. [DOI] [PubMed] [Google Scholar]
- 119.Kelso A, Groves P, Troutt AB, Francis K. Evidence for the stochastic acquisition of cytokine profile by CD4+ T cells activated in a T helper type 2-like response in vivo. Eur J Immunol. 1995;25:1168–75. doi: 10.1002/eji.1830250506. [DOI] [PubMed] [Google Scholar]
- 120.Bucy RP, Karr L, Huang GQ, et al. Single cell analysis of cytokine gene coexpression during CD4+ T-cell phenotype development. Proc Natl Acad Sci USA. 1995;92:7565–9. doi: 10.1073/pnas.92.16.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foucras G, Gapin L, Coureau C, Kanellopoulos JM, Guery JC. Interleukin 4-producing CD4 T cells arise from different precursors depending on the conditions of antigen exposure in vivo. J Exp Med. 2000;191:683–94. doi: 10.1084/jem.191.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–82. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+ CD45RC– cells and CD4+ CD8– thymocytes. J Exp Med. 1999;189:279–88. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–6. [PubMed] [Google Scholar]
- 129.Inobe J, Slavin AJ, Komagata Y, Chen Y, Liu L, Weiner HL. IL-4 is a differentiation factor for transforming growth factor-beta secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomyelitis. Eur J Immunol. 1998;28:2780–90. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 130.Groux H, O'garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 131.Hoyne GF, Le Roux I, Corsin-Jimenez M, et al. Serrate 1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4+ T cells. Int Immunol. 2000;12:177–85. doi: 10.1093/intimm/12.2.177. 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- 132.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–69. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Assenmacher M, Lohning M, Scheffold A, Richter A, Miltenyi S, Schmitz J, Radbruch A. Commitment of individual Th1-like lymphocytes to expression of IFN-γ versus IL-4 and IL-10: selective induction of IL-10 by sequential stimulation of naive Th cells with IL-12 and IL-4. J Immunol. 1998;161:2825–32. [PubMed] [Google Scholar]
- 134.Noble A, Pestano GA, Cantor H. Suppression of immune responses by CD8 T cells: 1. Superantigen-activated CD8 cells induce unidirectional Fas-mediated apoptosis of antigen-activated CD4 cells. J Immunol. 1998;160:559–65. [PubMed] [Google Scholar]
- 135.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–9. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 136.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–7. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 137.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–52. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 138.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 139.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 140.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–4. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 141.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 142.Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol Rev. 1998;165:287–300. doi: 10.1111/j.1600-065x.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 143.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–40. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Groux H, Bigler M, de-Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakata Y, Uzawa A, Suzuki G. Control of CD4 T cell fate by antigen re-stimulation with or without CTLA-4 engagement 24 h after priming. Int Immunol. 2000;12:459–66. doi: 10.1093/intimm/12.4.459. 10.1093/intimm/12.4.459. [DOI] [PubMed] [Google Scholar]
- 146.Kersh EN, Kersh GJ, Allen PM. Partially phosphorylated T cell receptor zeta molecules can inhibit T cell activation. J Exp Med. 1999;190:1627–36. doi: 10.1084/jem.190.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Utting O, Teh SJ, Teh HS. A population of in vivo anergized T cells with a lower activation threshold for the induction of CD25 exhibit differential requirements in mobilization of intracellular calcium and mitogen-activated protein kinase activation. J Immunol. 2000;164:2881–9. doi: 10.4049/jimmunol.164.6.2881. [DOI] [PubMed] [Google Scholar]
- 148.Grundstrom S, Dohlsten M, Sundstedt A. IL-2 unresponsiveness in anergic CD4+ T cells is due to defective signaling through the common gamma-chain of the IL-2 receptor. J Immunol. 2000;164:1175–84. doi: 10.4049/jimmunol.164.3.1175. [DOI] [PubMed] [Google Scholar]
- 149.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 150.Zhang X, Giangreco L, Broome HE, Dargan CM, Swain SL. Control of CD4 effector fate: transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J Exp Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 152.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kurts C, Heath WR, Kosaka H, Miller JF, Carbone FR. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J Exp Med. 1998;188:415–20. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–30. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Inaba K, Turley S, Yamaide F, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–73. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dai Z, Arakelov A, Wagener M, Konieczny BT, Lakkis FG. The role of the common cytokine receptor gamma-chain in regulating IL-2-dependent, activation-induced CD8+ T cell death. J Immunol. 1999;163:3131–7. [PubMed] [Google Scholar]
- 158.Wang R, Rogers AM, Rush BJ, Russell JH. Induction of sensitivity to activation-induced death in primary CD4+ cells: a role for interleukin-2 in the negative regulation of responses by mature CD4+ T cells. Eur J Immunol. 1996;26:2263–70. doi: 10.1002/eji.1830260944. [DOI] [PubMed] [Google Scholar]
- 159.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis – immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 160.Zhang X, Brunner T, Carter L, et al. Unequal death in T helper cell (Th) 1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–49. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–9. [PubMed] [Google Scholar]
- 162.Dooms H, Van Belle T, Desmedt M, Rottiers P, Grooten J. Interleukin-15 redirects the outcome of a tolerizing T-cell stimulus from apoptosis to anergy. Blood. 2000;96:1006–12. [PubMed] [Google Scholar]
- 163.Pilling D, Akbar AN, Girdlestone J, et al. Interferon-β mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 164.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon-γ eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–22. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eisenbraun MD, Tamir A, Miller RA. Altered composition of the immunological synapse in an anergic, age-dependent memory T cell subset. J Immunol. 2000;164:6105–12. doi: 10.4049/jimmunol.164.12.6105. [DOI] [PubMed] [Google Scholar]
- 166.Linton PJ, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891–900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–24. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–4. doi: 10.1016/s0167-5699(97)01079-7. 10.1016/S0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]