Abstract
In this study, we investigated the potential of a DNA vaccine expressing the minimal cytotoxic T lymphocyte (CTL) epitope gp33 of the lymphocytic choriomeningitis virus glycoprotein to protect against infection of a non-lymphoid organ and compared this to protection against a systemic infection. Furthermore, since immune stimulatory sequences have been shown to augment CTL responses, we examined the capacity of CpG DNA to enhance CTL memory. The data show that DNA vaccination with a gp33-based gene construct induced short-lived gp33-specific CTL which protected against a systemic infection but not against a peripheral infection. Immune stimulatory sequences were incapable of either prolonging CTL memory or promoting protection against infection of a peripheral organ.
Introduction
Gene vaccination is an attractive approach to circumvent the deleterious side-effects of traditional protein vaccines. DNA vaccines have been reported to elicit humoral as well as T-cell responses.1,2 While significantly elevated antibody and helper T-cell responses are usually readily detected after gene vaccination, in many cases primary cytotoxic T lymphocyte (CTL) responses are not very easily detected directly ex vivo, suggesting that the CTL responses triggered by DNA vaccination are often weak. Nevertheless, DNA vaccination has been shown to prime CTL responses in numerous viral systems.1,2 In many of these cases, repetitive boosts were required to confer antiviral protection, while single immunizations usually resulted in detectable antiviral CTLs but not long-lived antiviral protection. Besides repetitive application, immunostimulatory sequences (ISS) containing CpG motifs have been associated with effective immunization.3–5
While ISS are very effective in augmenting antibody responses when co-administered with protein antigen6–9 and while an expression plasmid devoid of one defined ISS sequence was a poor stimulator for a T-cell response,3 little is known as to whether ISS are capable of enhancing CTL memory. It is widely debated whether long-lived CTL memory requires persisting antigen or not.10,11 Whether CTL memory is found to be long lived or not, critically depends on the read out system used. While protection against a systemic viral challenge does not require homing of effector or memory CTL to the site of infection, a peripheral challenge infection can only be controlled rapidly if CTL can home immediately to the site of infection, which is a feature of recently activated effector CTL.10,12
In this study we investigated the capacity of DNA vaccination to exert protection against a systemic versus a peripheral infection and whether administration of ISS modulated the quality, i.e. the effector status of CTL to protect against infections delivered through the two different formulations.
Materials and methods
Gene construct
A DNA insert coding for the FLAG Tag (DYKDDDDK) and the gp33 minimal CTL epitope flanked N-terminally by three leucines and C-terminally by four alanines was synthesized as an 86-mer oligonucleotide (GATCTatggactacaaagacgatgacgacaagCTACTACTAAAAGCTGTGTACAATTTCGCCACCT-GTGCTGCTGCTGCTGGAGCT; where lower case letters indicate the FLAG; bold letters indicate the (Leu)3 or (Ala)4 repeats, respectively; italic letters indicate gp33; and the underlining shows the BglII and the SacI restriction site overhangs, respectively). The complementary single-stranded oligonucleotides were synthesized such that hybridization of the two oligonucleotides resulted in BglII and SacI overhangs, respectively. After hybridization, the insert was cloned into the BglII/SacI linearized pEGFP-N3 expression vector (Clontech, Palo Alto, CA). The resulting plasmid was designated pEGFPL33A.
Mice and virus
Six- to ten-week-old C57BL/6 mice were bred at the Institut für Labortierkunde of the University of Zurich and kept under specific pathogen-free conditions according to institutional guidelines. Each experiment contained groups of three mice which were analysed individually. The lymphocytic choriomeningitis virus (LCMV) WE strain was used in this study. Virus titres were determined in a LCMV infectious focus assay as previously described.13 Recombinant vaccinia virus Vacc-G2 was originally obtained from D. H. L. Bishop and Vacc-VSV IND from Dr B. Moss. Vaccinia virus titres were determined as previously described.14
Cell transfections
COS7 and MBL-2 cells were grown in Dulbecco’s modified Eagle’s minimal essential medium, (DMEM) containing 5% fetal calf serum (FCS) and transfected with Lipofectamine (Gibco-BRL, Basel, Switzerland) according to the manufacturer’s specifications. MBL-2 stable transfectants were selected in 1 mg/ml G418. EGFP expression levels were determined in cell clones by flow cytometry using a fluorescence-activated cell sorter (FACScan; Becton Dickinson, Basel, Switzerland).
DNA immunization
For intradermal immunization the Helios Gene Gun System (Biorad, Hercules, CA) was used. One-micrometre gold beads were coated with the expression plasmid pEGFPL33A according to the manufacturer’s specifications. For co-coupling of ISS and pEGFPL33A, 600 µg of the phosphorothionated oligonucleotide TCCATGACGTTCCTGATGCT (1668 pt; all linkages modified)15 was co-precipitated together with 100 µg of circular pEGFPL33A. Coupling of plasmid DNA was confirmed as described in the manufacturer’s specifications by eluting DNA from the beads and visualizing eluted plasmid DNA by gel electrophoresis. Co-coupling of ISS with plasmid DNA did not significantly alter the amount of plasmid DNA which could be eluted from the gold beads.
Precipitation of the ISS onto the gold beads was confirmed by eluting DNA from six gene gun cartridges into H2O. The DNA was precipitated with ethanol in the presence of glycogen, resuspended and end-labelled with [γ-32P]ATP using polynucleotide kinase and an aliquot of the reaction mixture was separated on a 19% polyacrylamide gel. The gel was exposed for 3 min and the autoradiograph was analysed with a phosphoimager. A theoretical yield of at least 18 µg bound ISS was calculated by comparing the signal intensities. For immunization, 1 µg of pEGFPL33A DNA was applied per discharge. Each mouse received three non-overlapping discharges onto the shaved abdomen. A discharge pressure of 400 psi was set.
In vitro and in vivo restimulation
Mice were analysed individually. For in vitro restimulation 5 × 106 spleen cells were co-cultured in Iscove's modified Dulbecco's minimal essential medium [IMDM; supplemented with 10% FCS, penicillin/streptomycin, glutamine and 5 × 10−5 m 2-mercaptoethanol; in some experiments medium was supplemented with 5% concanavalin A (Con A) supernantant] with 2 × 105 LCMV-infected peritoneal exudate macrophages in a volume of 2 ml (24-well tissue culture plate). Macrophages were obtained by injecting mice intraperitoneally (i.p.) with 1 ml of thioglycollate on day − 6 and with 200 plaque-forming units (PFU) of LCMV WE on day −4. After 5 days, 1 ml of medium was removed and 100 µl of these standard cultures was serially diluted in duplicate (four steps, threefold dilutions) in minimum essential medium (MEM) supplemented with 2% FCS in 96-well round bottom plates and specific cytotoxic activity was determined. For in vivo restimulation, immunized mice were challenged intravenously (i.v.) with 5 × 105 PFU LCMV WE. After 4 days, spleen cell suspensions were prepared, lymphocytes were counted and then serially diluted as described above.
51Cr release assay
Specific cytotoxicity was determined in a standard 51Cr release assay as described.16 EL4 cells were coated with LCMV glycoprotein peptide gp33 (KAVYNFATM) at a concentration of 10−6 m and labelled in a total volume of 300 µl MEM/2% FCS with 250 µCi 51Cr for 2 hr at 37° on a rocking platform. The labelled target cells were washed three times and 104 cells were added to the effector cells in a final volume of 200 µl. After a 5-hr incubation at 37°, 60 µl of the supernatants was harvested and counted with a gamma-counter.
Statistical analysis
For statistical analysis the data were subjected to a Mann–Whitney U-test.
Results and discussion
Construction of the expression plasmid and detection and processing of the minimal CTL epitope
To best reveal the effect of ISS on CTL responses and CTL memory, we used the LCMV system in which DNA vaccination with the LCMV glycoprotein (GP) has been shown to be suboptimal. We fused the LCMV GP minimal CTL epitope gp33 (H-2Db-restricted) to enhanced green fluorescence protein (eGFP) to allow simple detection of the gene product (Fig. 1a). As a backup, the FLAG-tag was fused N-terminally to the fusion protein. Furthermore, to omit unpredictable negative interference of newly introduced amino acids with antigen processing, we flanked the epitope N-terminally with three leucines and C-terminally with four alanines. These amino acid residues are not expected to affect proteasome processing negatively (M. Groettrup, personal communication). The resulting expression plasmid was named pEGFPL33A.
Figure 1.
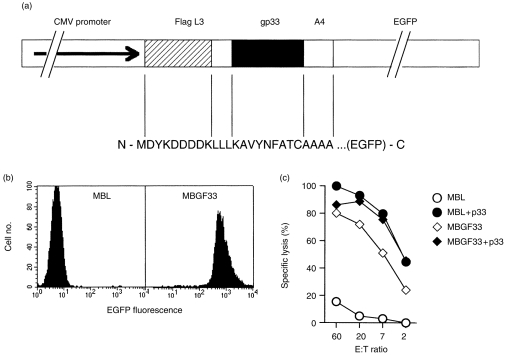
(a) The gp33 minimal epitope gene construct. The LCMV GP minimal epitope was flanked by three leucines and four alanines. For detection the FLAG tag was fused N-terminally and EGFP C-terminally to the minimal epitope. Expression of the fusion protein is driven by the cytomegalovirus (CMV) promoter. The expression plasmid was named pEGFPL33A. (b) Expression levels of the minimal epitope fusion protein. MBL-2 B-cell lymphoma cells were stably transfected with the fusion gene construct and expression levels of the minimal epitope gene product were determined by flow cytometry. One representative clone MBGF33 is shown. Non-transfected MBL-2 cells served as negative controls. (c) Specific lysis of MBGF33. Transfectants served as 51Cr-labelled target cells in a 5-hr cytotoxicity assay. LCMV-specific splenic effector CTL were obtained from mice which had been infected i.v. with 200 PFU of LCMV WE 8 days previously. To evaluate maximal antigen presentation and cytotoxicity the respective target cells were pulsed with gp33 peptide. Non-transfected MBL-2 cells served as controls. Spontaneous release was < 20% for all targets. A representative example of two independent experiments is shown.
To verify expression of the minimal epitope, COS7 fibroblasts were transiently transfected with the expression construct and analysed by fluorescence microscopy. Direct eGFP fluorescence was readily detected in transient transfectants (not shown). Expression of the fusion protein was also verified in immunohistological stainings of the same transfectants using the monoclonal antibody M2 directed against the FLAG-tag (data not shown). To assess whether the minimal epitope was correctly processed and presented on H-2Db, MBL-2 B-cell lymphoma cells were stably transfected with the expression construct. Expression levels of the fusion protein by individual clones was directly determined by monitoring EGFP fluorescence by flow cytometry and a representative clone was expanded (Fig. 1c). To assay for correct processing and presentation of the minimal gp33 CTL epitope, the stable transfectant was labelled with 51Cr and tested in a cytotoxicity assay using effector CTLs obtained from a mouse immunized with LCMV 8 days previously (Fig. 1b). Untransfected MBL-2 served as a negative control. MBGF33 were lysed and this lysis increased only marginally after addition of gp33, suggesting that these cells expressed fusion protein levels which were saturating to reach maximal sensitivity of target cells for CTL lysis. Thus, the minimal CTL epitope was correctly processed and presented.
CTL responses against gp33 are not enhanced by ISS
To assess whether DNA vaccination with pEGFPL33A primed gp33-specific CTL, mice were immunized once with three discharges of gold beads coated with the expression vector pEGFPL33A. Ten days after immunization spleen cells from individual mice were restimulated in vitro for 5 days with LCMV-infected macrophages. Mice that were immunized with LCMV 30 days previously served as positive controls for optimal priming (Fig. 2a, c). Two out of three DNA-vaccinated mice produced specific cytotoxic activities against gp33-coated target cells (Fig. 2a). After addition of 5% Con A supernatant to the cultures, spleen cell cultures from all mice generated specific cytotoxic activities (Fig. 2d), showing that one mouse was suboptimally primed. Thus, intradermal immunization with pEGFPL33A induced gp33-specific CTL. CTL and B-cell responses have been shown to be augmented by ISS, particularly when ISS were co-administered with protein or peptide antigen.6,8,17 Yet very little information is available on the capacity of ISS to specifically enhance CTL responses in conjunction with a DNA vaccine. To approach this question, we co-precipitated the expression plasmid pEGFPL33A, together with ISS (pEGFPL33A/ISS), on to gold beads, immunized mice as described above and restimulated spleen cells in vitro. Figure 2(b) shows that co-immunization with ISS did not significantly augment the gp33-specific CTL response when compared to Fig. 2(a) (P-values at dilution 1, 3, 9 and 27, respectively: P(1) = 0·27, P(3) = 0·27, P(9)= 0·51, P(27) = 0·83). Similarly, cultures prepared in the presence of Con A supernantant did not generate any significantly different responses if mice were primed with ISS (Fig. 2d,e: P(1) > 0·99, P(3) = 0·66, P(9) = 0·38, P(27) = 0·38). Weeratna et al. have recently shown that co-administration of expression plasmid and ISS may inhibit transgene expression, possibly through competition for cell surface receptors promoting DNA uptake.18 Although this may be of lesser importance for gene-gunned DNA because less DNA is administered and gold particles are thought to penetrate into the cell, we prepared cryosections from skin samples taken from the site of DNA application. Inspection by fluorescence microscopy for EGFP fluorescence confirmed that the fusion protein was produced in vivo at significant levels in the presence and absence of ISS (data not shown). Furthermore, to confirm that ISS were coupled to the beads, DNA was eluted from the beads, end-labelled with [γ-32P]ATP and separated on a polyacrylamide gel. As a control, 50, 10 and 1 pmol of ISS were labelled in parallel. Autoradiographs confirmed that ISS were coupled to the gold beads (Fig. 3). Thus, DNA vaccination resulted in the generation of gp33-specific CTL, but co-administration of ISS did not augment the CTL response.
Figure 2.
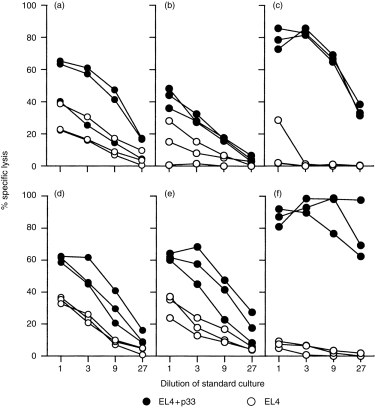
(a, d) DNA vaccination induces gp33-specific CTL. C57BL/6 mice were immunized once with pEGFPL33A-coated gold beads (three discharges on the shaved abdomen) using a Helios gene gun device. After 10 days spleen cells were restimulated in vitro for 5 days in medium without (a) or with (d) 5% Con A supernatant by co-culturing with LCMV-infected macrophages. Standard cultures were serially diluted and specific cytotoxicity was tested in a 5-hr 51Cr release assay on gp33-pulsed or unpulsed EL-4 target cells. Spontaneous release was < 20%. (b, e) ISS do not enhance the generation of gp33-specific CTL. Mice were immunized as described above with gold beads coated with pEGFPL33A and ISS. After 10 days spleen cells were restimulated for 5 days in the absence (b) or presence (e) of Con A supernatant and cultures were tested for gp33-specific cytotoxicity as described for (a). LCMV-immune mice (200 PFU LCMV i.v. 30 days before restimulation) served as controls (c, f). For statistical analysis the data were subjected to a Mann–Whitney U-test.
Figure 3.
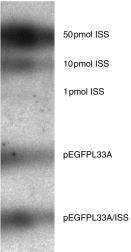
Detection of ISS coupled to the gold beads. Coupled DNA was eluted from the gold beads and radioactively end-labelled with [γ-32P]ATP using polynucleotide kinase. The labelled products were separated on a 19% polyacrylamide gel and detected in autoradiographs. In parallel the indicated amounts of ISS were end-labelled as controls.
ISS do not enhance CTL memory
To test whether gp33-specific CTL could be recovered after prolonged periods from mice immunized with the minimal epitope DNA vaccine and whether ISS had any beneficial effect on the longevity of these memory CTL, mice were immunized with pEGFPL33A- or pEGFPL33A/ISS-coated gold beads and restimulated in vivo 41 days after DNA vaccination. Figure 4(a) shows that gp33-specific CTL could be detected in a cytotoxicity assay and that co-immunization with ISS did not significantly increase this response (P-values at E:T ratios of 90, 30, 10 and 3, respectively: P(90) = 0·28, P(30) = 0·08, P(10) = 0·13, P(3) = 0·51). Thus, ISS did not seem to enhance the memory CTL response.
Figure 4.
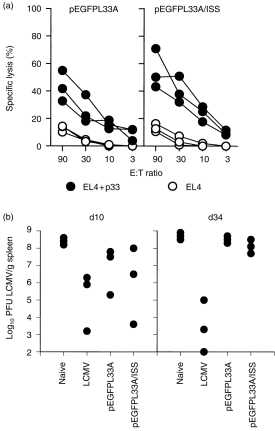
(a) Recovery of gp33-specific memory CTL after DNA vaccination. C57BL/6 mice were immunized with pEGFPL33A or pEGFPL33A/ISS as described for Fig. 2. After 41 days mice were restimulated in vivo by i.v. challenge with 5 × 105 PFU LCMV WE and 4 days thereafter spleen cells were assayed in a 5-hr 51Cr release assay. Since 4 days are not sufficient for effector CTL to develop from naive CTL, this read out system assays for memory CTL. EL-4 target cells were pulsed with gp33 peptide or were left unpulsed. Spontaneous release was < 20%. (b) Antiviral protection against systemic LCMV infection is short-lived after DNA vaccination. C57BL/6 mice were immunized with pEGFPL33A or pEGFPL33A/ISS as described for Fig. 2. LCMV infected (200 PFU LCMV i.v.) and naive mice served as controls. Ten or 34 days after immunization mice were challenged i.v. with 5 × 105 PFU LCMV WE and 3 days later LCMV titres were determined in the spleen. For statistical analysis the data were subjected to a Mann–Whitney U-test.
Memory CTL can be divided into two different populations, based on their capacity to mediate immediate effector function.19 While long-lived antigen-independent memory CTL display delayed effector function and are protective against systemic infections, they are not protective against infections of peripheral organs.10 On the other hand antigen-dependent memory CTL which phenotypically and functionally resemble effector CTL display immediate effector function and protect against peripheral infections. We therefore investigated whether DNA vaccination induced memory CTL which are protective against a systemic infection and/or infection of a peripheral organ and whether ISS enhanced the protective capacity of these responses. For this purpose, mice were vaccinated with pEGFPL33A or pEGFPL33A/ISS and challenged i.v. on day 10 or day 34 with 5 × 105 PFU LCMV for systemic infection. Three days later LCMV titres were measured in the spleen (Fig. 4b). The data show that the average LCMV titres were reduced by a factor of 10 in DNA-vaccinated (from 3·9 × 108 PFU in unprimed mice to 3·8 × 107 PFU in pEGFPL33A-primed mice and 3·5 × 107 PFU in pEGFPL33A/ISS-primed mice) if challenged 10 days after vaccination and that co-administration of ISS did not significantly augment antiviral protection when compared to mice vaccinated with pEGFPL33A alone (P = 0·83). Antiviral protection against this systemic infection was short-lived, since LCMV titres were not reduced in any of the DNA-vaccinated mice if challenged 34 days after immunization.
To test whether DNA vaccination induced (effector) memory CTL protective against infection of a peripheral organ, mice immunized with pEGFPL33A or pEGFPL33A/ISS were challenged i.p. after 10 days and 30 days with recombinant vaccinia virus expressing the LCMV GP (vacc-GP) and virus titres were measured in the ovaries. To rule out elimination of virus via a non-specific lymphokine-mediated mechanism, vaccinated mice were infected with an irrelevant recombinant vaccinia virus expressing the vesicular stomatitis virus glycoprotein (vacc-VSV). Only one out of six mice vaccinated 10 days before challenge had a reduced vacc-GP virus titre in the ovaries and ISS had no influence (Fig. 5). After 30 days none of the mice had reduced vacc-GP virus titres (not shown). Thus, despite co-immunization with ISS, DNA vaccination was not able to produce CTL protective against infection of a peripheral organ.
Figure 5.
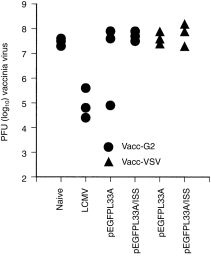
DNA vaccination fails to induce protection against infection of a peripheral organ. C57BL/6 mice were immunized with pEGFPL33A or pEGFPL33A/ISS as described for Fig. 2. Ten days after immunization mice were challenged i.p. with 2 × 106 PFU recombinant vaccinia virus expressing LCMV GP (Vacc-G2) or with irrelevant recombinant vaccinia virus (Vacc-VSV) as controls. LCMV-infected mice served as controls for virus clearance. Four days after the vaccinia challenge infection, vaccinia titres were measured in the ovaries.
This contrasts with recent studies showing that ISS improved the immunogenicity also of DNA vaccines.3,4 The difference may be explained by the possibility that ISS sequences need to be double stranded in order to exert an adjuvant effect. However, single-stranded oligonucleotide used herein readily activated resting B cells in vitro (Annette Oxenius, unpublished data). The observed difference may alternatively be explained by the read out system which was used to test for the effect of ISS. In most experiments, we used in vivo assays measuring the antivirally protective effect of DNA vaccination in the presence or absence of ISS. We believe that, particularly the measurement of protection of a peripheral organ against viral infection, from a biological point of view is the most significant parameter when evaluating the potential of a DNA vaccine, since many infections occur through a peripheral route. This is especially important in view of the fact that elevated CTLp frequencies measured after secondary restimulation in vitro do not necessarily correlate with antiviral protection in vivo.10 Antiviral protection by CTL against infection of solid, non-lymphoid tissue is more demanding, since the memory CTL need to be activated and have to home through solid tissue to the site of infection to exert antiviral effector function. In this respect our data points to one important feature of DNA vaccine-induced CTL memory. While DNA vaccination can induce memory CTL which are detectable after prolonged periods after secondary restimulation, these CTL promote limited antiviral protection against a systemic infection only if challenged within 10 days. Moreover, CTL induced after DNA vaccination were not able to protect against infection of a non-lymphoid solid organ. Thus, it appears that DNA vaccination induced mainly short-lived CTL protective against systemic infections. However, these CTL were not able to migrate to extra-lymphoid sites of infection to clear virus. This suggests that under the described conditions DNA vaccination leads to induction of resting but not effector (memory) CTL.19,20 Future potent vaccine strategies aiming at inducing antivirally protective CTL memory must therefore aim at generating effector memory CTL which protect not only against systemic but also against peripheral infections. This may be achieved by repetitive boosting21–23 with several antigenic epitopes, which however, is laborious, and/or by aiming at modulating the immune response by co-administration of lymphokine- and growth factor-producing expression plasmids24–26 or by ubiquitination.27 Yet, considering the emerging data, it seems that the two most important factors required to obtain potent long-lived antivirally protective CTL memory are the induction of initial CTL responses with a large clonal burst size28 and/or to ensure that antigen can persist.29 Thus, successful DNA vaccines for CTL memory should optimally aim at fulfilling these two prerequisites.
Acknowledgments
The authors would like to thank Professor Rolf Zinkernagel and Professor Hans Hengartner for their support and Dr Andrew MacPherson for helpful discussions. Special thanks to Karin Riem-Brduscha for technical assistance. This study was supported by the Kanton of Zurich.
References
- 1.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15(617):617. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 4.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635. [PubMed] [Google Scholar]
- 5.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 6.Sun S, Kishimoto H, Sprent J. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J Exp Med. 1998;187:1145. doi: 10.1084/jem.187.7.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman M, Martin-orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants [see comments] Nat Med. 1997;3:849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 8.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870. [PubMed] [Google Scholar]
- 9.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kündig TM, Bachmann MF, Oehen S, et al. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinkernagel RM, Bachmann MF, Kündig TM, Oehen S, Pircher H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996;14:333. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann MF, Kündig TM, Hengartner H, Zinkernagel RM. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without ‘memory T cells’? Proc Natl Acad Sci USA. 1997;94:640. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates [published errata appears in J Virol Methods 1991;35(1):115 and 1992;38(2):263] J Virol Methods. 1991;33:191. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 14.Binder D, Kündig TM. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J Immunol. 1991;146:4301. [PubMed] [Google Scholar]
- 15.Sparwasser T, Miethke T, Lipford G, et al. Bacterial DNA causes septic shock [letter] Nature. 1997;386:336. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 16.Hany M, Oehen S, Schulz M, et al. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol. 1989;19:417. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- 17.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 18.Weeratna R, Brazolot Millan CL, Krieg AM, Davis HL. Reduction of antigen expression from DNA vaccines by coadministered oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998;8:351. doi: 10.1089/oli.1.1998.8.351. [DOI] [PubMed] [Google Scholar]
- 19.Oehen S, Brduscha Riem K. Differentiation of naive CTL to effector and memory CTL: Correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338. [PubMed] [Google Scholar]
- 20.Zimmermann C, Brduscha-riem K, Blaser C, Zinkernagel RM, Pircher H. Visualisation, characterization and turnover of CD8+ memory T cells in virus-infected host. J Exp Med. 1996;183:1367. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarozinski CC, Fynan EF, Selin LK, Robinson HL, Welsh RM. Protective CTL-dependent immunity and enhanced immunopathology in mice immunized by particle bombardment with DNA encoding an internal virion protein. J Immunol. 1995;154:4010. [PubMed] [Google Scholar]
- 22.Martins LP, Lau LL, Asano MS, Ahmed R. DNA vaccination against persistent viral infection. J Virol. 1995;69:2574. doi: 10.1128/jvi.69.4.2574-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama M, Zhang J, Whitton JL. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sin JI, Kim JJ, Boyer JD, Ciccarelli RB, Higgins TJ, Weiner DB. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen DL, Dybdahl-sissoko N, McGregor MW, et al. Coadministration of DNA encoding interleukin-6 and hemagglutinin confers protection from influenza virus challenge in mice. J Virol. 1998;72:1704. doi: 10.1128/jvi.72.2.1704-1708.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez F, An LL, Harkins S, et al. DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size [see comments] Nature. 1994;369:652. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 29.Kündig TM, Bachmann MF, Ohashi PS, Pircher H, Hengartner H, Zinkernagel RM. On T cell memory: arguments for antigen dependence. Immunol Rev. 1996;150:63. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]


