Abstract
Substance-dependent individuals show disadvantageous decision-making, as well as alterated frontocortical recruitment when performing experimental tasks. We investigated whether substance-dependent patients (SDP) would show blunted recruitment of posterior mesofrontal cortex (PMC) by a conflict between concurrently-increasing reward and risk of penalty in a monetary game of “chicken.” SDP and controls performed: motor control (no reward) trials, guaranteed reward trials in which reward was not at risk, and risky trials where subjects were required to terminate their reward accrual before a secret varying time limit or else “bust” and forfeit that trial’s winnings (low penalty) or the current trial’s winnings plus an equal amount of previous winnings (high penalty). Reward accrual duration at risk of “busting” correlated negatively with trait neuroticism. The contrast between winning guaranteed reward versus non-reward activated the caudate head bilaterally in SDP but not controls. Accumulation of money at risk of low- or high-penalty (contrasted with accumulating guaranteed money) activated the PMC in both groups, but with a greater magnitude and more anterior extent in controls. Pre-decision signal increase in a PMC volume of interest negatively correlated with risk-taking in low-penalty trials, and was blunted in SDP relative to controls under both penalty conditions after controlling for individual differences in actual risk taking and the higher neuroticism of SDP. These data suggest that SDP are characterized by a combination of: a) striatal hypersensitivity to reward, and b) under-recruitment of the specialized conflict-monitoring circuitry of the PMC when reward entails potential penalties.
Keywords: Risk-taking, Decision-making, Reward, Anterior cingulate, Posterior mesofrontal cortex, Substance dependence, Alcohol, Cocaine, Striatum
1.0 Introduction
Poor impulse control correlates with current substance use (Barnes et al., 1999; Donovan and Jessor, 1985) and predicts future substance use (Masse and Tremblay, 1997; Myers et al., 1995) and dependence (Caspi et al., 1996; Moffitt et al., 2002). Comorbidity between substance use disorder (SUD) and attention-deficit hyperactivity disorder (Wilens, 2004), as well as antisocial (Sher and Trull, 2002) and borderline (Trull et al., 2000) personality disorders has been attributed to heritable traits underlying poor behavior control (Kreek et al., 2005; Slutske et al., 1998), such as dysfunctional frontal cortex (Jentsch and Taylor, 1999). For example, SUD subjects opt for rewards at risk of disproportionately severe penalties (Bechara et al., 2001) similar to subjects with frontal lobe lesions (Bechara et al., 1994).
Frontocortical dysfunction during decision-making in SUD is of interest because: 1) SUD is characterized by decisions to become intoxicated despite potential psychosocial, medical, and legal consequences, 2) SUD therapy invokes mental representations of the consequences of intoxication versus abstinence, and 3) substance abuse itself damages the frontal cortex (Bartzokis et al., 2002; Pfefferbaum et al., 1995). Functional magnetic resonance imaging (fMRI) studies consistently reveal frontocortical recruitment by behavior control tasks (Horn et al., 2003; Li et al., 2006a; Li et al., 2006b; Ridderinkhof et al., 2004), such as risk-taking (Rogers et al., 2004). SUD subjects also show blunted frontocortical glucose utilization (Gilman et al., 1990; Samson et al., 1986) and blood flow (Bolla et al., 2003), that correlate with reaction times (Dao-Castellana et al., 1998) and risky choices (Bolla et al., 2003; Fishbein et al., 2005) in decision-making tasks. These findings suggest a possibility that higher-order cortical regions that maintain or integrate representations of potential penalties for immediately rewarding behavior are impaired in persons with SUD.
When they are given an opportunity to obtain rewards at progressively increasing probability of penalty, might substance dependent patients (SDP) show reduced recruitment of frontocortical circuitry specialized for monitoring a risk/reward incentive conflict? We scanned SDP and controls while they performed a recently-introduced monetary risk-taking task (RTT) (Bjork et al., 2007). In the RTT, subjects passively accrued potential rewards, but were required to voluntarily terminate the accrual before a secret, varying time limit or they would “bust” and suffer a penalty of either non-reward or money loss. This contingency was intended to reflect two aspects of drug-taking: 1) the probability of a bad outcome (e.g. an overdose) can rise in conjunction with consumption magnitude, and 2) the subject is aware that bad outcomes are possible, but their specific probability is not signaled. The RTT also included motor-control (nonrewarded) and guaranteed reward trials. The RTT thus enabled two primary analyses. First, it enabled detection of brain activation by reward accrual itself, which may be normal (or increased) in SPD-- by contrasting fMRI signal change during guaranteed reward trials with signal change during motor control trials. Second, it enabled isolation of risk/reward conflict-elicited brain activation, which may be lower in SDP -- by contrasting signal change during risky reward accrual with signal change during reward accrual with no risk of a bad outcome (Bjork et al., 2007).
In an initial investigation of whether SUD is characterized by deficient contingency conflict-monitoring neurocircuitry, we assessed whether SDP show reduced risk/reward conflict-elicited recruitment of the posterior mesofrontal cortex (PMC). The PMC encompasses the supragenual anterior cingulate cortex (ACC) (Brodmann area 24), and extends superiorly and posteriorly to Brodmann areas 8, 6, and 32. The PMC features extensive connectivity with cortical regions that subserve cognitive control and motor execution, as well as amygdala and mesial orbitofrontal and striatal regions shown to govern motivation (Bush et al., 2002; Margulies et al., 2007; Paus, 2001). PMC is thus well-positioned anatomically to perform as a specialized integrator of both the emotional/motivational and cognitive calculation-based elements of a response conflict.
Accordingly, the PMC is reliably recruited by pre-decision conflicts (Ridderinkhof et al., 2004) as well as error avoidance (Magno et al., 2006) and feedback (Ullsperger and von Cramon, 2004). Critically, activity in this region is sensitive to the motivational and emotional aspects of conflict-monitoring (Taylor et al., 2006). Previous studies with response-conflict tasks have shown mesofrontal activation deficits in current (Kaufman et al., 2003) and abstinent (Li et al., 2006b) cocaine users, marijuana users (Gruber and Yurgelun-Todd, 2005), and opiate-dependent subjects (Forman et al., 2004).
Minimizing groupwise differences in bad outcomes is critical for interpreting functional activation of PMC in that error notifications also activate PMC (Ridderinkhof et al., 2004). For example, were SDP to experience significantly more error outcomes from their choices (as they do when performing the Iowa gambling task suboptimally (Bechara et al., 2001)), trait-like PMC pre-decision activation deficits in SDP might be masked by enhanced activation due to increased salience of (and motivation to avoid) aversive stimuli. To mitigate this, the RTT exploits how humans avoid risk to preserve modest gains (Kahneman and Tversky, 1979) to reduce individual differences in risk-taking and errors. In a previous variant of this task, SDP and controls took similar, minimal risks (Bjork et al., 2004a).
Because cocaine users show altered frontocortical activity during decision-making (Bolla et al., 2003) and typically drink heavily (Grant and Harford, 1990), we first applied the RTT to alcohol-dependent patients who also met lifetime criteria for cocaine abuse or dependence. We hypothesized that: 1) both SDP and controls would bust infrequently in the RTT to preserve existent winnings, and 2) SDP would show reduced recruitment of PMC while they decided “when to say ‘when’” in risky trials-- either analyzed singly or as a linear contrast with recruitment by guaranteed rewards.
2.0 Methods
2.1 Subjects
Procedures were approved by the Institutional Review Board of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). All participants provided written informed consent. Subjects were right-handed and free of neurological disease or other significant histories of illness as determined by physical examination and medical interviews conducted at the National Institutes of Health Clinical Center (CC) in Bethesda, MD. Control subjects (n = 17), age 23–46 (10 male; mean age 33.5), were recruited with community advertisement, and were free of any mental illness history as determined by structured clinical interviews for DSM-IV. SDP (n = 17), age 18–43 (10 male; mean age 32.9) were recruited from the inpatient alcoholism treatment unit at the CC. Patients with history of seizures, IQ<80, psychosis, or craniofacial features indicative of fetal alcohol syndrome (FAS) were excluded. Mood and behavior disorders were not exclusion criteria. All SDP met DSM-IV criteria for alcohol dependence. All SDP had a lifetime history of either cocaine dependence (n = 16) or abuse (n = 1), and most also had a lifetime history of either cannabis dependence (n=10) or abuse (n =1).1 All SDP reported alcohol misuse as the primary reason for hospitalization. Subjects were only scanned after complete withdrawal (> 1 week of sobriety in hospital).
2.2 The risk-taking task (RTT)
Stimuli were back-projected onto a screen and viewed using a head coil mirror. RTT trials were contiguously and pseudorandomly presented, 14 s in duration, and required the subject to press a button on a small button box twice during each of four types of trials (described below; Figure 1). A cumulative winnings counter was continuously displayed in black characters in the upper middle of the screen. Across three concatenated scanning runs, subjects completed 24 trials of each type. To facilitate task comprehension, trial types were denoted by screen background colors that reflected the hazards of proceeding at a traffic light.
Figure 1.
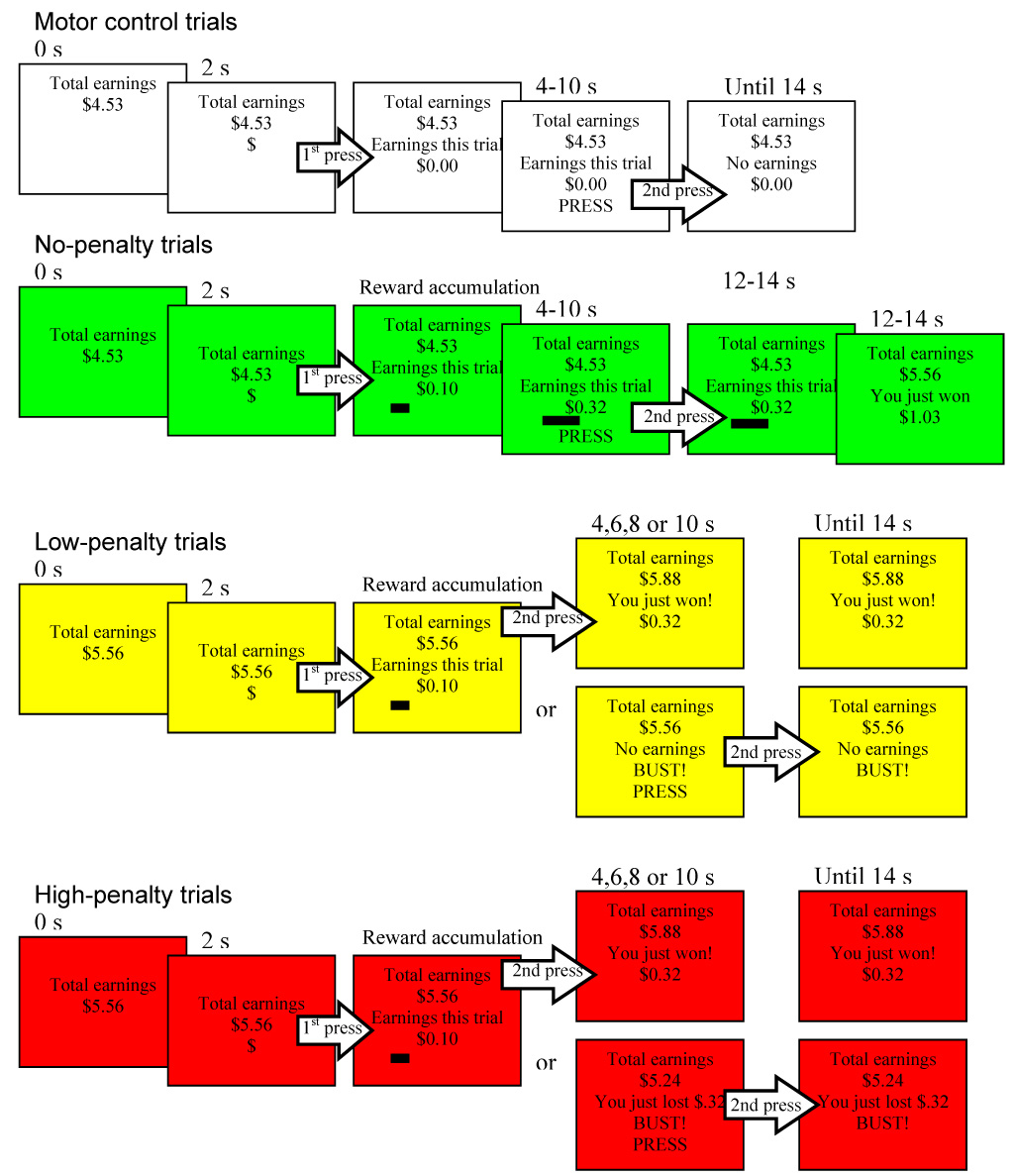
The risk taking task presented subjects with four types of pseudorandomly-presented trials (duration 14 s, n = 24 ea). In motor control trials, subjects pressed on cue twice (to the “$” and to the word “press”) for no incentive. In no-penalty trials, subjects began accruing money after pressing in response to the “$” cue, and accumulated winnings throughout the trial with no chance of penalty. In low-penalty trials, each trial was assigned a secret time limit of either 4, 6, 8, or 10 seconds after the $ cue, during which the subject was allowed to accumulate money. If the subject voluntarily stopped reward accrual before the secret time limit (top bifurcated outcome) he or she added accrued trial winnings to total winnings. If he or she failed to stop reward accrual before the secret time limit (bottom bifurcated outcome), he or she “busted” and forfeited all winnings that trial, and was instructed to press a second time. In high-penalty trials, subjects were also required to terminate reward accrual before the secret varying time limit, but busts resulted in subtraction of trial-accumulated winnings from previous winnings.
2.2.1 Motor Control trials
(white screen) Two seconds into the trial, a “$” appeared at the bottom middle of the screen, at which time subjects were instructed to press the response button. After responding, “Earnings this trial: $0.00” was displayed in the center, and the “$” disappeared. After a pseudo-randomized delay of 4, 6, 8, or 10 s after presentation of the $ cue, the word “press” appeared, at which time the subject was instructed to press the button the second time. Twelve s after trial onset, the words “No earnings this trial” appeared.
2.2.2 No-penalty trials
(green screen) Two seconds into the trial, a “$” appeared at the bottom middle of the screen. After responding to the “$”, the subject began accruing earnings. First, the “Earnings this trial:” money counter was displayed in the center of the screen, and just below it, a numerical counter began advancing like the display on a gasoline pump. Earnings accumulation accelerated slightly across the trial. Second, a horizontal bar positioned in the lower left of the screen lengthened in a rightward direction in proportion to accumulating trial earnings. Four, 6, 8, or 10 s later, the word “press” replaced the dollar sign, at which time the subject was to press the button the second time, but money continued to accumulate until 10 s after trial onset, at which time the cumulative earnings counter was increased by that trial’s earnings, and the words “You just won $x.xx” appeared below the cumulative counter.
2.2.3 Low-penalty trials
(yellow screen) Two s into the trial, the “$” appeared at the bottom middle of the screen, at which time subjects were instructed to press the response button to begin accruing earnings as in the non-penalty reward trials. However, the duration during which the subject was allowed to accrue winnings was variable and covert. To earn money in the low-penalty trial, the subject was required to voluntarily press the button a second time to terminate accrual of winnings before a secret time limit (also an even distribution of 4–10 seconds after the presentation of the $ cue) was reached. If the subject pressed the button again prior to that trial’s secret time limit, accrual stopped, the animated bar stopped lengthening, and the cumulative earnings counter advanced by the trial’s winnings. If the covert time limit was reached without the subject having pressed the button again, the subject “busted” and won no money for the trial. The words “No earnings this trial” and “BUST” appeared in place of the trial counter, along with the word “PRESS” to elicit a second motor response.
2.2.4 High-penalty trials
(red screen) This trial type was identical to the low-penalty trial type in all respects but with a doubled bust penalty. Whereas busts in the low-penalty reward trials simply resulted in no winnings for that trial, if a subject busted in the high-penalty reward trial (by not pressing the button a second time prior to the covert time limit), the subject did not win any money, and the winnings on the trial counter at the time of the bust were deducted from previous cumulative winnings on the “Total earnings” counter.
2.3 Task training
Before scanning, subjects: 1) were read an instruction script which explained the contingencies of each trial type but not the distribution of secret time limits, 2) viewed an envelope containing cash and were reminded that they would actually receive task winnings, and 3) performed a practice version of the task for no consequence. Each subject busted at least once while practicing.
2.4 Imaging data collection and analysis
2.4.1 FMRI acquisition
Subjects were scanned in a 3 T General Electric MRI scanner (General Electric, Milwaukee, WI) and a quadrature head coil. We collected 24 3.8-mm-thick axial slices sequentially from inferior to superior, with a 1mm gap, using a T2*-sensitive echoplanar sequence with a repetition time (TR) = 2000 msec, echo time (TE) =40 msec, flip = 90°. In-plane resolution was 3.75 × 3.75 mm. Structural scans for coregistration were acquired using a T1-weighted MP-RAGE sequence (TR, 100 msec; TE, 7 msec; flip, 90°). Head motion was minimized with a deflateable head restraint cushion.
2.4.2 FMRI Preprocessing
Functional data were preprocessed as follows: 1) voxel time series were interpolated to correct for nonsimultaneous slice acquisition within each volume, 2) volumes were concatenated across task sessions; 3) volumes were corrected for head motion in three-dimensional space. No participant's head moved more than 1.5 mm in any dimension from one volume acquisition to the next or more than 3 mm overall. We applied a 4 mm FWHM isotropic smoothing kernel, followed by a despiking algorithm and bandpass filtration of signal fluctuations (either greater than 0.011/sec or less than 0.15/sec) uncharacteristic of a hemodynamic response.
2.4.3 Individual statistical maps
The regression model featured six regressors of interest (motor control, no-penalty, low-penalty wins, low-penalty busts, high-penalty wins, high-penalty busts), with additional regressors modeling residual motion, and baseline and linear trends. Regressors of interest were convolved with a canonical gammavariate blood-oxygen level-dependent (BOLD) hemodynamic responses time-locked to the presentation of the ($) cue. Because penalty trials do not have outcome notifications that are temporally separated from the pre-decision period, and because they elicit a protracted activation beginning at the time of the $ cue that precludes separate deconvolution of notifications (Bjork et al., 2007), notifications were not modeled. Time series correlations with modeled responses were linearly contrasted (LC) between trial types.
2.4.4 Groupwise statistical maps
Individual maps of contrast t-statistics were warped into Talairach space and combined in a random-effects analysis for each subject group separately. Activations are reported where voxels: 1) each exceeded a significance threshold of p < .0001, and 2) were part of a contiguous cluster of sufficient size (5 voxels, or 337.5 ul) to obtain a family-wise corrected type I error rate ≤ 0.05 using Monte Carlo simulation, and 3) were not within 20 mm of a more activated voxel.
2.4.5 Volume of interest (VOI) analysis of PMC signal change
Signal was normalized as a percent change, averaged by trial type, and translated into Talairach space. Trial-averaged signal was passed through a VOI mask drawn a priori in the midsagittal plane across an area that encompassed pre-decision conflict-elicited activation maxima of previous studies (as diagrammed in (Ridderinkhof et al., 2004)) and extended 4mm bilaterally. Using an inter-group extension of an automated, voxel-based method (Momenan et al., 2004), the mask excluded voxels that were not segmented (Momenan et al., 1997) as gray matter in all subjects with a probability of at least 75%. Trial-averaged signal change was baseline-corrected by subtraction of signal at trial onset.
2.5 Behavioral and psychometric measures
On a separate day prior to the scan, subjects completed the NEO five-factor personality inventory (Costa and McCrae, 1992). We restricted consideration a priori to its five main personality factors and the impulsivity facet. In particular, the neuroticism subscale was calculated to control for the greater negative affect characteristic of SDP, while still providing a range of scores in healthy asymptomatic controls suitable for cross-group statistical analysis. Task engagement was inferred from the mean reaction time (RT) to respond to the “$” cues after trial onset, and risk-taking was inferred as the mean reward accrual time in non-busted penalty trials. After scanning, subjects rated from 0 to 3 how “happy,” “sad,” “anxious,” and “bored” they were when playing each trial type.
3.0 Results
3.1 Psychometric and behavioral data
3.1.1 Personality scores
NEO scores were not available from two controls. The SDP had significantly higher scores than controls in the neuroticism and extraversion factors, as well as the impulsivity facet of the NEO, but significantly lower scores in the agreeableness and conscientiousness factors2 (on-line supplemental Table 2). There was no group difference in openness.
3.1.2 Task behavior
Both SDP and controls earned approximately $35 (n.s.). Latency to respond to the “$” cue did not differ between SDP and controls in any trial type, but was slower for the motor control compared to other trial types (main effect of trial type F(3,90) = 7.806, P ≤ .01). In low-penalty trials, SDP terminated reward accrual significantly sooner than controls (Figure 2A; main effect of group F(1,32) = 5.305, P ≤ .05). Across runs of the experiment, risk-taking (s of reward accrual) increased slightly in SDP, but decreased in controls (group X time interaction F(2,64) = 3.839, P ≤ .05). However, adjusted mean reward accrual times were similar (P > .5) between SDP (4.61 s) and controls (4.89 s) when NEO-neuroticism scores were entered as a covariate in multiple regression. Accordingly, mean reward accrual time in low-penalty trials correlated negatively with NEO-neuroticism (Spearman r = .407, P ≤ .05); subjects with high neuroticism took less risk. Accrual time did not significantly correlate with NEO-impulsivity. On average, controls busted in three more trials (mean 9.6 ± 3.6) than did SDP (6.7 ± 3.6 busts; main effect of group F(1,32) = 5.191, P ≤ .05). There was a main effect of scanning run on busts, with fewest busts in the second run in both groups (F(2,64) = 3.806), P ≤ .05), but there was no group by time interaction.
Figure 2.
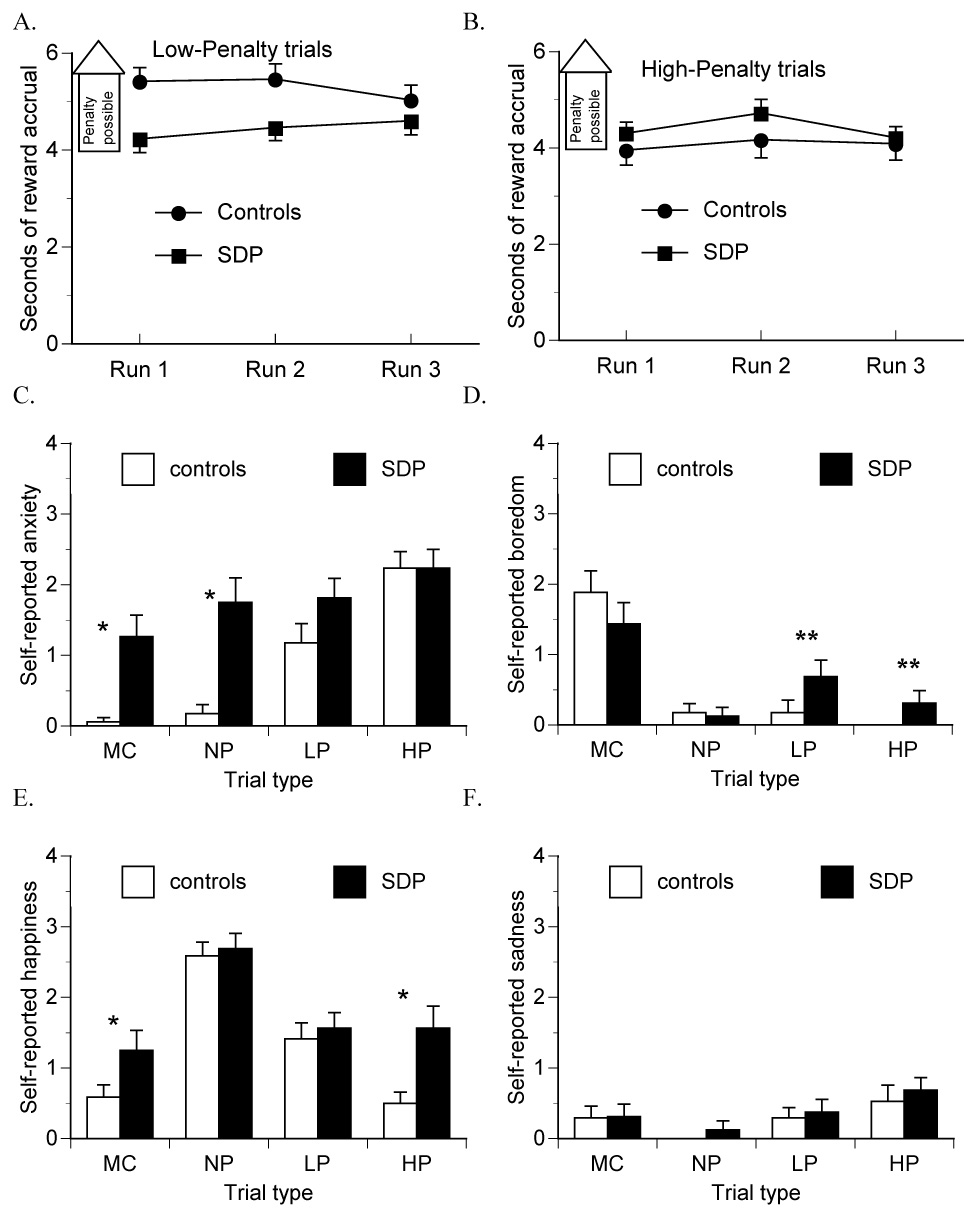
Controls showed significantly longer mean reward accrual duration (in non-busted trials) than substance-dependent patients (SDP) in low- (A) not high- (B) penalty trials. In a post-scan questionnaire, subjects rated their mood responses to motor control (MC), no-penalty (NP), low-penalty (LP) and high-penalty (HP) trials of task. Self-reported anxiety (C) and boredom (D) differed as a function of trial type more in controls than in SDP (group X trial type P < .05). Self-reported happiness (E) also tended to be more trial type-sensitive in controls compared to SDP (group X trial type P < .10). Self-reported sadness was minimal in both groups (F). * denotes P < .05; ** P < .10.
In high-penalty trials, there was no main effect of group or group X time interaction effect on either reward accrual time (Figure 2B) or busts (controls: mean 5.8 ± 4.2 busts, SDP: 5.4 ± 3.2 busts). There was a main effect of time on busts, with fewer busts in the second run compared to the first and third runs across both groups of subjects (F(2,64) = 7.981), P ≤ .001). Mean reward accrual time in high-penalty trials did not correlate with either NEO-neuroticism or impulsivity.
Size of the possible penalty affected risk taking among the controls but not among the SDP. There was a main effect of penalty magnitude on reducing risk exposure (F(1,32) = 9.570, P ≤ .01), where in high-penalty trials, controls (P ≤ .01), but not SDP, terminated reward accrual sooner than in low-penalty trials (group X trial type interaction (F(1,32) = 5.790, P ≤ .05).
To examine whether busting reduced subsequent risk-taking, we compared mean reward accrual time in trials that followed a win in the preceding low- or high- penalty trial versus those that followed a bust. In low-penalty trials, there was a main effect of previous trial outcome (F(1,32) = 7.417), P ≤ .05), with shorter risk exposure times in trials that followed a bust in the previous low-penalty trial (4.64 ± 1.1 s) compared to trials that followed a win (5.02 ± 1.3 s). There was no interaction effect of previous outcome with subject group (P > .6). Within high-penalty trials, there were no main or interaction effects of previous trial outcome on reward accrual.
3.2 Task-elicited affect
Self-reported anxiety reflected the magnitude of potential reward and penalty in controls but not in SDP (Figure 2C; group X trial type interaction (F(3,90) = 6.048, P ≤ .001). SDP reported significantly more anxiety than controls when playing motor-control and non-penalty trials, and thus did not show an orderly increase in anxiety as a function of risk like the controls. Boredom ratings reflected probabilities of reward and penalty in controls but not in SDP (Figure 2D; group X trial type interaction (F(3,90) = 2.721, P ≤ .05). SDP showed a trend (P ≤ .1) toward being more bored than controls when playing both low- and high-penalty trials. Self-reported happiness reflected the relative reward/penalty ratio in controls but not in SDP (Figure 2E), with a trend toward a group X trial type interaction (F(3,90) = 2.473, P ≤ .10), where SDP were more happy than controls when playing both motor control and high-penalty trials. Self-reported sadness was minimal in both groups (Figure 2F).
3.3 Brain activation by linear contrasts
3.3.1 Activation by guaranteed reward (no conflict or risk)
Accruing reward in no-penalty trials (contrasted with motor-control trials) activated the caudate head bilaterally in SDP, with activated voxels extending ventrally toward left nucleus accumbens (Figure 3A), and additional activation in occipital cortex (Table 1). There was no suprathreshold activation by this contrast in controls (figure 3B). To characterize this activation, we extracted trial-averaged BOLD signal data from 3mm radius spheres centered at the caudate activation maxima in SDP. This indicated that suprathreshold LC-elicited activation in SDP but not controls resulted from: 1) nonsignficantly greater signal decrease under motor-control conditions in SDP compared to controls in left caudate (Figure 23) a trend for greater peak signal increase under no-penalty reward conditions in SDP compared to controls in right caudate.4
Figure 3.
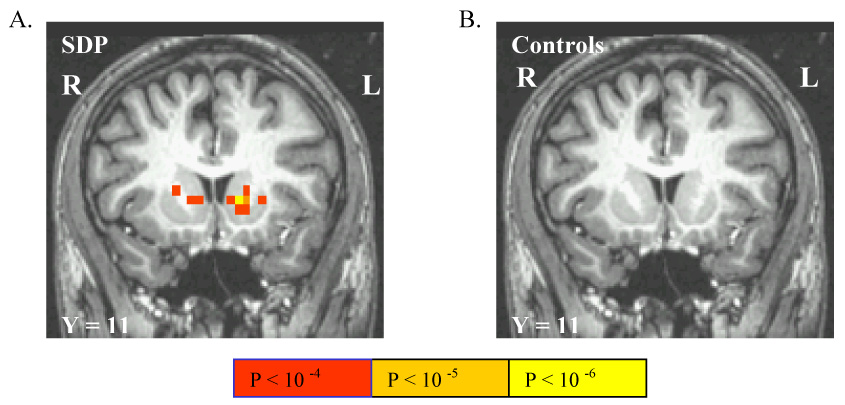
The linear contrast between reward accrual in non-penalty trials versus cue-elicited responses for no incentive in motor control trials activated the caudate head bilaterally in SDP (A) but not controls (B). Image reversed per radiological convention.
Table 1.
Activations by pursuit of guaranteed reward versus motor control (non-reward)
| Contrast | Region | Talairach Coordinates: | t-value | uncorrected p* | ||
|---|---|---|---|---|---|---|
| Controls | No activations | |||||
| SDP | L Caudate head | −11 | 11 | −1 | 8.503 | <.000001 |
| R Caudate head | 10 | 14 | −1 | 6.423 | <.00001 | |
| L Posterior cingulate gyrus | −4 | −30 | 29 | 5.762 | <.0001 | |
| R Middle occipital gyrus | 38 | −79 | 0 | 6.801 | <.00001 | |
Activations reported in this and subsequent tables are maxima of clusters with volume sufficient to survive a family-wise type I error correction of p < 0.05 using Monte Carlo simulation.
3.3.2 Activation during conflicted decision making by reward at risk of penalty
Accruing reward in low-penalty trials, contrasted with accruing (guaranteed) reward during no-penalty trials, activated PMC in both SDP (Figure 4A), and controls (Figure 4B), where controls showed more anteroventral activation as well as activation in occipital cortex (Table 2). Accruing reward in high-penalty trials, contrasted with accruing reward in non-penalty trials, activated PMC in both SDP and controls (Figures 4C and 4D, respectively; Table 3), where PMC activation extended more anteroventrally in controls. The high- versus no-penalty contrast activated similar regions of cortex in controls as did the low- versus no-penalty contrast, and also activated occipital and frontal cortex in SDP. Finally, winning reward at risk of high- versus low- penalty activated only mesial occipital lobe in both SDP and controls (Table 4).
Figure 4.
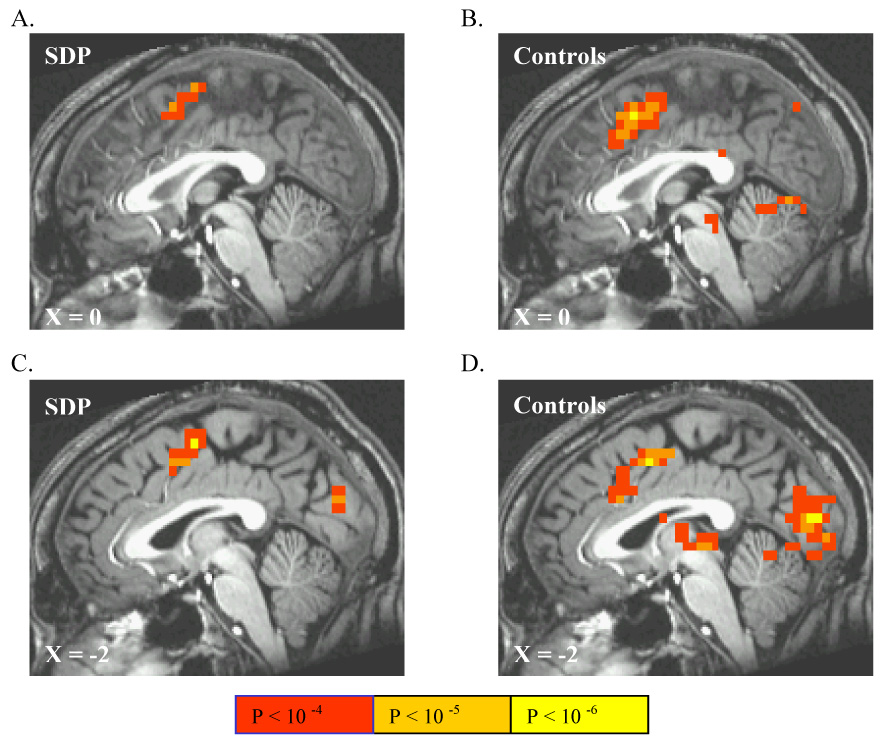
The linear contrast between reward accrual at risk of winning nothing in low-penalty trials versus winning guaranteed reward in non-penalty trials activated portions of posterior mesofrontal cortex (PMC) in SDP (A) and controls (B). Similarly, the linear contrast between reward accrual at risk of losing previous winnings in high-penalty trials versus winning guaranteed reward in no-penalty trials also activated MPC in SDP (C) and controls (D), with additional activation of mesial occipital cortex in both groups.
Table 2.
Brain activation by pursuit of reward at risk of low-penalty versus no-penalty
| Region | Talairach Coordinates: | t-value | uncorrected p* | |||
|---|---|---|---|---|---|---|
| Controls | L Putamen | 19 | 11 | −4 | 10.294 | <.000001 |
| R Putamen | 9 | 8 | 5 | 9.125 | <.000001 | |
| L Thalamus | 15 | −23 | 5 | 10.088 | <.000001 | |
| R Thalamus | 8 | −19 | 5 | 8.968 | <.000001 | |
| L Inferior occipital gyrus | 38 | −68 | −4 | 8.482 | <.000001 | |
| L Posterior mesofrontal cortex | 4 | 11 | 44 | 8.192 | <.000001 | |
| L Inferior parietal lobule | 41 | −38 | 53 | 7.445 | <.00001 | |
| 26 | −49 | 39 | 6.587 | <.00001 | ||
| L Middle temporal gyrus | −30 | −71 | 20 | 5.677 | <.0001 | |
| R Middle temporal gyrus | 38 | −53 | 0 | 7.445 | <.00001 | |
| Dorsomesial cerebellum | 4 | −56 | −4 | 7.207 | <.00001 | |
| L Middle frontal gyrus | −38 | 26 | 29 | 6.892 | <.00001 | |
| R Middle frontal gyrus | 38 | 30 | 24 | 7.137 | <.00001 | |
| 30 | −4 | 58 | 6.261 | <.0001 | ||
| R Posterior mesofrontal cortex | 11 | 4 | 58 | 6.867 | <.00001 | |
| L Insula | −30 | −15 | 20 | 6.800 | <.00001 | |
| R Superior occipital gyrus | 26 | −71 | 39 | 6.774 | <.00001 | |
| L Precentral gyrus | −30 | −8 | 44 | 6.229 | <.0001 | |
| R Posterior cingulate | 4 | −34 | 24 | 5.799 | <.0001 | |
| L Superior parietal lobule | −15 | −60 | 53 | 5.703 | <.0001 | |
| R Precuneus | 8 | −75 | 48 | 5.633 | <.0001 | |
| R Superior frontal gyrus | 34 | 49 | 15 | 5.268 | <.0001 | |
| SDP | R Posterior mesofrontal cortex | 8 | −4 | 58 | 8.877 | <.000001 |
| L Posterior mesofrontal cortex | −4 | 8 | 44 | 7.372 | <.00001 | |
| R Putamen | 15 | 0 | 0 | 5.882 | <.0001 | |
| L Superior parietal lobule | −19 | −68 | 39 | 5.350 | <.0001 | |
Table 3.
Brain activation by pursuit of reward at risk of high-penalty versus no-penalty
| Region | Talairach Coordinates: | t-value | uncorrected p* | |||
|---|---|---|---|---|---|---|
| Controls | L Middle frontal gyrus | −34 | 34 | 20 | 5.647 | <.0001 |
| −26 | 38 | 37 | 5.567 | <.0001 | ||
| R Middle frontal gyrus | 19 | 0 | 53 | 9.317 | <.000001 | |
| 26 | 34 | 39 | 6.899 | <.00001 | ||
| R Superior occipital gyrus | 27 | −68 | 39 | 8.980 | <.000001 | |
| L Putamen | −19 | 11 | 4 | 8.627 | <.000001 | |
| R Thalamus | 12 | −12 | 15 | 8.533 | <.000001 | |
| L Thalamus | −19 | −26 | 15 | 8.353 | <.000001 | |
| L Cuneus | −4 | −83 | 15 | 8.175 | <.000001 | |
| L Postcentral gyrus | −49 | −30 | 48 | 7.765 | <.000001 | |
| −26 | −11 | 48 | 7.495 | <.00001 | ||
| L Superior frontal gyrus | −11 | −4 | 63 | 7.728 | <.000001 | |
| L Posterior mesofrontal cortex | −4 | 4 | 44 | 7.708 | <.000001 | |
| R Substantia Nigra | 11 | −19 | −4 | 7.424 | <.00001 | |
| L Anterior cingulate cortex | −4 | 19 | 24 | 7.200 | <.00001 | |
| R Inferior parietal lobule | 41 | −45 | 53 | 7.147 | <.00001 | |
| Dorsomesial cerebellum | 0 | −60 | −4 | 6.971 | <.00001 | |
| L Middle occipital gyrus | −41 | −68 | −4 | 6.696 | <.00001 | |
| −30 | −75 | 24 | 5.518 | <.0001 | ||
| R Middle occipital gyrus | 38 | −64 | 10 | 5.607 | <.0001 | |
| L Superior parietal lobule | −11 | −64 | 58 | 6.533 | <.00001 | |
| R Lingual gyrus | 19 | −53 | 4 | 6.390 | <.00001 | |
| 8 | −90 | −4 | 5.621 | <.0001 | ||
| SDP | L Posterior mesofrontal cortex | −4 | −4 | 53 | 7.734 | <.000001 |
| L Caudate head | −11 | 11 | 4 | 7.067 | <.000001 | |
| R Putamen | 19 | 11 | 0 | 6.982 | <.00001 | |
| L Cuneus | −4 | −79 | 24 | 6.877 | <.00001 | |
| R Middle frontal gyrus | 23 | −8 | 48 | 6.542 | <.00001 | |
| L Middle occipital gyrus | −38 | −75 | 5 | 6.279 | <.0001 | |
| R Precuneus | 19 | −60 | 44 | 5.712 | <.0001 | |
| L Anterior cingulate | −6 | 15 | 34 | 5.603 | <.0001 | |
| L Precentral gyrus | −34 | −23 | 48 | 5.583 | <.0001 | |
Table 4.
Activations by reward accrual at risk of high-penalty versus reward at risk of low-penalty
| Contrast | Region | Talairach Coordinates: | t-value | uncorrected p | ||
|---|---|---|---|---|---|---|
| Controls | L Cuneus | −4 | −79 | 15 | 7.373 | <.00001 |
| R Cuneus | 15 | −71 | 15 | 7.507 | <.000001 | |
| L Lingual gyrus | −11 | −56 | 5 | 6.004 | <.0001 | |
| R Lingual gyrus | 11 | −49 | 5 | 5.478 | <.0001 | |
| SDP | L Cuneus | −4 | −79 | 20 | 6.430 | <.00001 |
| R Cuneus | 11 | −60 | 10 | 6.155 | <.0001 | |
3.3.3 Error-correlated activation
In a post hoc analysis, penalty trials with win and bust outcomes were separately remodeled and contrasted. Random-effect analyses did not reveal any significant outcome-correlated activation in either group or when groups and penalty trial types were combined. In order to examine potential effects of errors on increasing PMC activation in the subsequent trial, in a second analysis, data from penalty trials were also re-modeled based on the outcome of the previous non-busted trial of that type. This contrast also did not reveal significant activation.
3.4 Penalty trial signal change in the PMC volume of interest
Hemodynamic responses in motor-control and no-penalty trials were nearly identical in SDP and controls. In low-penalty trials, Controls had significantly greater signal change than SDP in the two acquisitions prior to potential busts (Figure 5A), resulting in a trend for a group X time interaction effect (F(5,160) = 1.852, P = .1). A post hoc voxel-wise t-test of the activation by the low- versus no-penalty LC identified PMC voxels with significantly reduced recruitment in the SDP compared to controls (Figure 5B). Critically, in a simultaneous multiple regression analysis, the total AUC of the hemodynamic response (as the dependent variable) was still significantly blunted in SDP after entering individual differences in reward-accrual time and NEO-neuroticism scores as covariates into model fitting (group effect Beta = .564, P ≤ .01). In addition, reward-accrual time, but not NEO-neuroticism, also independently correlated with PMC signal increase (Beta = −.534, P ≤ .01).
Figure 5.
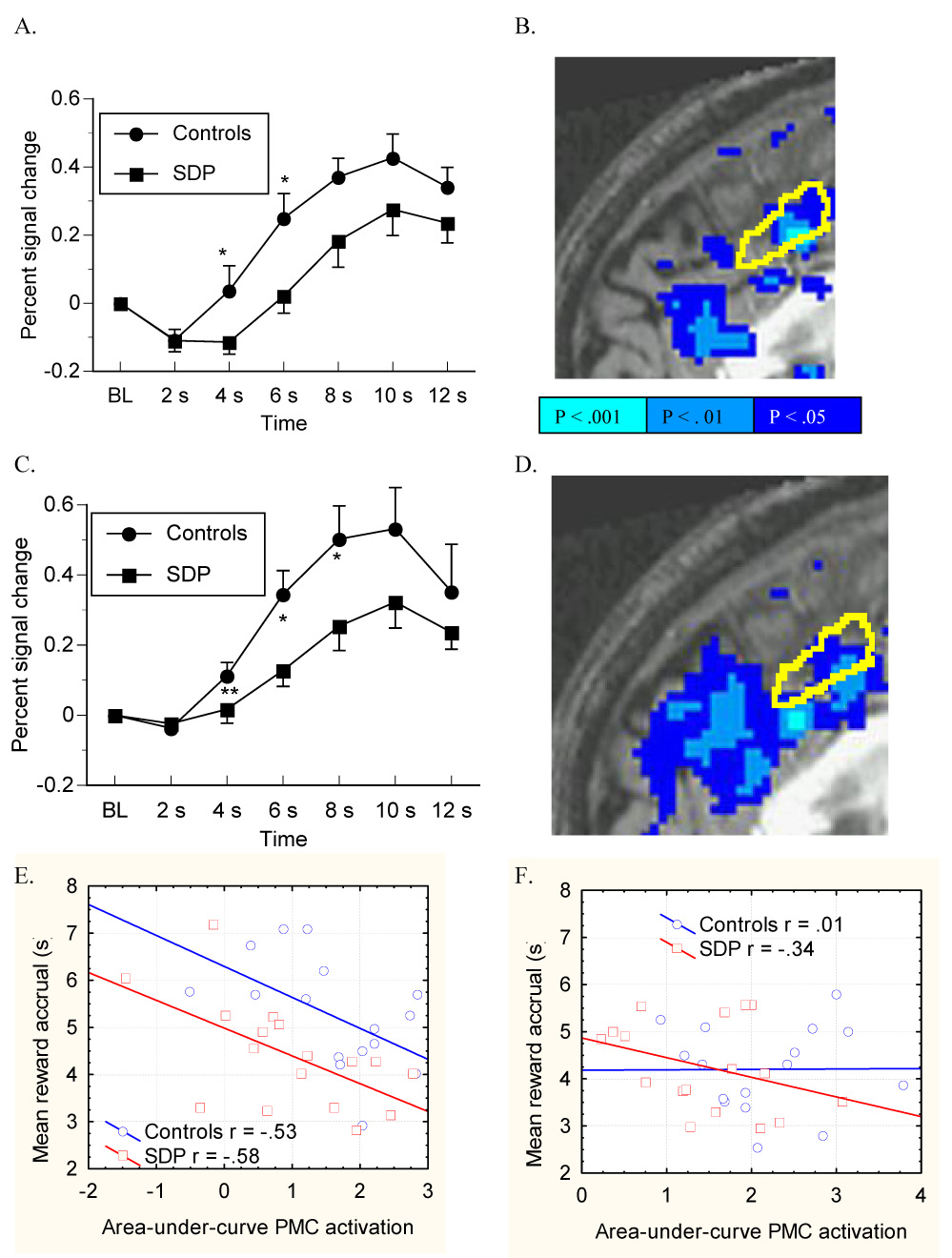
Trial-type-averaged BOLD signal was extracted from PMC in a midsagittal volume of interest mask (yellow outline) drawn to encompass the activation maxima previously reported in several experiments on pre-decision conflict (see ref. (Ridderinkhof et al., 2004)). In low-penalty trials, SDP showed blunted BOLD signal change in PMC relative to controls following the onset of risky reward accrual (the 2 s timepoint) as seen in the hemodynamic response itself (A) and in a voxel-wise t-test of the group difference in activation by the low-penalty versus no-penalty contrast, where reduced activation in SDP is depicted in blue (B). In high-penalty trials, SDP also showed a blunted hemodynamic response to risky reward (C), with significantly lower anterior cingulate activation (per voxel-wise t-test) by high-penalty trials contrasted with no-penalty trials (D). Area-under-curve activation of the PMC by risky reward accrual correlated negatively with risk-taking behavior in both SDP and controls in low-penalty trials (E), but not in high-penalty trials (F).
Controls also had significantly greater hemodynamic responses than SDP in high-penalty trials (Figure 5C), as inferred from the group X time interaction effect (F(5,160) = 2.312, P ≤ .05), single acquisition timepoints, and in the voxel-wise t-test of activation by the high- versus no-penalty LC (Figure 5D). As with low-penalty trials, the total AUC of the hemodynamic response in high-penalty trials was also significantly blunted in SDP (group effect Beta = .524, P ≤ .05) after controlling for reward-accrual time and NEO-neuroticism scores as covariates. Neither NEO-neuroticism nor reward-accrual time independently correlated with signal increase.
In simple bivariate correlation, reward accrual time inversely correlated with the area-under-curve (AUC) of the hemodynamic response in both SDP (Beta = −.58, P ≤ .05) and controls (Beta = −.53, P ≤ .05) in low-penalty trials (Figure 5E) but not in high-penalty trials (P > .1) (Figure 5F).
4.0 Discussion
4.1 General findings
These data extend findings that individuals with SUD are characterized by altered frontocortical recruitment while decision-making (Bolla et al., 2003; Fishbein et al., 2005; Forman et al., 2004; Kaufman et al., 2003). As we hypothesized, cocaine-abusing alcoholics showed blunted PMC recruitment by a reward/risk conflict that models drug-taking behavior in its juxtaposition of progressively-increasing reward and penalty likelihood within a single behavioral sequence. Deficient conflict-elicited PMC activation in SDP was evident both in the LC between guaranteed and risky reward accrual, as well as in the trial-averaged hemodynamic responses during low- and high-penalty trial types extracted and analyzed singly. This deficit was most evident in portions of anterior cingulate cortex consistently recruited by pre-decision conflict in numerous experiments. Conversely, there were minimal group differences in posterior aspects of PMC linked more specifically to intention to generate self-initiated motor responses (Lau et al., 2004). In addition, SDP also showed caudate head activation by guaranteed reward accrual itself. Finally, controls demonstrated orderly, intuitive affective reactions to risk and reward contingencies across trial types but SDP did not.
4.2 Risk-taking in the task
Between-subject and between-trial differences in reward accrual were greater in low-relative to high-penalty trials. For example, busting in low-penalty trials on average reduced the reward accrual time in the subsequent (non-busted) low-penalty trial, but this did not occur in high-penalty trials. In addition, NEO-neuroticism negatively correlated with risky behavior in low penalty trials, but not in high-penalty trials. We suspect that the high-penalty trials engendered a more facile strategy to avoid risking previous winnings altogether, where pre-decision conflict was essentially avoided when subjects terminated reward accrual at the timepoint when busts could begin to occur (Fig 2B). Thus, the low-penalty trials were likely better suited to examination of the relationship between risk-taking and other variables. Because risk-taking in low-penalty trials correlated with individual differences in NEO-neuroticism, but not with impulsivity, this suggests that task behavior was likely influenced more by sensitivity to unpleasant stimuli (busts) and less by impulsivity that would promote risk-seeking.
Finally, PMC activation in low-penalty trials correlated negatively with actual risk-taking but not directly with NEO-neuroticism. It seems likely that PMC activation by a specific task conflict or threat would be more proximally related to avoidance behavior within that task relative to a correlation between activation and a more global psychometric measure. Moreover, the relationship between trait responsiveness to aversive stimuli and exaggerated PMC recruitment may also have been altered (or perhaps mitigated by) the availability of risk-avoiding responses. Future variants of this paradigm could present subjects with a similar risk-reward conflict over time, but parametrically vary the availability of penalty avoidance responses.
While both groups behaved similarly in high-penalty trials, an unexpected finding was that SDP were more cautious in low-penalty trials. We suspect that despite having more impulsive personalities, the SDP were less willing to take risks in the tasks by virtue of their greater sensitivity to aversive stimuli, as measured by trait neuroticism. Notably, the main effect of group on risk-taking was eliminated after controlling for NEO-neuroticism scores. Risk averse behavior may also have been attractive to SDP by virtue of its lower cognitive demand. Notably, in the healthy brain, choosing guaranteed rewards activates frontocortical and parietal voxels less than choice of a risky alternative (Gonzalez et al., 2005), and persons with SUD show reflexive lose-switch responses to error outcomes (Paulus et al., 2002). For example, heroin users readily adopted a “play it safe” strategy following bad outcomes in a similar risk-taking task, where this risk avoidant behavior correlated with reduced ACC activation (Ersche et al., 2006). Thus, SDP subjects may have played it safe to reduce cognitive conflict. Parenthetically, we note that after busting in another variant of this task (Bjork et al., 2004a), SDP frequently vocalized anger then adopted a conservative strategy in subsequent trials, suggesting both affective and cognitive underpinnings of error avoidance.
4.3 Brain activation by guaranteed rewards
Guaranteed reward accrual in no-penalty trials elicited significant caudate head activation in SDP but not controls. In previous reports with healthy adults (Bjork et al., 2004b; Elliott et al., 2000; Yacubian et al., 2006), caudate head was recruited by notification of monetary rewards. Considered together with their activation decrement in penalty trials, this suggests that SDP may show disproportional recruitment of motivational circuitry by positive, relative to negative, behavior contingencies. This combination characterizes decision-making deficits of SDP while performing the Iowa Gambling Task, where SDP more frequently choose to pick cards from “decks” containing high-reward cards that are laden with disproportionately larger penalties (Bechara et al., 2001; Bechara et al., 2002).
4.4 Brain activation by reward at risk of penalty
In accord with our hypothesis, SDP showed a blunted pattern of conflict-specific brain activation compared to controls despite intact penalty avoidance, with subnormal activation in ACC voxels that are frequently recruited by pre-decision behavior conflicts (Ridderinkhof et al., 2004). This was evident both in the linear time-series contrast between risky versus guaranteed reward accrual, and in the contour of the hemodynamic impulse response across penalty trial types in a mask drawn a priori in the PMC. This cortical response to our behavioral challenge suggests that SUD is characterized by under-recruitment of specialized frontocortical response conflict-monitoring circuitry. This may in turn represent a generalized neurobiological correlate of a reduced potential of the addicted brain to reference potential negative consequences for drug-taking behavior.
BOLD signal in penalty trials was already increased by the time penalties became possible and before most subjects responded to stop accrual (~ 6 s). We therefore surmise that PMC activation was engendered primarily by pre-decision processing, not by outcome monitoring. We do not believe that group-wise activation differences resulted from group-wise differences in task-behavior for two reasons. First, SPD demonstrated a PMC activation deficit during high-penalty trials in the absence of differences from controls in either busts or reward-accrual duration. Second, in both penalty trials, the SDP deficit remained significant after individual differences in reward accrual and proneness to negative affect (neuroticism) were controlled for. Finally, the similar latency to respond to begin accruing reward after the “$” cue between SDP and controls does not suggest that SDP had subnormal PMC activation because they were simply less engaged in the task.
During penalty trials, we did not find PMC activation differences as a function of either the outcome of the current trial or activation differences based on the outcome of the previous trial. We offer two explanations for this. First, since outcomes further bifurcate the 24 trials of each penalty type, there may not have been enough trial events (especially busts) to adequately model outcomes. Second, we suspect that since PMC activations were engendered during the reward accrual and before notifications, subjects were uniformly motivated to avoid errors in every penalty trial they encountered, resulting in relatively similar PMC activation across trials.
Interestingly, the PMC recruitment deficit in SDP resembles that found in healthy adolescents (Bjork et al., 2007), raising the possibility that chronic alcohol/drug intoxication by the SDP resulted in stunted development of the PMC. In clinical interviews, most SDP reported onset of regular heavy drinking by late adolescence. Notably, frontal lobe dysmorphology is detectable by young adulthood in persons with adolescent-onset alcohol dependence (De Bellis et al., 2005). It is also possible that delayed premorbid PMC development may contribute to the development and progression of substance abuse.
4.5 PMC activation decrements in the absence of increased task errors
We desired roughly similar rates of error outcomes in this experiment in order to avoid interpretive confounds in that PMC is recruited not only be pre-decision conflict, but also by error notification and monitoring (Ridderinkhof et al., 2004). The PMC activation deficit in SDP did not translate into greater errors in either penalty trial type. It may be that this dissociation occurred with the RTT because minimal PMC activation was sufficient to minimize errors when using a facile, risk-avoidant strategy. Many subjects commented after scanning that they had adopted set strategies for responding in the penalty trials. Moreover, the RTT presented vivid threats (to tangibly-represented assets) that likely artificially enhanced vigilance to facilitate penalty avoidance in SDP despite alterations in frontocortical circuitry.
Conversely, lateral parietal lobe, which was recruited in both groups, may have subserved actual cost-benefit calculations during decision-making (Dehaene et al., 1999; Sugrue et al., 2005) to successfully avoid penalties. We suspect that the blunted PMC activity in SDP resulted instead from disordered motivation-related (Taylor et al., 2006) components of contingency-conflict monitoring, where controls were more intently processing the risk-reward conflict. Another possibility is that the SDP activation deficit partly reflected a reduced appraisal of self-involvement (agency) (Moran et al., 2006) in the conflict. Both of these explanations are consistent with the less orderly and intuitive effects of trial contingencies on self-reported emotion among the SDP.
We believe that a functional reorganization away from optimized frontocortical conflict-monitoring circuitry in the service of adequate laboratory task performance is clinically meaningful in SUD, especially if it suggests inefficient processing. For example, Yucel et al (Yucel et al., 2007) recently reported increased recruitment of frontal and parietal cortex in opiate-dependent subjects in service of normative performance of a response-conflict task. Altered frontal and parietal activation while performing normally in a working memory task has also been reported in adults (Desmond et al., 2003) and adolescents (Caldwell et al., 2005; Tapert et al., 2004) with alcohol use disorders. These additional activations have been interpreted as compensatory adaptations in SUD. Compensatory adaptations in brain disorders may be ultimately insufficient, however, when either the difficulty of a laboratory task is parametrically increased (Tan et al., 2006), or when the subject is in real-world situations with less salient or less explicitly framed behavioral contingencies.
4.6 Study limitations and avenues of future research
This experiment had three key limitations. First, the RTT was not temporally configured to disentangle pre-decision activation from outcome notification activation. Future variants of this task could separate these two components of decision-making while still retaining ambiguity of outcome probabilities. For example, the decision-making period during reward accrual could be programmed to elicit a second, self-initiated response without immediately implying an outcome. The actual programmed time-limit could then be graphically revealed to the subject after a temporally-jittered delay, with retroactive calculation (and feedback) of trial outcome.
Second, it is not possible to disentangle the degree to which activation deficits in SDP resulted from premorbid PMC dysfunction relative to the effects of chronic polydrug exposure. Although the VOI mask included only voxels likely containing likely gray matter in every subject, it is nonetheless possible that some component of the risk-elicited PMC activation decrement in SDP resulted from morphological effects of comorbid chronic alcohol and cocaine abuse (Bartzokis et al., 2002; Rogers and Robbins, 2001). We suspect, however, that the activation deficit in SDP reported here reflects premorbid cortical traits conferring impulsivity and risk of substance abuse—possibly compounded by morphological effects of many years of heavy alcohol exposure. To indirectly address the causality issue, future research may explore contingency conflict-elicited PMC recruitment in drug-naïve, at-risk adolescent populations, such as children of alcoholics.
Third, there was extensive comorbidity with affective disorders in the SUD subjects, and affective disorders themselves relate to dysfunctional frontocortical blood flow (Videbech, 2000). However, the deficit in PMC signal increase during risky reward accrual in the SDP remained significant after controlling for their greater neuroticism. Similarly, it is not possible to isolate independent correlates of alcohol abuse versus cocaine abuse with regional brain recruitment by risk and reward in these comorbid patients. Moreover, most SDP also abused at least one other drug besides cocaine and alcohol. Future experiments should feature recruitment of diagnostically-pure patient populations to characterize PMC recruitment by risk appraisal in different psychiatric syndromes. Finally, these findings from detoxified treatment-seeking subjects may not generalize to actively-using subjects.
In conclusion, in SDP, a conflict between positive and negative contingencies within the same behavioral sequence elicited deficient recruitment in a region of cortex that (in healthy adults) is consistently recruited by tasks that require monitoring and successful resolution of a response conflict (Ridderinkhof et al., 2004). The global PMC activation decrement in SDP did not translate here into increased rates of poor outcomes when the possibility of penalty was explicitly signaled in a simple, artificial task. We suspect, however, that dysfunctional PMC activation in substance dependence is a meaningful indicator of deficient cortically-mediated risk-appraisal, which may in turn confer vulnerability to bad decisions in more ambiguous real-world situations.
Supplementary Material
Footnotes
Supplementary data tables are presented in the online version of this paper at http://dx.doi.org by entering doi:xxxxxxxx.
Full SDP characteristics are presented in Table 1 of the online version of this paper at http://dx.doi.org by entering doi:xxxxxxxx.
Full data on the NEO-Five Factor Inventory scores are presented in Table 2 of the online version of this paper at http://dx.doi.org by entering doi:xxxxxxxx.
Supplemental data as shown in Figure 1A of the online version of this paper can be viewed at http://dx.doi.org by entering doi:xxxxxxxx.
Data shown in Figure 1D of the online version of this paper can be viewed at http://dx.doi.org by entering doi:xxxxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes GM, Welte JW, Hoffman JH, Dintcheff BA. Gambling and alcohol use among youth: influences of demographic, socialization, and individual factors. Addict Behav. 1999;24:749–767. doi: 10.1016/s0306-4603(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004a;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004b;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory and NEO Five-Factor Inventory: Professional manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Jessor R. Structure of problem behavior in adolescence and young adulthood. J Consult Clin Psychol. 1985;53:890–904. doi: 10.1037//0022-006x.53.6.890. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Roiser JP, Fryer TD, London M, Robbins TW, Sahakian BJ. Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology (Berl) 2006;188:364–373. doi: 10.1007/s00213-006-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res. 2005;23:119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, Kroll P, Brunberg JA. Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol. 1990;28:775–785. doi: 10.1002/ana.410280608. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Dana J, Koshino H, Just M. The framing effect and risky decisions: Examining cognitive functions with fMRI. J Econ Psychol. 2005;26:1–20. [Google Scholar]
- Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory: An analysis of decision under risk. Econometrica. 1979;47:263–292. [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006a;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006b;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Masse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54:62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Dev Psychopathol. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Momenan R, Hommer D, Rawlings R, Ruttimann U, Kerich M, Rio D. Intensity-adaptive segmentation of single-echo T1-weighted magnetic resonance images. Human Brain Mapping. 1997;5 doi: 10.1002/(SICI)1097-0193(1997)5:3<194::AID-HBM4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Momenan R, Rawlings R, Fong G, Knutson B, Hommer D. Voxel-based homogeneity probability maps of gray matter in groups: assessing the reliability of functional effects. Neuroimage. 2004;21:965–972. doi: 10.1016/j.neuroimage.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Myers MG, Brown SA, Mott MA. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcohol Clin Exp Res. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Samson Y, Baron JC, Feline A, Bories J, Crouzel C. Local cerebral glucose utilisation in chronic alcoholics: a positron tomographic study. J Neurol Neurosurg Psychiatry. 1986;49:1165–1170. doi: 10.1136/jnnp.49.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Substance use disorder and personality disorder. Curr Psychiatry Rep. 2002;4:25–29. doi: 10.1007/s11920-002-0008-7. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiatr Clin North Am. 2004;27:283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, Roffel K, Clarke K, Wood SJ, Forman SD, Pantelis C. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12(611):691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


