Abstract
Owing to its skin-sensitizing and fluorochromatic properties, fluorescein isothiocyanate (FITC) is employed frequently as an experimental hapten in mechanistic studies of contact allergy, particularly in the context of the role of migration and activation of Langerhans’ cells. In this study we demonstrated that topical exposure of mice to FITC results in the selective development of activated lymph node cells (LNC) expressing a preferential type 2 cytokine-secretion profile, with high levels of interleukin (IL)-4 and IL-10, but low levels of interferon-γ (IFN-γ). Negative selection (complement depletion) identified CD4+ T helper (Th)2-type cells as the primary source in activated LNC of the type 2 cytokines IL-4 and IL-10, whereas the low levels of IFN-γ produced were derived exclusively from CD8+ T cytotoxic (Tc) 1-type cells. A biphasic pattern of cutaneous inflammatory reactions was elicited by exposure to FITC, the early phase of which could be transferred passively with serum (presumably immunoglobulin E [IgE] antibody), whereas adoptive transfer experiments demonstrated that Th2-type CD4+ cells were responsible for the delayed-type component of the dermal hypersensitivity reaction. In contrast with contact allergic reactions induced by other sensitizing haptens, which are considered to be largely Th1/Tc1-mediated immune processes regulated by Th2-type cells, these results suggest therefore that the skin lesions provoked in mice by FITC are primarily a result of the activation of Th2-type cells.
Introduction
The development of allergic responses is, to a large extent, orchestrated by T-cell subsets and their cytokine products. Delayed-type hypersensitivity (DTH) reactions, such as chemical contact allergy, are regarded usually as cell-mediated immune responses, and T helper (Th)1-type cells have been implicated frequently, but not invariably, as the principal effector cell.1–3 Thus, cutaneous inflammatory responses to soluble, particulate and allogeneic antigens have been adoptively transferred by Th1 clones, but not by Th2 clones of identical specificity.1,2 Furthermore, such DTH responses have been shown to be dependent upon production of the type 1 cytokines interferon-γ (IFN-γ) and interleukin (IL)-2,4,5 and in mice lacking the CD4 gene, contact sensitization is suboptimal.6 Conversely, Th2 cells and their cytokine products are associated generally with the induction of immediate-type hypersensitivity reactions such as asthma, with the type 2 cytokines IL-4 and IL-5 promoting, respectively, the production of immunoglobulin E (IgE) antibody and the development and recruitment of eosinophils,7–9 and Th2-cell clones transferring passively airway hyper-responsiveness and pulmonary eosinophilia in experimental animals.10 Indeed, T cells derived from the airways of patients with allergic asthma (by using bronchoalveolar lavage or bronchial biopsy) have been shown to express elevated levels of mRNA for these cytokines, such that the frequency of type 2 cells correlated positively with tissue eosinophilia.11,12
In recent years, however, it has become apparent that CD8+ T cytotoxic (Tc) cells also display functional heterogeneity analogous to that observed for CD4+ Th cells, with two populations designated Tc1 and Tc2 that exhibit differentiated cytokine phenotypes comparable with Th1 and Th2 cells, respectively.13–15 There is evidence also that CD8+ cells may play a role in the pathogenesis of allergic disease, particularly in the expression of chemical contact hypersensitivity. It has been shown, for example, using in vivo monoclonal antibody depletion, that whereas DTH responses to protein and cellular antigens are mediated by CD4+ effector cells, with CD8+ cells having a down-regulatory function, responses to chemical haptens such as dinitrofluorobenzene (DNFB) were effected by both CD4+ and CD8+ cells, with a subpopulation of CD4+ cells playing a down-regulatory role.16 It has been reported also that at least two different T-cell subsets are required for the successful transfer of contact sensitivity to chemical allergens, a CD4+ αβ+ IL-2-producing subset and a CD8+ γδ+ IFN-γ-producing subset.17,18 Other investigators, however, have observed that the cutaneous hypersensitivity responses provoked by contact allergens such as DNFB or trinitrochlorobenzene are mediated by IFN-γ-producing CD8+ cells exclusively, with Th2-type CD4+ cells down-regulating this response,19–21 and that the inflammatory reaction was mediated through the cytotoxic activity of the CD8+ T cells.22 It should be recognized also that under certain circumstances both Tc1 and Tc2 cells may contribute to dermal hypersensitivity reactions. Therefore, allospecific Tc1 and Tc2 cells, when injected into the footpads of naive allogeneic recipient mice, stimulated antigen-specific inflammation of comparable levels and with similar kinetics.23
CD8+ T cells may also influence allergic respiratory hypersensitivity reactions. There is some limited evidence to suggest that Tc2-type cells may be necessary for ovalbumin-induced airway reactions in mice, as CD8+-cell depletion prevented antigen-induced airway hyper-responsiveness and lung eosinophilia in this model.24 There is, however, a substantial body of evidence that CD8+ cells are able to inhibit IgE antibody responses, particularly those provoked by inhaled protein allergen. Here, down-regulation of IgE production has been shown to be largely or wholly a function of a small population of γδ+ T cells that express high levels of IFN-γ upon in vitro challenge with specific antigen.25 There is little or no information available as to the probable contributions of these cells to the development of respiratory hypersensitivity to chemical respiratory allergens.
Clearly, the evidence to date suggests that both CD4+- and CD8+-cell subsets may have the potential to play influential roles in chemical allergy. In the current investigations we have characterized in greater detail the cellular basis for cutaneous immune and inflammatory responses provoked by the skin-sensitizing fluorochrome, fluorescein isothiocyanate (FITC). As a result of its fluorochromatic properties, which facilitate, for example, the tracking of antigen-bearing cells from the skin to the draining lymph nodes, this material has been used for many years as an experimental probe in mechanistic studies of chemical contact hypersensitivity.26,27 However, it has been demonstrated recently, that under conditions where FITC induces a DTH cutaneous response, restimulation in vitro of activated draining lymph node cells (LNC) resulted in a predominance of IL-4-secreting cells compared with IFN-γ-expressing cells, suggestive of a type 2 phenotype of cytokine expression, although the cellular provenance of cytokine production was not examined.28 In the current series of experiments, the relative contributions of CD4+ and CD8+ cells to the cytokine-secretion profile and cutaneous inflammatory reactions induced by FITC were examined in detail. The cytokine production phenotype elicited by FITC was compared with the type 1 and type 2 profiles stimulated by the reference contact allergen 2,4-dinitrochlorobenzene (DNCB) and the reference respiratory allergen trimellitic anhydride (TMA), respectively.29,30
Materials and methods
Mice
Young adult (6–12 weeks old) female BALB/c mice (Harlan Seralab, Bicester, Oxfordshire, UK) were used throughout these studies. Mice were housed in metal cages, and food (SDS PCD pelleted diet; Special Diets Services Ltd, Witham, Essex, UK) and water were available ad libitum. The ambient temperature was maintained at 21 ± 2° and relative humidity was 55 ± 10% with a 12-hr light/dark cycle. All experiments were carried out under the provisions of the Animals (Scientific Procedures) Act, 1986.
Chemicals
DNCB (98% pure) and FITC isomer I (90% pure) were obtained from Sigma Chemical Co. (St Louis, MO). TMA (97% pure) was supplied by Aldrich Chemical Co. (Gillingham, Dorset, UK). FITC and TMA were stored under anhydrous conditions. TMA and DNCB were dissolved in 4 : 1 acetone : olive oil (AOO) and FITC was prepared in 1 : 1 dibutylphthalate : acetone (DBP). Solutions were prepared freshly immediately prior to dosing.
Sensitization of mice for LNC preparation
Groups of mice (n = 5 for chemical; n = 10 for vehicle) received 50 µl of different concentrations of FITC in DBP, 10% TMA in AOO or 1% DNCB in AOO, bilaterally on each shaved flank. Control animals were treated concurrently with AOO or DBP vehicle alone. Five days later this treatment was repeated. After a further 5 days, 25 µl of chemical or vehicle alone was applied to the dorsum of both ears, daily for three consecutive days.
Preparation of cells from draining lymph nodes
Thirteen days after the initiation of treatment, draining auricular lymph nodes were excised and pooled for each experimental group. A single-cell suspension of LNC was prepared under aseptic conditions by gentle mechanical disaggregation through sterile 200-mesh stainless steel gauze. Viable cell counts were performed by exclusion of 0·5% Trypan Blue, and LNC were cultured in RPMI-1640 growth medium (Gibco, Paisley, Strathclyde, UK) supplemented with 25 mm HEPES, 400 µg/ml of streptomycin, 400 µg/ml of ampicillin and 10% heat-inactivated fetal calf serum (FCS) (RPMI-FCS).
Complement depletion of CD4+ and CD8+ T cells from LNC
LNC, prepared as described above, were incubated at a concentration of 2 × 107 cells/ml at 4° for 45 min with 4 µg/ml of rat monoclonal anti-L3T4 (anti-mouse CD4; clone YTS 191.1.2) or anti-Lyt.2 (anti-mouse CD8; clone YTS 169.4) diluted in RPMI-FCS. Both antibodies were of immunoglobulin G2b (IgG2b) isotype and were obtained from Harlan SeraLab (Crawley Down, Sussex, UK). Control preparations were incubated with the same concentration of rat IgG2b myeloma protein (Serotec, Kidlington, Oxfordshire, UK) diluted in RPMI-FCS. Lymphocyte populations were washed once and resuspended in RPMI-FCS supplemented with 10% low-toxicity rabbit complement (Harlan SeraLab, Crawley Down, UK) and incubated for a further 45 min at 37° in a humidified atmosphere of 5% CO2 in air. Cells were washed twice and viable cell counts were performed by exclusion of 0·5% Trypan Blue. The extent of CD4+ and CD8+ depletion was assessed by flow cytometric analysis. Aliquots of cells (1 × 106) were incubated (for 30 min in the dark at 4°) with 5 µl of anti-CD4-phycoerythrin, 5 µl of anti-CD8-FITC or 5 µl of isotype-matched controls (all antibodies were obtained from Serotec). Cells were washed twice (at 200 g for 5 min) with phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) and 0·5 ng of propidium iodide, and then resuspended in 500 µl of PBS containing 1% paraformaldehyde. Labelled populations were analysed in a fluorescence-activated cell sorter (FACsCalibur flow cytometer; Becton-Dickinson, San Jose, CA) using cellquest software. Non-viable (propidium iodide permeable) cells were excluded from the analysis.
Adoptive transfer of LNC and measurement of cutaneous inflammation
Naive recipient animals (n = 3) received (by intravenous injection) 500 µl of RPMI-FCS medium alone or different concentrations of CD4+ depleted, CD8+ depleted or control populations of FITC-activated LNC. The ear thickness of all mice was measured using an engineers’ micrometer (Wright & Moore, Sheffield, South Yorkshire, UK). Twenty-four hours later, all animals were challenged on the dorsum of both ears with 25 µl of 0·5% FITC in DBP and challenge-induced increases in ear thickness were measured at various time-points thereafter. The inflammatory response was calculated as a function of challenge-induced increases in ear thickness according to the following formula:
 |
A two-tailed Student’s t-test was used for statistical evaluation of responses.
Culture of LNC
Cells (1-ml aliquots), at a concentration of 107 cells/ml in RPMI-FCS, were seeded into 24-well tissue culture plates and maintained at 37° in a humidified atmosphere of 5% CO2 in air in the presence or absence of 2 µg/ml of concanavalin A (Con A) (Sigma). Culture was terminated after 24–120 hr and the supernatants were collected, centrifuged at 100 g for 5 min and stored at −70° prior to analysis.
Cytokine determinations
IL-4
The IL-4 content of culture supernatants derived from Con A-stimulated LNC was measured by using sandwich enzyme-linked immunsorbent assay (ELISA), as described previously.29,30 Plastic microtitre plates (Nunc, Copenhagen, Denmark) were coated by overnight incubation at 4° with 2·5 µg/ml of rat monoclonal anti-IL-4 antibody (Genzyme, Cambridge, MA) in 0·1 m carbonate buffer (pH 9·6). The plates were then blocked for 30 min at 37° with 10% FCS in PBS. Recombinant murine IL-4 (specific activity 1–2 × 107 U/mg; Genzyme) diluted in RPMI-FCS was added to triplicate wells and samples of conditioned medium diluted to different extents in RPMI-FCS were added to duplicate wells and the plates incubated for 2 hr at room temperature. Plates were then incubated for 2 hr at room temperature with 8 µg/ml of goat anti-mouse IL-4 (R & D Systems Europe, Abingdon, Oxon, UK) diluted in RPMI-FCS, followed by a further 2-hr incubation at room temperature with a 1 : 1000 dilution in RPMI-FCS of peroxidase-conjugated donkey anti-goat IgG (Serotec). Enzyme substrate (o-phenylenediamine and urea hydrogen peroxide) was added and the reaction terminated after 15 min by the addition of 0·5 m citric acid. Between each incubation stage the plates were washed with PBS containing 0·05% Tween-20. The absorbance at 450 nm was measured using an automated reader (Multiskan; Flow Laboratories, Irvine, Ayrshire, UK). A standard curve derived using murine recombinant IL-4 and associated computer software for microplate-based assays (Genesis, Life Sciences International Ltd, Basingstoke, Hampshire, UK) was used to calculate the IL-4 concentration in supernatants. The limit of detection was 300–600 pg/ml. Standard errors were less than 10% in most experiments.
IFN-γ
A sandwich ELISA was used to measure the IFN-γ content of culture supernatants. Plastic microtitre plates (Nunc) were coated by overnight incubation at 4° with 0·5 µg/ml of hamster monoclonal anti-IFN-γ antibody (Genzyme) in 0·1 m carbonate buffer (pH 9·6). The plates were then blocked for 30 min at 37° with 10% FCS in PBS. Recombinant murine IFN-γ (specific activity 1 × 107 U/mg; Genzyme) diluted in RPMI-FCS was added to triplicate wells, and samples of conditioned medium diluted to different extents in RPMI-FCS were added to duplicate wells and the plates incubated for 2 hr at room temperature. Plates were then incubated for 2 hr at room temperature with 4 µg/ml of goat anti-mouse IFN-γ (Genzyme) diluted in RPMI-FCS, followed by a further 2-hr incubation at room temperature with a 1 : 1000 dilution in RPMI-FCS of peroxidase-conjugated donkey anti-goat IgG (Serotec). Enzyme substrate (o-phenylenediamine and urea hydrogen peroxide) was added and the reaction terminated after 15 min by the addition of 0·5 m citric acid. Between each incubation stage the plates were washed with PBS containing 0·05% Tween-20. The absorbance at 450 nm was measured using an automated reader (Multiskan). A standard curve derived using murine recombinant IFN-γ and associated computer software for microplate-based assays (Genesis) was used to calculate the IFN-γ concentration in supernatants. The limit of detection was 75–150 pg/ml. Standard errors were less than 10% in most experiments.
IL-10
The IL-10 content of culture supernatants was analysed using a dual-monoclonal sandwich ELISA. Plastic microtitre plates (Nunc) were coated by overnight incubation at 4° with 4 µg/ml of rat monoclonal anti-IL-10 antibody (Pharmingen, San Diego, CA, USA) in 0·1 m carbonate buffer (pH 9·6). The plates were then blocked for 90 min at room temperature with 10% FCS in PBS. Recombinant murine IL-10 (specific activity 5 × 105 U/mg; Genzyme) diluted in RPMI-FCS was added to triplicate wells and samples of conditioned medium diluted to various extents in RPMI-FCS were added to duplicate wells and the plates incubated for 6 hr at room temperature. Plates were then incubated overnight at 4° with 2 µg/ml of biotinylated rat anti-mouse IL-10 (Pharmingen), followed by a further 90-min incubation at room temperature with 2·5 µg/ml of ExtrAvidin peroxidase (Sigma), both diluted in PBS containing 10% FCS. Enzyme substrate (tetramethyl benzidine and hydrogen peroxide) was added and the reaction terminated after 10 min by the addition of 2 m sulphuric acid. Between each incubation stage, the plates were washed with PBS containing 0·05% Tween-20. The absorbance at 450 nm was measured using an automated reader (Multiskan). A standard curve derived using murine recombinant IL-10 and associated computer software for microplate-based assays (Genesis) was used to calculate the IL-10 concentration in supernatants. The limit of detection was 300–600 pg/ml. Standard errors were less than 10% in most experiments.
Sensitization of mice for antibody production and cutaneous inflammatory responses
Groups of mice (n = 5) received 50 µl of 0·5% FITC in DBP bilaterally on each shaved flank. Seven days later this treatment was repeated. After a further 7 days, animals were killed by cardiac puncture and pooled serum samples were prepared and stored at −20° until analysis of FITC immune serum for specific IgE antibody content. In some experiments, naive recipient mice (n = 4) received an intradermal injection of 30 µl of FITC immune serum or normal mouse serum into the dorsum of the ear. Forty-eight hours later, animals were challenged by an intravenous injection of 250 µl of physiological saline containing 1 mg/ml FITC-BSA conjugate or BSA carrier protein alone (Sigma). Cutaneous inflammatory responses were measured as a function of challenge-induced increases in ear thickness, as described previously. Alternatively, FITC-sensitized and naive control animals were challenged on the dorsum of both ears with 25 µl of 0·5% FITC in DBP. Cutaneous inflammatory responses were measured as a function of challenge-induced increases in ear thickness, as described above. A two-tailed Student’s t-test was used for statistical evaluation of responses.
Measurement of FITC-specific IgE antibody
The presence of IgE antibodies in serum was detected by using homologous passive cutaneous anaphylaxis (PCA) assay. Pooled serum samples diluted to different extents in physiological saline (30 µl total volume) were injected into the dermis of the ears of naive recipient mice (n = 4). Two days later, 0·25 mg of FITC-BSA or BSA, together with Evans Blue dye (1·25 mg), in 250 µl of physiological saline were injected intravenously. Thirty minutes following challenge, mice were killed and the diameter of cutaneous reactions measured. IgE titre was recorded as the lowest serum dilution that resulted in a blue lesion > 3 mm in the skin in the majority of recipient animals upon challenge.
Results
Cytokine-secretion profile of FITC-activated LNC
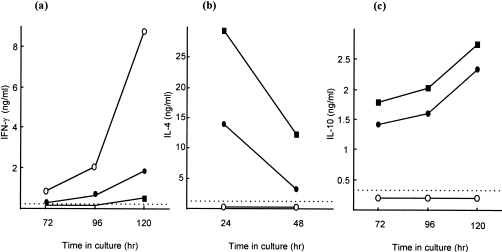
In preliminary experiments, the cytokine phenotype of LNC isolated following prolonged topical exposure of mice to FITC was investigated (Fig. 1). Concurrent control animals were exposed to the reference contact allergen DNCB or to the reference respiratory allergen TMA in order to induce, respectively, type 1 and type 2 cytokine-secretion profiles for comparative purposes. As reported previously,29,30 LNC derived after treatment with DNCB expressed high levels of the type 1 cytokine IFN-γ, but levels of IL-4 and IL-10 were below the limits of detection. The converse phenotype was observed for TMA-activated LNC, with vigorous IL-10 and mitogen-inducible IL-4 production, but little IFN-γ. Exposure to 0·5% FITC resulted in a type 2 cytokine-secretion pattern analogous to that provoked by TMA, with expression of all three cytokines reaching similar levels and following identical kinetics to TMA-stimulated LNC. Furthermore, the phenotype displayed by FITC-activated LNC was independent of the dose applied, with concentrations ranging between 2% and 0·2% all inducing the preferential expression of type 2 cytokines (data not shown). In addition, the cytokine-secretion pattern of FITC-stimulated LNC was unaffected by the isomer of FITC used, with FITC isomers I and II stimulating identical cytokine profiles (data not shown). LNC derived from vehicle (AOO or DBP)-treated mice failed to express detectable levels of either type 2 cytokine and produced low levels of IFN-γ only (data not shown).
Figure 1.
Cytokine-secretion profiles of unfractionated, allergen-activated lymph node cells (LNC). Mice were exposed topically to 10% trimellitic anhydride (TMA) in acetone : olive oil (AOO) (•), 1% 2,4-dinitrochlorobenzene (DNCB) in AOO (○), or 0·5% fluorescein isothiocyanate (FITC) in dibutyl phthalate : acetone (DBP) (▪). Thirteen days after the initiation of exposure, draining auricular lymph nodes were excised and a single-cell suspension of LNC isolated. Supernatants were prepared after culture of LNC for different periods of time in the absence (interferon-γ [IFN-γ] and interleukin [IL]-10) or presence (IL-4) of 2 µg/ml of concanavalin A (Con A). IFN-γ (a), IL-4 (b) and IL-10 (c) concentrations were measured by using cytokine-specific enzyme-linked immunosorbent assay (ELISA). Results from a single representative experiment are presented. The limit of detection for each ELISA is indicated by the horizontal broken line.
Contribution of CD4+ and CD8+ T lymphocytes to LNC cytokine-production profiles
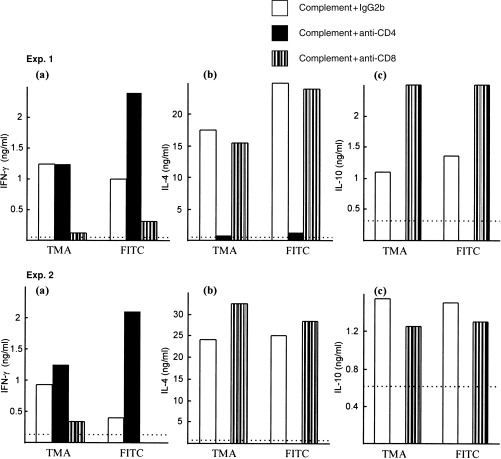
In subsequent experiments, the contribution of CD4+ and CD8+ T cells to the cytokine phenotype induced by FITC was examined by negative selection (complement depletion). Comparisons were performed with LNC populations derived from mice treated with TMA or DNCB. Flow cytometric analyses revealed that unfractionated LNC derived from allergen-treated mice contained 10·8–17·4% CD8+ cells and 28·9–38·5% CD4+ cells. Depletion of CD4+ cells resulted routinely in populations with < 1·9% CD4+ cells and 17·6–25·2% CD8+ cells, whereas CD8+-depleted fractions contained < 1·7% CD8+ cells and 34·6–44% CD4+ cells. Examination of the cytokine phenotypes of FITC- and TMA-stimulated LNC populations demonstrated that the high levels of IL-10 and mitogen-inducible IL-4 were associated predominantly with CD4+ T cells, with unfractionated and CD8+-depleted fractions expressing large amounts of these cytokines, whereas CD4+-cell depletion abrogated IL-4 and IL-10 production almost completely (Fig. 2; two independent experiments). In contrast, the low levels of IFN-γ secreted by LNC from FITC- or TMA-exposed mice were found to be primarily a property of CD8+ cells. Thus, CD8+-depleted fractions expressed little of this cytokine while, in the majority of experiments, populations depleted of CD4+ cells elaborated higher levels of IFN-γ than did unfractionated LNC, presumably as a result of either the loss of inhibitory type 2 cytokines or because of the effective increase in CD8+ cell numbers. The association of type 2 cytokine expression with CD4+ cells, and of IFN-γ production with CD8+ cells, in FITC-activated LNC populations, is consistent with previous observations with other respiratory chemical allergens such as diphenylmethane diisocyanate (MDI).30 The provenance of the high levels of IFN-γ secreted by LNC derived from exposure to chemical contact allergens such as DNCB is, however, very different. Peak (120 hr) IFN-γ expression by unfractionated DNCB-activated LNC was 4·1 and 8·7 ng/ml in two independent experiments. In each case, IFN-γ production was maintained in both CD4+- and CD8+-depleted fractions, with peak levels of secretion of this cytokine for the former population of 5·3 and 7·8 ng/ml and for the latter population of 4·7 and 5·6 ng/ml, respectively, in the two independent experiments.
Figure 2.
Contribution of CD4+ and CD8+ cells to the cytokine-secretion profile induced by trimellitic anhydride (TMA) and fluorescein isothiocyanate (FITC). Draining lymph node cells (LNC) were isolated following topical exposure to 10% TMA in acetone : olive oil (AOO) or 0·5% FITC in dibutyl phthalate : acetone (DBP). Cells were treated with complement and an isotype-control preparation of rat immunoglobulin G2b (IgG2b) myeloma protein, anti-CD4 antibody or anti-CD8 antibody. Supernatants were prepared following culture of LNC for 24 hr in the presence of 2 µg/ml of concanavalin A (Con A) (interleukin [IL]-4) or for 120 hr in the absence of Con A (interferon-γ [IFN-γ] and IL-10). IFN-γ (a), IL-4 (b) and IL-10 (c) concentrations were measured by using cytokine-specific enzyme-linked immunosorbent assay (ELISA). Results from two independent experiments (Exp. 1 and Exp. 2) are shown. The limit of detection for each ELISA is indicated by the horizontal broken line.
Dermal hypersensitivity reactions induced by FITC
Given the fact that topical exposure to FITC resulted in a preferential type 2 cytokine-secretion phenotype (the cellular source of which was analogous to those responses provoked by allergens such as TMA and MDI, which are associated in humans with immediate-type hypersensitivity reactions such as respiratory allergy), the kinetics of dermal hypersensitivity reactions induced by FITC was analysed (Fig. 3; two independent experiments). Inflammatory responses were measured as a function of challenge-induced increases in ear thickness in control (naive) animals or in animals that had been exposed previously to 0·5% FITC in DBP (sensitized). Challenge of control animals with 0·5% FITC in DBP failed to stimulate an inflammatory response at any time-point measured (changes in ear thickness of less than 5·7% were recorded). In contrast, sensitized mice displayed vigorous hypersensitivity reactions that were biphasic in nature. Significant increases in ear thickness were observed as early as 45 min after challenge, which then reached maximal levels 24 hr after challenge. A similar biphasic pattern of cutaneous hypersensitivity responses has been reported previously for the respiratory allergen TMA, although in the same series of experiments topical exposure to the contact allergen DNCB under identical conditions failed to provoke the early component of the dermal reaction.31
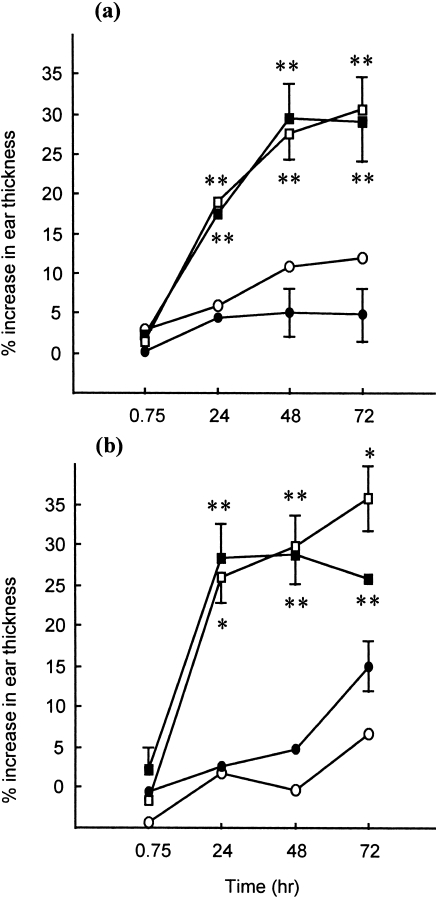
Figure 3.
Kinetics of fluorescein isothiocyanate (FITC)-induced dermal hypersensitivity responses. Animals (n = 5) received 50 µl of 0·5% FITC in dibutyl phthalate : acetone (DBP) on the shaved flanks on days 0 and 7. Fourteen days after the initiation of exposure, the ear thickness of sensitized (□) and control (naive) (▪) animals was measured, using an engineers’ micrometer, immediately prior to challenge on the dorsum of both ears with 25 µl of 0·5% FITC in DBP. Ear thickness was re-evaluated at various time-points thereafter, and elicitation reactions recorded as the mean percentage increase in ear thickness (± SE) relative to prechallenge values. Results from two independent experiments (a) and (b) are presented. At all time-points, significant (P < 0·01) increases in mean ear thickness relative to concurrent controls were observed.
Contribution of humoral and cell-mediated responses to dermal hypersensitivity reactions
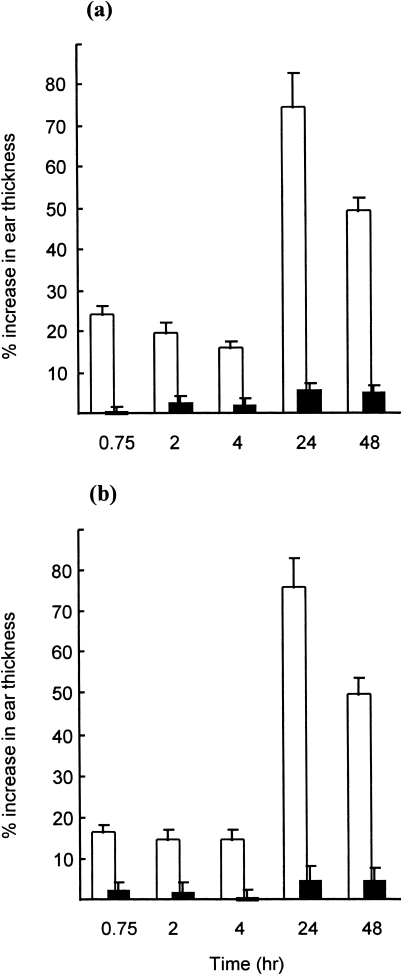
In order to assess the contribution of antibody- and cell-mediated responses to the cutaneous inflammatory reaction provoked by sensitization with FITC, adoptive transfer experiments were performed. The immediate component of the dermal hypersensitivity response was transferred successfully to naive recipient mice with serum derived from FITC-sensitized animals (Fig. 4; two independent experiments). Thus, injection of FITC-immune serum caused a significant and very rapid increase in ear thickness following intravenous injection of FITC-BSA conjugate; the increase in ear thickness peaked at 15–30 min and declined thereafter, reaching background levels by 24 hr. Challenge of recipients of FITC-immune serum with BSA carrier protein alone failed to provoke dermal hypersensitivity reactions at any time-point investigated, and adoptive transfer of naive mouse serum failed to induce significant differences in ear thickness when recipients were challenged with either FITC-BSA conjugate or BSA carrier protein alone (Fig. 4). These experiments indicate that the early component of the cutaneous hypersensitivity response to FITC is serum mediated and presumably attributable to the production of specific IgE antibody. This interpretation is supported by analysis of the specific IgE content of FITC-immune sera by homologous PCA assay. In three independent experiments, pooled sera drawn 14 days after the initiation of topical exposure to 0·5% FITC in DBP were found to contain relatively high-titre anti-FITC IgE antibody, with titres of 1/32, 1/128 and 1/128 obtained in the three individual experiments. Pooled sera isolated from naive animals and tested concurrently with FITC-immune sera failed to elicit a positive response in the PCA assay, even when undiluted. The titres for anti-FITC IgE antibody are similar to those recorded following topical exposure of mice to other chemical respiratory allergens such as phthalic anhydride or TMA.32,33 Furthermore, in animals sensitized 5, rather than 14, days prior to challenge, a time-frame before which systemic IgE antibody production is manifest, significant DTH (24 hr) reactions only were observed (data not shown).
Figure 4.
Dermal hypersensitivity following passive sensitization with fluorescein isothiocyanate (FITC) immune serum. Groups of mice (n = 4) received a 30 µl intradermal injection (into both ear pinnae) of pooled serum from FITC-sensitized mice (• ▪) or from naive mice (○ □). Two days later the ear thickness of all mice was measured immediately prior to challenge with intravenous injection of 250 µl of physiological saline containing 0·25 mg of an FITC-bovine serum albumin (BSA) conjugate (• ○) or of BSA alone (▪ □). Ear thickness was re-evaluated at various time-points thereafter and elicitation reactions recorded as the mean percentage increase in ear thickness (± SE) relative to prechallenge values. Results from two independent experiments (a) and (b) are presented. SE values of < 1·0 are not shown. *P < 0·05; **P < 0·01: significant increase in mean ear thickness relative to controls receiving normal serum and challenged with FITC-BSA conjugate.
Adoptive transfer of LNC populations derived from FITC-exposed mice resulted in dermal inflammatory reactions with a different kinetic profile. Intravenous administration to naive syngeneic recipients (n = 3) of 6 × 107 unfractionated FITC immune cells and topical challenge 24 hr later with FITC failed to stimulate significant local inflammation 45 min after challenge compared with challenge of control mice which had received RPMI-FCS medium alone (mean increases in ear thickness ± SE of −0·2% ± 2·3 and −0·25% ± 2·3, respectively). However, significant increases in ear thickness were observed 24 and 48 hr after challenge (15·9% ± 4·9 and 16·9% ± 1·6, and −0·7% ± 2·7 and 0% ± 3·0, for animals receiving FITC-immune cells and for control animals, respectively). Challenge of recipient animals with irrelevant hapten (10% TMA in AOO) failed to provoke significant cutaneous hypersensitivity responses at any time-point investigated (data not shown).
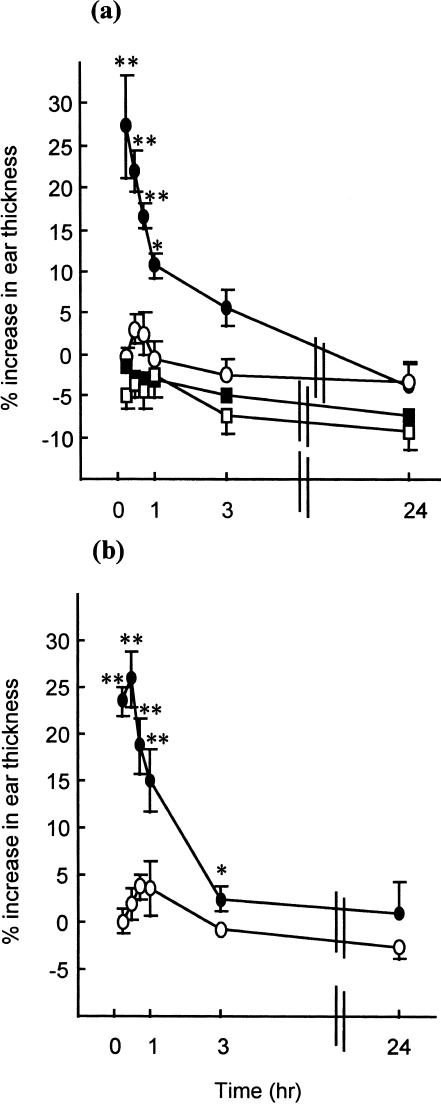
In further experiments, the relative contributions of CD4 and CD8 cells to the delayed hypersensitivity response were examined (Fig. 5; two independent experiments). Animals (n = 3) received 6 × 107 unfractionated FITC-immune cells (containing ≈ 39% CD4+ and 12% CD8+ cells), CD4+-depleted cells (consisting of < 2% CD4+ and 21% CD8+ cells) or CD8+-depleted cells (containing < 2% CD8+ cells and 41% CD4+ cells). Control animals received an intravenous injection of RPMI-FCS medium alone. As demonstrated previously, in each of the two independent experiments, the delayed (but not the early) component of the dermal inflammatory response was transferred successfully with unfractionated LNC. Interestingly, the CD4+-depleted fraction consistently failed to transfer dermal hypersensitivity reactions, whereas administration of the CD8+-depleted fraction was, in each experiment, associated with virtually identical inflammatory reactions to those induced by the unfractionated LNC population. The cytokine-secretion profiles of the CD4+- and CD8+-depleted fractions confirmed that the residual populations represented Tc1- (IFN-γ secreting) and Th2- (IL-4 and IL-10 expressing) type cells, respectively (data not shown). Finally, experiments were performed with increased numbers of CD4+-depleted cells in order to examine whether the differences observed between the two negatively selected populations were qualitative rather than quantitative. Naive recipient animals (n = 3) received 12 × 107 CD4+-depleted cells (the negative-selection procedure in this experiment reduced CD4+ cells from 35·7 to 1·8% and enriched CD8+ cells from 15·0 to 22·9%) or RPMI-FCS medium alone, and were challenged with FITC on the dorsum of both ears, as described previously. At no time-point measured (45 min to 72 hr) was a significant increase in ear swelling observed relative to concurrent controls (data not shown).
Figure 5.
The kinetics of dermal hypersensitivity reactions following passive sensitization with allergen-activated lymph node cells (LNC): role of CD4+ and CD8+ cells. Groups of mice (n = 3) received an intravenous injection of 500 µl of medium alone (○), or 6 × 107 unfractionated (□), CD4+-depleted (•) or CD8+-depleted (▪) populations of fluorescein isothiocyanate (FITC)-activated LNC. The ear thickness of all mice was measured using an engineers’ micrometer. Twenty-four hours later, all animals were challenged on the dorsum of both ears with 25 µl of 0·5% FITC in dibutyl phthalate : acetone (DBP). Challenge-induced increases in ear thickness were measured at various time-points thereafter and elicitation reactions were recorded as the mean percentage increase in ear thickness (± SE) relative to prechallenge values. Results from two independent experiments (a) and (b) are presented. SE values of < 3·0 are not shown. *P < 0·05; **P < 0·01: significant increase in mean ear thickness relative to controls (medium alone).
Discussion
Topical exposure to the sensitizing fluorochrome FITC resulted in the development of a selective type 2 cytokine-secretion profile, in agreement with a previous report.28 The high levels of IL-4 and IL-10 derived from CD4+ Th2-type cells, whereas the comparatively low levels of IFN-γ were associated with CD8+ Tc1-type cells. The exposure regimen used in these experiments is one designed to induce polarized cytokine-secretion phenotypes following topical exposure to different classes of chemical allergen.29,30 Thus, concurrent identical treatment with the known human contact allergen DNCB resulted in preferential type 1 cytokine expression, with high levels of IFN-γ production, but relatively little of the type 2 cytokines IL-4 and IL-10. The type 2 cytokine pattern elicited by FITC is unlike that usually provoked following treatment with other chemical contact allergens, including DNFB,28,29,34 oxazolone18,29 or trinitrochlorobenzene,34,35 where a preferential IFN-γ/IL-2 (type 1) profile is observed. Consistent with the predominant type 2 cytokine phenotype, topical exposure to FITC resulted also in the production of a vigorous specific IgE antibody response. Challenge of sensitized animals elicited a biphasic dermal hypersensitivity response. The early (45 min) component was mediated by serum (presumably IgE antibody) and the delayed (24–48 hr) component was effected by the CD4+ Th2-type fraction of FITC-stimulated LNC, with no apparent contribution from CD8+ cells. These data do not formally exclude an obligatory role for an early initiating component of the DTH response mediated by immunoglobulin M (IgM) antibody produced by the B1-cell subset, as described by Askenase et al., for contact allergens such as oxazolone and trinitrochlorobenzene.36 However, the fact that a vigorous DTH (24–72 hr) reaction is transferred by CD4+ cells in the absence of a detectable acute (45 min) cutaneous inflammatory response suggests that, under these circumstances, an IgM antibody-mediated early reaction is not necessary for the development of FITC-induced DTH reactions. In contrast to FITC, where cutaneous inflammatory responses have been shown (in the present work) to be effected by CD4+ type 2 cells, reactions to other chemical contact haptens such as DNFB or trinitrochlorobenzene have been reported variously to be mediated by combinations of type 1 CD4+ and/or CD8+ effector cells16–21 and down-regulated by type 1 or type 2 CD4+ cells.16,19–21 Taken together, these data defining the cellular basis for FITC-induced inflammatory responses suggest that FITC may be an unrepresentative experimental probe for mechanistic studies of chemical contact allergy. Alternatively, it is possible that immune responses to contact allergens are somewhat more variable in character than has been previously supposed based on analyses of a relatively few potent skin sensitizers.
While it is generally accepted, as described above, that contact sensitization is effected primarily by type 1 cells and associated cytokines,1–6 it is clear that type 2 cells and their cytokine products may play an important role in the elicitation of contact dermatitic reactions. In particular, there is a considerable body of evidence to suggest that IL-4 plays a role in the elicitation of contact allergic reactions. It has been demonstrated that cellular transfer of DTH is inhibited by blocking the action of IL-4 using either anti-IL-4 antibody or antisense oligonucleotides,37 indicating a mandatory requirement for IL-4 in contact sensitization. However, other investigators, using the same experimental approach, have shown that inhibition of IL-4 results instead in more vigorous DTH responses and up-regulates IFN-γ expression, suggestive of a down-regulatory role for this cytokine in sites of contact allergen-induced inflammation.38 The basis for these apparently conflicting data is not known, but may be a result of various factors, including the strain of mice utilized, the chemical allergen used for sensitization or the nature of the exposure regimen. It has been reported, for example, that mice deficient in IL-4 exhibit normal DTH responses to oxazolone, but that the magnitude and duration of contact allergy to DNCB is compromised significantly.39 With regard to the possibility that the exposure regimen is important, it has been demonstrated that whereas applied hapten concentration is without effect on the nature and vigour of the type 1 response induced by topical exposure to trinitrochlorobenzene, administration of the hapten by patching alters the type1/type 2 balance, stimulating the development of IL-4-producing CD4+ Th2-type cells and down-regulating the contact hypersensitivity reaction.35 Furthermore, the longevity of exposure to chemical contact allergen appears to influence markedly the induced cytokine milieu, with repeated exposure resulting in a shift of cutaneous cytokine expression away from a Th1-dominated response (where IFN-γ and IL-2 production predominates) to a Th2 (IL-4 and IL-10)-dominated response.40 However, it must be noted that the exposure regimen used in the current series of experiments did not, by any means, result in a Th2-biased response for all allergens examined; concurrent identical treatment with the known human contact allergen DNCB resulted in a preferential Th1-type cytokine-secretion phenotype. Thus, the information available to date indicates that the expression of IL-4 (and possibly other type 2 cytokines), particularly at sites of dermal challenge, regulates what is considered to be a largely Th1/Tc1-dependent immune process, although the factors governing whether such is up- or down-regulated are still unclear.
Taken together with previous investigations it is clear that low-molecular-weight allergens can induce, in mice, immune responses of varying characteristics. An important question is the nature of the factors that cause polarization. In this context the functional heterogeneity of dendritic cells (DC) may be a critical factor. In theory, selectivity of T-cell responses could be achieved either by interaction with discrete functional subpopulations of DC41,42 or by the adaptive acquisition by DC of properties that encourage polarization.43 With respect to the former, Maldonado-Lopez et al.41 have reported that lymphoid CD8α+ DC selectively induce Th1-cell differentiation, whereas CD8α– DC of myeloid origin favour Th2-type responses. Such heterogeneity may be amplified further by the changing phenotype of DC during maturation.43 It remains to be determined whether, and to what extent, the development in mice of polarized responses to sensitizing chemicals is a function of the innate and/or acquired characteristics of the DC populations with which the chemicals interact.
The data contained within this report reveal that not only does FITC induce a selective Th2-type immune response, but also that these cells are able to effect the DTH reaction. The assumption is that FITC-specific CD4+ Th2-type cells are able to gain access to the relevant skin tissues in response to challenge. The consensus view is that the homing of Th1-effector cells to sites of inflammation, including the skin, is facilitated by their expression of ligands for E- and P-selectin.44–46 In the absence of competition from Th1 cells, FITC-specific Th2-effector cells are presumably able to localize in the skin. The directed movement of Th2 cells to sites of cutaneous challenge may be effected, at least in part, through their possession of appropriate chemokine receptors, the expression of which may be further modified by the cytokine environment.47,48 Specifically it can be assumed that the recruitment of Th2 effector cells into skin sites will be favoured by the expression of CCR3 and CCR4 chemokine receptors and the absence of CCR7.47–49
In conclusion, the results reported here reveal that FITC is able to induce, in mice, a selective Th2-type CD4+ T-lymphocyte response characterized by the increased expression, by LNC, of type 2 cytokines and the production of IgE antibody. The data demonstrate also that CD4+ Th2-effector cells are able to cause DTH reactions in skin-sensitized mice.
Acknowledgments
This work was supported, in part, by a grant from the Health and Safety Executive, UK.
Abbreviations
- AOO, acetone
olive oil
- DBP, dibutyl phthalate
acetone
- DNCB
2,4-dinitrochlorobenzene
- DNFB
2,4-dinitrofluorobenzene
- DTH
delayed-type hypersensitivity
- FITC
fluorescein isothiocyanate
- LNC
lymph node cell
- MDI
diphenylmethane diisocyanate
- PCA
passive cutaneous anaphylaxis
- TMA
trimellitic anhydride
References
- 1.Cher DJ, Mosmann TR. Two types of murine helper T cell clones. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138:442–451. [PubMed] [Google Scholar]
- 2.Ohta A, Sato N, Yahata T, et al. Manipulation of Th1/Th2 balance in vivo by adoptive transfer of antigen-specific Th1 or Th2 cells. J Immunol Methods. 1997;209:85–92. doi: 10.1016/s0022-1759(97)00152-x. [DOI] [PubMed] [Google Scholar]
- 3.Dieli F, Asherson GL, Colonna Romano G, Sireci G, Gervasi F, Salerno A. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cell lines: role of γδ cells. J Immunol. 1994;152:2698–704. [PubMed] [Google Scholar]
- 4.Diamanstein T, Eckert R, Volk H-D, Kupier-Weglinski J-W. Reversal by interferon-γ of inhibition of delayed-type hypersensitivity induction by anti-CD4 or anti-interleukin 2 receptor (CD25) monoclonal antibodies. Evidence for the physiological role of the CD4+ TH1+ subset in mice. Eur J Immunol. 1988;18:2101–3. doi: 10.1002/eji.1830181237. [DOI] [PubMed] [Google Scholar]
- 5.Fong TAT, Mosmann TR. The role of IFN-γ in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989;143:2887–93. [PubMed] [Google Scholar]
- 6.Kondo S, Beissert S, Wang B, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene-targeted mouse. J Invest Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 7.Finkelman FD, Katona IM, Urban JF, Holmes J, Ohara T, Tung AS, Sample JG, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–41. [PubMed] [Google Scholar]
- 8.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwami T, Nagai H, Tsuruoka N, Koda A. Effect of murine recombinant interleukin-5 on bronchial reactivity in guinea-pigs. Clin Exp Allergy. 1993;23:32–8. doi: 10.1111/j.1365-2222.1993.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 10.Li XM, Schofield BH, Wang QF, Kim KH, Huang SK. Induction of pulmonary allergic responses by antigen-specific Th2 cells. J Immunol. 1998;160:1378–84. [PubMed] [Google Scholar]
- 11.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, Kay AB, Hamid Q. Cytokine messenger RNA expression for IL-3, IL-4, IL-5 and granulocyte/macrophage colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to eosinophilia. J Immunol. 1992;148:2390–4. [PubMed] [Google Scholar]
- 12.Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 13.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Gros G, Erard F. Non-cytotoxic, IL-4, IL-5, IL-10 producing CD8+ T cells: their activation and effector functions. Curr Opin Immunol. 1994;6:453–7. doi: 10.1016/0952-7915(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 15.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 16.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–8. [PubMed] [Google Scholar]
- 17.Ptak W, Askenase PW. γδ+ T cells assist αβ+ T cells in adoptive transfer of contact sensitivity. J Immunol. 1992;149:3503–8. [PubMed] [Google Scholar]
- 18.Dieli F, Asherson GL, Sireci G, Dominici R, Scire E, Salerno A. Development of IFN-γ-producing CD8+ γδ+ T lymphocytes and IL-2-producing CD4+ αβ+ T lymphocytes during contact sensitivity. J Immunol. 1997;158:2567–75. [PubMed] [Google Scholar]
- 19.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production. Interferon-γ-producing (Tc1) effector CD8+ T cells and interleukin (IL)-4/IL-10 producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Banerjee A, DiIulio NA, Fairchild RL. Development of effector CD8+ T cells in contact hypersensitivity occurs independently of CD4+ T cells. J Immunol. 1997;158:4721–8. [PubMed] [Google Scholar]
- 21.Bouloc A, Cavani A, Katz SI. Contact hypersensitivity in MHC class II-deficient mice depends on CD8 T lymphocytes primed by immunostimulatory Langerhans’ cells. J Invest Dermatol. 1998;111:44–9. doi: 10.1046/j.1523-1747.1998.00236.x. [DOI] [PubMed] [Google Scholar]
- 22.Kehren J, Desvignes C, Krasteva M, et al. Cytotoxicity is mandatory for CD8+ T cell-mediated contact hypersensitivity. J Exp Med. 1999;189:779–86. doi: 10.1084/jem.189.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Sad S, Kagi D, Mosmann TR. CD8 Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol. 1997;158:4152–61. [PubMed] [Google Scholar]
- 24.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, Larsen GL, Gelfand EW. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a murine model of airway sensitization. J Exp Med. 1996;183:1719–29. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 26.Macatonia SE, Knight SC. Dendritic cells and T cells transfer sensitization for delayed-type hypersensitivity after skin painting with contact sensitizer. Immunology. 1989;66:96–101. [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Imai Y, Irimura T. Contribution of dermal macrophage trafficking in the sensitization phase of contact hypersensitivity. J Immunol. 1998;161:6835–44. [PubMed] [Google Scholar]
- 28.Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA4 Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J Immunol. 1996;157:117–25. [PubMed] [Google Scholar]
- 29.Dearman RJ, Basketter DA, Kimber I. Differential cytokine production following chronic exposure of mice to chemical respiratory and contact allergens. Immunology. 1995;86:545–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Dearman RJ, Moussavi A, Kemeny DM, Kimber I. Contribution of CD4+ and CD8+ T lymphocyte subsets to the cytokine secretion patterns induced in mice during sensitization to contact and respiratory chemical allergens. Immunology. 1996;89:502–10. doi: 10.1046/j.1365-2567.1996.d01-778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dearman RJ, Mitchell JA, Basketter DA, Kimber I. Differential ability of occupational chemical contact and respiratory allergens to cause immediate and delayed dermal hypersensitivity reactions in mice. Int Arch Allergy Immunol. 1992;97:315–21. doi: 10.1159/000236139. [DOI] [PubMed] [Google Scholar]
- 32.Dearman RJ, Kimber I. Differential stimulation of immune function by respiratory and contact chemical allergens. Immunology. 1991;72:563–70. [PMC free article] [PubMed] [Google Scholar]
- 33.Dearman RJ, Kimber I. Divergent immune responses to respiratory and contact chemical allergens: antibody elicited by phthalic anhydride and oxazolone. Clin Exp Allergy. 1992;22:241–50. doi: 10.1111/j.1365-2222.1992.tb03079.x. [DOI] [PubMed] [Google Scholar]
- 34.Bellinghausen I, Brand U, Enk AH, Knop J, Saloga J. Signals involved in the early TH1/TH2 polarization of an immune response depending on the type of antigen. J Allergy Clin Immunol. 1999;103:298–306. doi: 10.1016/s0091-6749(99)70505-1. [DOI] [PubMed] [Google Scholar]
- 35.Wang L-F, Sun C-C, Wu J-T, Lin R-H. Epicutaneous administration of hapten through patch application augments TH2 responses which can downregulate the elicitation of murine contact hypersensitivity. Clin Exp Allergy. 1999;29:271–9. doi: 10.1046/j.1365-2222.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 36.Askenase PW, Kawikova I, Paliwal V, Akahira-Azuma M, Gerard C, Tugli T, Tsuji R. A new paradigm of T cell allergy: requirement for the B-1 cell subset. Int Arch Allergy Immunol. 1999;118:145–9. doi: 10.1159/000024052. [DOI] [PubMed] [Google Scholar]
- 37.Salerno A, Dieli F, Sireci G, Bellavia A, Asherson GL. Interleukin-4 is a critical cytokine in contact sensitivity. Immunology. 1995;84:404–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Asada H, Linton J, Katz SI. Cytokine gene expression during the elicitation phase of contact sensitivity: regulation by endogenous IL-4. J Invest Dermatol. 1997;108:406–11. doi: 10.1111/1523-1747.ep12289700. [DOI] [PubMed] [Google Scholar]
- 39.Traidl C, Jugert F, Krieg T, Merk H, Hunzelmann N. Inhibition of allergic contact dermatitis to DNCB but not to oxazolone in interleukin-4-deficient mice. J Invest Dermatol. 1999;112:476–82. doi: 10.1046/j.1523-1747.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 40.Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Sihohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484–91. [PubMed] [Google Scholar]
- 41.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8α+ and CD8α– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rissoan M-C, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu Y-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–8. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 43.Kalinski P, Hilkens CMU, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 44.Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–3. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 45.Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162:3193–201. [PubMed] [Google Scholar]
- 46.Chu A, Hong K, Berg EL, Ehrhardt RO. Tissue specificity of E- and P-selectin ligands in Th1-mediated chronic inflammation. J Immunol. 1999;163:5086–93. [PubMed] [Google Scholar]
- 47.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Nature. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]