Abstract
Experiments reported herein demonstrate that activation of bovine B cells via surface immunoglobulin M (sIgM) cross-linking, analogous to T-cell independent (TI-2) antigenic stimulation, results in the expression of CD5. Interestingly, in the presence of CD40 ligand, sIgM-mediated induction of CD5 on B cells was inhibited. These findings indicate that activation of bovine B cells via B-cell receptor (BCR) cross-linking results in a CD5+ B-cell phenotype and that CD40 signalling is inhibitory to this process. Analysis of cytokine mRNA indicates that bovine B cells constitutively express tumour necrosis factor-α (TNF-α) and interleukin (IL)-1β transcripts in vitro, while IL-10 mRNA expression is induced following sIgM cross-linking. IL-12 p40 transcripts were produced by B cells activated by CD40, but not by BCR, ligation. Analysis of cytokine receptor mRNA indicates that activation through CD40, in the presence or absence of IgM cross-linking, results in increased IL-4 receptor-α (IL-4Rα), IL-13Rα1 and interferon-α receptor 1 (IFN-αR1) mRNA levels. Overall, these findings suggest that activation of bovine B cells through BCR cross-linking yields an activation phenotype that differs substantially from that of B cells activated through CD40.
Introduction
B-cell responses to both T-cell-independent (TI) and T-cell dependent (TD) antigens play an integral role in the generation of protective humoral immune responses to various pathogens. In contrast to TD antigens, TI antigens are capable of activating B cells in the absence of major histocompatibility complex (MHC) class II-restricted T-cell help. TI-2 antigens, unlike TI-1 antigens, are inherently non-mitogenic and activate B cells by cross-linking surface immunoglobulin (sIgM) via repeating epitopes.1 Examples of pathogen-derived TI-2 antigens include trypanosome variable surface glycoprotein (VSG), vesicular stomatitis virus (VSV) glycoprotein and bacterial capsular polysaccharides.1 In contrast to TI-2 responses, the generation of humoral responses to TD antigens is thought not to require antigen cross-linking of surface immunoglobulin.2 Instead, these responses are dependent upon direct interactions between cell-surface receptors expressed by T and B cells, the most critical of which is the CD40–CD40 ligand (CD40–CD40L) interaction.3
Studies conducted on mouse splenic B cells have demonstrated that TI-2 activation (via sIgM cross-linking),4 unlike TD antigen activation, results in the expression of CD5 on B cells.5,6 CD5 is a membrane glycoprotein expressed on T cells and a subset of B cells, termed B-1 cells.7–9 CD5+ B cells have been reported to exist in a number of species, although the proportion of B cells expressing CD5, as well as their anatomical localization, varies among species. In some species, such as rabbit10 and chicken,11 nearly all B cells express CD5. Bovine CD5+ B cells have been reported to constitute up to 30% of the adult peripheral blood lymphocyte population, yet are absent from Peyer’s patches and lymph nodes.12 Interestingly, a recent study conducted in sheep, a species closely related to the bovine, has suggested that although the CD5+ CD11b+ B cells found in sheep display a B-1-like B-cell phenotype, based on their behaviour, sheep B-1 and B-2 subsets cannot be considered to be truly homologous to the B-cell subpopulations described in mice.13
CD5+ B cells are known to play a role in bovine immune responses to pathogens such as Trypanosoma congolense12,14 and bovine leukaemia virus (BLV).15 However, studies aimed at elucidating the origination and function of this B-cell population during these infections, as well as other infections in cattle, are lacking. Additionally, few studies have examined the potential influence that particular pathways of activation can have on the phenotype of an activated bovine B cell. Given the relative importance that both TI-2 and TD B-cell responses play in eliciting protective immunity to the broad range of pathogens encountered by cattle, we sought to investigate the effect of TI-2 stimulation (via B-cell receptor [BCR] cross-linking) and CD40 ligation (the critical signal required to drive the TD B-cell responses) on B-cell phenotype. We present evidence that bovine CD5– B cells express CD5 and CD11b in response to activation by BCR cross-linking but not in response to CD40 ligation. In addition, we report for the first time that, depending on the activation stimulus, bovine B cells can be activated to express mRNA for a variety of cytokines and cytokine receptors. Ultimately, these data demonstrate that activation of bovine B cells through BCR cross-linking yields a different phenotype than that observed for B cells activated through CD40.
Materials and methods
Animals
Blood donors were healthy Bos taurus animals between 4 and 12 months of age. Four separate animals, one heifer and three steers, were used for the following experiments. All animals were housed separately in an indoor facility.
CD5– B-cell isolation
Blood was collected in anticoagulant and incubated for 25 min at 37° with carbonyl iron (Sigma, St. Louis, MO), at a final concentration of 0·1 mg/ml, to remove phagocytic cells. Following 20 min of centrifugation at 900 g, buffy coats were harvested and residual red blood cells were lysed in red-blood cell lysis buffer. B cells were then selected by passive panning, as previously described.16 Briefly, peripheral blood mononuclear cells (PBMCs) were resuspended at a concentration of 1 × 107 cells/ml in panning solution: 3% bovine serum albumin (BSA), 10 m m Tris, 50 µg/ml of gentamicin, 0·0025 m m CaCl2 and 0·002 m m MgCl2 in Hanks’ balanced salt solution (HBSS), and allowed to adhere to plastic tissue culture plates for 1 hr. Non-adherent cells were removed by washing twice with HBSS, and adherent cells (enriched B cells) were harvested by vigorous pipetting. Residual CD3+ T cells were removed from enriched B cells by treatment with an antibody to bovine CD3, MM1A (Washington State University Monoclonal Antibody Center [WSU MAb Center], Pullman, WA), followed by magnetic depletion using sheep anti-mouse immunoglobulin G (IgG)-coated magnetic beads (Dynal Inc., Lake Success, NY). CD5-expressing T and B cells were further depleted by treatment with a mouse monoclonal antibody (mAb) specific for bovine CD5, CACT105A (WSU MAb Center), followed by magnetic bead depletion. Cells were routinely 90–95% IgM+ and less than 5% CD5+, as determined by flow cytometric analysis.
B-cell culture conditions
B cells were cultured, at a concentration of 2 × 106 cells/ml, in cRPMI containing 10% fetal calf serum (FCS). F(ab′)2 fragments of goat anti-bovine IgM (Kirkegaard and Perry Laboratories, Gaithersburg, MD) were generated using pepsin cleavage (Immobilized Pepsin; Pierce, Rockford, IL). To ensure that the antibody preparation was endotoxin free, cleaved F(ab′)2 fragments were incubated with immobilized polymyxin B (Sigma) and subjected to the Limulus amoebocyte assay (endotoxin levels were ≤ 0·7 ng per 1 µg of antibody) (Associates of Cape Cod, Falmouth, MA). Surface IgM cross-linking was carried out using F(ab′)2 fragments of goat anti-bovine IgM. In some cases, IgM was cross-linked using polyclonal goat anti-bovine IgM or monoclonal mouse anti-bovine IgM (BM-23; Sigma) coupled to agarose avidin D beads (Vector Laboratories, Burlingame, CA) at a concentration of 50–60 µl of 1 mg/ml antibody-coated beads per ml of culture. Culture of B cells with a murine fibroblast cell line, DAP3, stably transfected with bovine CD40L (boCD40L–DAP3) was carried out at a ratio of one CD40L–DAP3 cell to every four B cells. Transfected cells and non-transfected DAP3 cells were treated with 50 µg/ml of mitomycin C for 30 min at 37°, washed three times in HBSS and allowed to adhere to the plastic wells for at least 1–2 hr. Before B cells and cross-linking antibody reagents were added to the wells, residual unbound fibroblasts were removed. Cells to be used for RNA analysis were harvested, washed in HBSS and pelleted. Supernatants were removed and cell pellets were snap-frozen in liquid nitrogen and stored at −70°.
Cell proliferation assays were conducted on B cells cultured (in triplicate) at a concentration of 1 × 105 B cells per well in a 96-well plate. B cells were pulsed with 1 µCi of [3H]thymidine (NEN-DuPont, Boston, MA) following 72 hr of culture and harvested 18 hr later onto Skatron filter mats (Skatron Instruments, Lier, Norway) using a cell harvester (Skatron Instruments). Thymidine incorporation was determined by scintillation counting (Beckman Instruments, Fullerton, CA).
Flow cytometry
The following antibodies specific for bovine antigens were used for staining: CC17 recognizing CD5 (Serotec, Raleigh, NC); MM10A recognizing CD11b and MM1A recognizing CD3 (WSU MAb Center); F(ab′)2 goat anti-bovine IgM-conjugated fluorescein isothiocyanate (FITC) (Kirkegaard and Perry Labs; F(ab′)2 fragments generated as described above); and monoclonal mouse anti-bovine IgM (BM-23; Sigma). Secondary detection antibody reagents used included rat anti-mouse IgG1-conjugated phycoerythrin (PE) (Becton-Dickinson, San Jose, CA) and rat anti-mouse IgG2a/b–FITC (Pharmingen, San Diego, CA). Antibodies used as negative staining controls for establishing proper gates included the secondary detection antibodies (listed above), alone and in conjunction with isotype-matched mouse IgG1 and mouse IgG2a (Sigma). Cells were fixed in 2% buffered paraformaldehyde and analysed using a fluorescence-activated cell sorter (FACS) Vantage flow cytometer and cellquest acquisition and analysis programs (Becton-Dickinson).
Ribonuclease protection assay
The ribonuclease protection assay (RPA) was performed on 3–5 µg of RNA extracted using an RNeasy RNA extraction kit (Qiagen, Chatsworth, CA). Probes were synthesized using the Riboprobe T7 System (Promega, Madison, WI) and [α32P]-UTP (3000 Ci/mmol) supplied by NEN-DuPont. Radiolabelled antisense RNA probes were generated using cDNA fragments of bovine cytokines, receptors and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cloned into the EcoRI/HindIII site of pGEM-4 (Promega) as templates. Probes were hybridized to RNA samples (as well as a tRNA control) overnight at 56°. Samples were RNase-treated, phenol–chloroform extracted and ethanol precipitated. Protected fragments were analysed on a 5% denaturing (8 m urea) polyacrylamide gel. Gels were dried and exposed to X-ray film overnight in the presence of intensifying screens. Where indicated, protected fragments were quantified by radioanalytical detection (AMBIS 4000; Ambis Inc., San Diego, CA).
Results
Surface IgM cross-linking induces CD5 protein and mRNA expression in bovine CD5– B cells
CD5+ B cells have been found to expand in number during certain viral and parasitic infections in cattle.12,14,15 Whether this expansion reflects the outgrowth of a pre-existing CD5+ B-cell population, or simply the acquisition of the CD5 marker as a result of B-cell activation, is unknown. To explore the potential likelihood of the latter, we sought to determine whether bovine CD5– B cells could be induced to express CD5 upon activation. In order to determine this, highly purified bovine CD5– B cells (> 90% IgM+, ≤ 5% CD5+) were obtained and cultured for 42 hr in medium alone or in the presence of 50 µg/ml of F(ab′)2 goat anti-bovine IgM (Fig. 1a, 1b). Flow cytometric analysis of these cells indicated that at this concentration of anti-IgM, greater than 50% of the B cells in culture were induced to express CD5 (53%, Fig. 1b). CD5 expression was also observed to be induced on B cells following activation with whole polyclonal goat anti-bovine IgM coupled to agarose avidin D beads, as well as with whole monoclonal mouse anti-bovine IgM (data not shown). In addition, as depicted in Fig. 1(c), RNase protection analysis indicated that IgM cross-linking results in the accumulation of CD5 mRNA over a 24-hr period.
Figure 1.
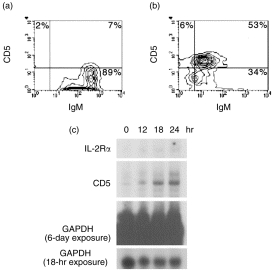
Induction of CD5 expression on bovine CD5– B cells by surface immunoglobulin M (sIgM) cross-linking. CD5– B cells (> 90% IgM+) were cultured in medium alone (a) or in the presence of goat anti-bovine IgM F(ab′)2 (50 µg/ml) (b) for 42 hr and then analysed for CD5 expression by flow cytometry. Results are representative of similar experiments carried out in three different animals. (c) Surface IgM cross-linking results in the accumulation of CD5 mRNA transcripts. B cells were cultured in the presence of polyclonal goat anti-bovine IgM conjugated to agarose avidin D beads for 12, 18, or 24 hr. Unstimulated B cells (time 0) and stimulated B cells were analysed by ribonuclease protection assay (RPA). A probe detecting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts was used to control for equal loading. Results are representative of two similar experiments. IL-2Rα, interleukin-2 receptor-α.
B-cell stimulation with a boCD40L-expressing transfected cell line inhibits anti-IgM-induced CD5 expression
Upon establishing that bovine CD5– B cells express CD5 in response to anti-IgM-mediated activation, a model TI-2-type stimulus, we investigated whether stimulation of B cells by CD40 ligation, the critical signal required for TD activation, would affect the expression of CD5. Unlike surface IgM cross-linking (Fig. 2c), activation of bovine CD5– B cells by a bovine CD40L-expressing DAP3 cell line16 did not result in an increase in the expression of CD5 protein over that observed for cells cultured with non-transfected DAP3 cells (Fig. 2a, 2b). Interestingly, when B cells were cocultured with CD40L transfectants and anti-IgM, the induction of CD5 surface expression was partially inhibited (Fig. 2d). At a lower concentration of goat anti-bovine IgM F(ab′)2 (10 µg/ml), B cells cocultured with CD40L–DAP3 transfectants were completely inhibited from expressing CD5, while at a higher concentration (50 µg/ml), the inhibition by CD40 ligation was almost completely overcome (data not shown).
Figure 2.
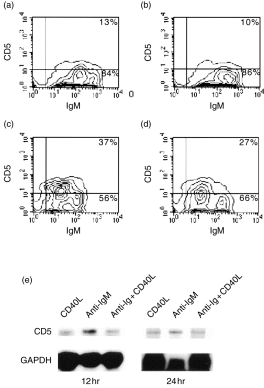
Activation of B cells by surface immunoglobulin M (sIgM) cross-linking, but not by CD40 ligation, results in the induction of CD5 expression. B cells were cultured for 42 hr in the presence of DAP3 cells alone (a), in the presence of CD40 ligand (CD40L)–DAP3 transfectants (b), in the presence of goat anti-bovine IgM F(ab′)2 (25 µg/ml) (c), or in the presence of both goat anti-bovine IgM and CD40L–DAP3 cells (d), and then analysed by flow cytometry. Results are representative of four independent experiments performed on three separate animals. (e) Activation of B cells by sIgM cross-linking results in a higher level of CD5 mRNA expression compared to levels observed for B cells activated through CD40 or CD40 combined with sIgM cross-linking. B cells were cultured, for 12 or 24 hr, in the presence of CD40L–DAP3 transfectants, in the presence of goat anti-bovine IgM F(ab′)2 (10 µg/ml), or in the presence of both goat anti-bovine IgM and CD40L–DAP3 cells, and analysed for CD5 mRNA expression by using the RNase protection assay. This experiment is representative of results obtained from three separate ribonuclease protection assays (RPAs) and one reverse transcription–polymerase chain reaction (RT–PCR) experiment.
Although B cells were inhibited from expressing CD5 when cultured under conditions in which IgM cross-linking is accompanied by ligation of CD40, they appeared to be synergistically activated by CD40 and sIgM stimulation, as demonstrated by proliferative responses (Table 1). Whereas small resting (percoll-fractionated) B cells cultured with mitomycin C-treated DAP3 cells only displayed a low level of proliferation in the presence or absence of goat anti-bovine IgM F(ab′)2, B cells cocultured with CD40L–DAP3 cells alone proliferated to levels that were fourfold greater than background proliferation. While anti-IgM did not result in significant B-cell proliferation, when combined with CD40 ligation, B-cell proliferation occurred at levels that were 10-fold greater than background proliferation. Thus, although the simultaneous stimulation of B cells with TD and TI-2 activators resulted in a higher degree of activation than either stimulation alone, signalling via CD40 interferes with anti-IgM-induced CD5 expression.
Table 1.
B cell proliferation at 4 days following stimulation with CD40L-transfectants and/or 25 μg/ml goat anti-bovine IgM F(ab’)2
| Treatment | Mean c.p.m.±SE | Stimulation index* |
|---|---|---|
| B cells+DAP3 cells | 352±4 | 1.0 |
| B cells+CD40L–DAP3 cells | 1342±373 | 3.8 |
| B cells+anti-IgM+DAP3 cells | 357±18 | 1.0 |
| B cells+anti-IgM+CD40L–DAP3 | 3502±802 | 9.9 |
| DAP3 cells only | 277±89 | – |
| CD40L–DAP3 cells only | 322±92 | – |
Stimulation index=experimental counts per minute (c.pm.)/c.p.m. of B cells+DAP3 cells.
To examine the possibility that CD40-mediated B-cell activation inhibited the induction of CD5 expression at the level of transcription, the effect of CD40 ligation on CD5 mRNA transcription in B cells was examined. CD5 mRNA expression was analysed by using the RPA for B cells cultured for 12 and 24 hr in the presence of either anti-IgM or CD40L–DAP3 cells alone, or in the presence of both anti-IgM and CD40L-DAP3 cells. As shown in Fig. 2(e), B cells cross-linked with goat anti-bovine IgM F(ab′)2 showed greater detectable levels of CD5 mRNA transcripts than B cells stimulated with CD40L-expressing transfectants. Phosphorimage analysis of protected CD5 and GAPDH fragments indicated that at 24 hr poststimulation, the ratio of CD5 to GAPDH signals for B cells treated with anti-IgM was nearly twofold greater than the ratio determined for B cells cocultured with CD40L-expressing DAP3 cells, regardless of whether anti-IgM was present. Thus, CD40 signalling may function to inhibit IgM-mediated CD5 protein expression at the level of transcription.
In an attempt to further characterize phenotypic changes that occur in bovine CD5– B cells following different modes of activation, we chose to determine the effect that these stimuli had on CD11b (Mac-1) expression. Mac-1 expression is a phenotypic characteristic of murine peritoneal B-1 cells.7 Additionally, CD11b is expressed on a subpopulation of both normal bovine and human peripheral blood B cells.12,17 In cattle, CD11b is often found to be coexpressed with CD5 on peripheral blood B cells.12In vitro, bovine CD5– B cells cultured in the presence of anti-IgM were marginally induced to express CD11b (14%) compared to B cells cultured with CD40L–DAP3 cells in the presence or absence of anti-IgM cross-linking (5 and 4%, respectively) (data not shown). Thus, unlike the large CD5 induction observed for B cells stimulated by sIgM cross-linking, only a small induction of CD11b was observed. CD11b induction on B cells may require additional signals for optimal expression. Notably, not all anti-IgM-mediated increases in surface marker expression were inhibited by ligation of CD40. The increase in MHC class II expression on bovine B cells observed following BCR cross-linking, for example, is not inhibited but is instead enhanced by costimulation through CD40 (data not shown).
Cytokine receptor expression by bovine CD5– B cells
In order to determine the potential for responsiveness to various cytokines, we analysed the expression of several cytokine receptors expressed by activated bovine B cells at the mRNA level. A representative mRNA analysis performed on B cells activated through IgM and/or CD40 for 12 hr is shown in Fig. 3. The mRNA for interferon-α receptor 1 (IFN-αR1), a component of the type I IFN receptor for cytokines such as IFN-α, -β and -τ, was up-regulated following 12 hr of stimulation with CD40L–DAP3 cells, either in the presence or in the absence of IgM cross-linking. IgM cross-linking alone, however, did not enhance the level of IFN-αR1 mRNA over that detected in B cells cultured with non-transfectants only. Similarly, interleukin-4 receptor-α (IL-4Rα) and IL-13Rα1 mRNA transcripts were found to be increased in B cells stimulated through CD40, but not through IgM. Compared to the level of IL-13Rα1 mRNA detected in unstimulated bovine PBMCs, IL-13Rα1 mRNA expression in bovine B cells stimulated through CD40 was low. Similar to the findings reported here, human tonsillar B cells activated through CD40 have been shown to up-regulate both IL-4Rα and IL-13Rα1.18–20 Unlike the differences seen in IFN-αR, IL-4Rα and IL-13Rα1 levels, the level of mRNA detected for the common γ-chain (γc chain), a component of a variety of cytokine receptors, including the IL-2, IL-4, IL-7, IL-9, IL-13 and IL-15 receptor complexes, remained relatively constant in B cells under the experimental conditions of this study. Similar cytokine receptor mRNA patterns were obtained when B cells, activated for 24 hr, were analysed.
Figure 3.
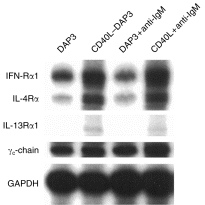
Analysis of B-cell cytokine receptor mRNA production by the RNase protection assay using various modes of activation. mRNA was extracted and analysed from CD5 B cells, cultured for 12 hr in the presence of: DAP3 cells, CD40 ligand (CD40L)–DAP3 cells, goat anti-bovine IgM F(ab′)2 (25 µg/ml), or both CD40L transfectants and anti-immunoglobulin M (IgM). Results are representative of three experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; γc-chain, common γ-chain; IFN-αR1, interferon-α receptor 1; IL-4Rα, interleukin-4 receptor-α; IL-13Rα, interleukin-13 receptor-α.
Bovine CD5– B-cell cytokine mRNA expression following sIgM cross-linking and CD40 ligation
In addition to characterizing the receptor phenotype of CD5– B cells activated by various stimuli, we were interested in characterizing the potential for cytokine production by bovine B cells following activation. Given the paucity of reagents available for bovine cytokine protein analysis, we investigated the production of cytokine mRNA by bovine B cells, 12 and 24 hr after activation, by using the RNase protection assay. As shown in Fig. 4(a), B cells produce tumour necrosis factor-α (TNF-α) mRNA constitutively in vitro, and little difference between treatments was observed for TNF-α:β-actin ratios, as determined by using densitometry (data not shown). IL-1β mRNA was observed to be produced by B cells constitutively in vitro and was slightly increased following anti-IgM activation, where densitometric determination of IL-1β:actin signals indicated a 1·5-fold increase in anti-IgM-treated cells compared with cells cultured in medium alone (Fig. 4a and data not shown). Surface IgM cross-linking, however, was observed to result in the production of IL-10 mRNA by B cells to levels at least twofold higher (IL-10:actin) than the levels detected for cells cultured in medium alone or in the presence of CD40–DAP3 cells. The up-regulation of IL-10 transcription appeared to be inhibited by CD40 stimulation, as the IL-10:actin signal ratio calculated for B cells activated by sIgM cross-linking alone was 2–2·5-fold higher than that determined for B cells activated by both anti-IgM and CD40 ligation at 12 and 24 hr after stimulation, respectively. Other cytokines that were examined for, but not detected, at these time-points included IL-4, IL-5, IL-2, IL-1α and IFN-γ (data not shown). In addition to producing IL-10, we demonstrated that bovine B cells are capable of producing interleukin-12 p40 mRNA. As shown in Fig. 4(b), bovine B cells activated through CD40 for 12 hr produced detectable transcripts for IL-12 p40. Interestingly, activation of B cells through both CD40 and IgM, for either 12 or 24 hr, synergized to result in levels of IL-12 p40 mRNA that appeared to be slightly higher than those detected in B cells stimulated through CD40 or IgM alone (≈ 1·5-fold higher based on IL-12 p40:GAPDH densitometric readings; data not shown). Importantly, human B cells activated through CD40, but not through IgM, have been reported to produce bioactive IL-12.21
Figure 4.
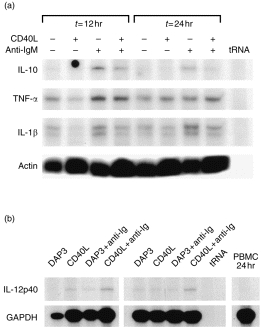
Analysis of B-cell cytokine mRNA production by the RNase protection assay. (a) CD5– B cells were cultured for 12 or 24 hr in medium alone, in the presence of CD40 ligand (CD40L)–DAP3 cells, in the presence of mouse anti-bovine immunoglobulin M (IgM) conjugated to agarose avidin D beads, or in the presence of both CD40L transfectants and anti-IgM. RNA was extracted and analysed for mRNA encoding interleukin (IL-10, IL-1β, tumour necrosis factor-α (TNF-α) and actin. Transcripts for interferon-γ (IFN-γ), IL-1α, IL-2, IL-4 and IL-5 were not detected (results not shown). (b) IL-12 p40 transcripts were measured in B cells cultured for 12 or 24 hr in the presence of: control DAP3 cells, CD40L transfectants, anti-IgM and DAP3 cells, or CD40L-transfectants along with anti-IgM. Peripheral blood mononuclear cells (PBMCs) cultured for 24 hr did not produce IL-12 p40 mRNA. Results are representative of two independent experiments.
Discussion
Herein, we present evidence that bovine B cells can be activated in vitro to express a CD5+ phenotype. Extrapolating to B-cell activation in vivo, our findings suggest that bovine B cells encountering microbial or parasitic TI-2 antigens in vivo may be induced to express CD5 and, potentially, CD11b, under certain conditions. Expansion of the CD5+ CD11b+ B-cell population has been observed to occur in cattle following infections with both BLV15 and T. congolense.12 During the immune response to these infections, parasite or virally derived TI-2 antigens may activate bovine B cells (via surface IgM cross-linking) to express CD5, thereby resulting in the observed increase in the number of CD5+ B cells. Importantly, concomitant ligation of CD40 (via interaction of B cells with activated T cells) would potentially prevent bovine B cells from expressing B-1-like markers, yet would probably result in enhanced B-cell activation and proliferation.
The consequences of CD5 induction on B cells are not completely known, although it has been suggested that CD5 may negatively regulate signalling through the BCR.22 A study conducted in the rabbit, however, has suggested that CD5 may bind immunoglobulin framework regions and thus potentially influence B-cell selection.23 Although a variety of functions can be hypothesized, further studies, including those aimed at identifying the ligand(s) for bovine CD5, are required in order to determine the significance of CD5 expression on bovine B cells.
Our findings with cytokine receptor mRNA expression indicate that CD40 signalling, unlike IgM signalling, activates bovine B cells to up-regulate IFN-αR1, IL-4Rα and IL-13Rα1 at the mRNA level. Whether these increases also occur at the protein level is presently unknown. However, if this were indeed the case, B cells activated by CD40–CD40L interactions would be expected to become more responsive to the cytokines that bind these receptors compared to those activated by surface receptor cross-linking alone. This a reasonable hypothesis with respect to IL-4-driven B-cell responses, as bovine B cells activated through CD40 exhibit higher levels of proliferation and immunoglobulin secretion in response to IL-4 than B cells activated by BCR cross-linking and IL-4 alone.24 Further studies, however, are required to determine if these observed increases in cytokine receptor mRNA transcripts, caused by CD40 ligation, correspond to increased B-cell responsiveness to cytokines, such as IL-4, IL-13 and type I IFNs.
Studies conducted in the mouse have suggested that CD5+ B cells, peritoneal B cells in particular, are the primary producers of B-cell-derived IL-10.25,26 Production of IL-10 by B-1 cells has been proposed to serve as an autocrine growth factor, as depletion of IL-10 results in the reduction of B-1 cells.27,28 We present evidence that, in addition to acquiring a CD5+ B-cell phenotype, bovine B cells produce IL-10 mRNA in response to IgM cross-linking. Conversely, CD40 ligation appears to stimulate bovine B cells to produce IL-12 p40 mRNA. These findings suggest that bovine B cells may participate in skewing the immune response through cytokine production. However, in order to develop a more complete understanding of the significance of bovine B-cell cytokine production in response to CD40 ligation or IgM cross-linking, cytokine production, like cytokine receptor expression, must be further analysed at the protein level.
Acknowledgments
This work was supported by the United States Department of Agriculture NRICGP project no. 96-35204-3584 and by the University of Missouri Agricultural Experiment Station. We thank Louise Barnett for her technical assistance in FACS analysis and the Limulus amoebocyte assay. We also thank Wendy Trigona for providing several of the cytokine receptor probes, and Bill Davis for supplying some of the antibodies against bovine antigens.
Glossary
Abbreviations
- BCR
B-cell receptor
- BLV
bovine leukaemia virus
- CD40L
CD40 ligand
- CR3
complement receptor 3
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PE
phycoerythrin
- RPA
ribonuclease protection assay
- sIgM
surface immunoglobulin M
- TD
T-cell dependent
- TI
T(thymus)-cell independent
References
- 1.Mond JJ, Lees A, Snapper CA. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 2.Tony HP, Phillips NE, Parker DC. Activation of murine B lymphocytes by anti-immunoglobulin is an inductive signal leading to immunoglobulin secretion. J Exp Med. 1980;162:1695. doi: 10.1084/jem.152.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle R. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:5911. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 4.Parker DC, Wadsworth DC, Schneider GB. Activation of murine B lymphocytes by anti-immunoglobulin is an inductive signal leading to immunoglobulin secretion. J Exp Med. 1980;152:138. doi: 10.1084/jem.152.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying ZIC, Rabin E, Wortis HH. Treatment of murine CD5– B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int Immunol. 1991;3:467. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- 6.Wortis H, Teutsch M, Higer M, Zheng J, Parker D. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci USA. 1995;92:3348. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monohar V, Brown E, Leiserson WE, Chused TM. Expression of Lyt-1 by a subset of B lymphocytes. J Immunol. 1982;129:532. [PubMed] [Google Scholar]
- 8.Huang H-JS, Jones NH, Strominger JL, Herzenberg LA. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu1/T1 (CD5) Proc Natl Acad Sci USA. 1987;84:204. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor A. A new nomenclature for B cells. Immunol Today. 1991;12:388. doi: 10.1016/0167-5699(91)90135-G. [DOI] [PubMed] [Google Scholar]
- 10.Chander R, Knight KL. CD5+ B cells predominate in peripheral tissues of rabbit. J Immunol. 1992;149:3858. [PubMed] [Google Scholar]
- 11.Koskinen R, Gobel TWF, Tregaskes CA, Young JR. The structure of avian CD5 implies a conserved function. J Immunol. 1998;160:4943. [PubMed] [Google Scholar]
- 12.Naessens J, Williams DJL. Characterization and measurement of CD5+ B cells in normal and Trypanosoma congolense-infected cattle. Eur J Immunol. 1992;22:1713. doi: 10.1002/eji.1830220708. [DOI] [PubMed] [Google Scholar]
- 13.Chevallier N, Berthelemy M, Laine V, et al. B-1-like cells exist in sheep. Characterization of their phenotype and behaviour. Immunology. 1998;85:178. doi: 10.1046/j.1365-2567.1998.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buza J, Sileghem M, Gwakisa P, Naessens J. CD5+ B lymphocytes are the main source of antibodies reactive with non-parasite antigens in Trypanosoma congolense-infected cattle. Immunology. 1997;92:226. doi: 10.1046/j.1365-2567.1997.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meirom R, Brenner J, Trainin Z. BLV-infected lymphocytes exhibit two patterns of expression as determined by Ig and CD5 markers. Vet Immunol Immunopathol. 1993;36:179. doi: 10.1016/0165-2427(93)90106-e. [DOI] [PubMed] [Google Scholar]
- 16.Estes DM, Tuo W, Brown WC, Goin J. Effects of typeI/typeII interferons and transforming growth factor-β on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology. 1998;95:604. doi: 10.1046/j.1365-2567.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5– B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690. [PMC free article] [PubMed] [Google Scholar]
- 18.Siepmann K, Wohlleben G, Gray D. CD40-mediated regulation of interleukin-4 signaling pathways in B lymphocytes. Eur J Immunol. 1996;26:1544. doi: 10.1002/eji.1830260721. [DOI] [PubMed] [Google Scholar]
- 19.Graber P, Gretener D, Herren S, et al. The distribution of IL-13 receptor alpha 1 on B cells, T cells and monocytes and its regulation by IL-13 and IL-4. Eur J Immunol. 1998;28:4286. doi: 10.1002/(SICI)1521-4141(199812)28:12<4286::AID-IMMU4286>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 20.Lee CE, Kim HI. Differential regulation of interleukin-4 receptor in normal and transformed B cells. Molecule Cells. 1995;5:493. [Google Scholar]
- 21.Schultze JL, Michalak S, Lowne J, et al. Human non-germinal center B cell interleukin-12 (IL-12) production is primarily regulated by T cell signals CD40 ligand, interferon-gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 23.Pospisil R, Fitts MG, Mage RG. CD5 is a potential selecting ligand for B cell surface immunoglobulin framework region sequences. J Exp Med. 1996;184:1279. doi: 10.1084/jem.184.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano A, Brown WC, Estes DM. Cloning, expression, and biological function of the bovine CD40 homologue: role in B-lymphocyte growth and differentiation in cattle. Immunology. 1997;90:294. doi: 10.1046/j.1365-2567.1997.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’garra A, Stapleton G, Dhar V, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 26.O’garra A, Chang R, Go N, Haughton G, Howard M. Ly-1 (B-1) B cells are the main source of B-cell-derived interleukin 10. Eur J Immunol. 1992;22:711. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 27.Ishida H, Hastings R, Thompson-snipes L, Howard M. Modified immunological status of anti-IL-10 treated mice. Cell Immunol. 1993;148:371. doi: 10.1006/cimm.1993.1119. [DOI] [PubMed] [Google Scholar]
- 28.Ishida H, Hastings R, Howard M. Multiple IL-10 antibody treatment blocks the development of Ly-1 lineage B cells. Ann N Y Acad Sci. 1992;651:264. doi: 10.1111/j.1749-6632.1992.tb24622.x. [DOI] [PubMed] [Google Scholar]


