Abstract
These experiments investigated the role of the α2-adrenoceptors of the basolateral nucleus of the amygdala (BLA) in modulating the retention of inhibitory avoidance (IA). In Experiment 1, male Sprague Dawley rats implanted with bilateral cannulae in the BLA received microinfusions of a selective α2-adrenoceptor antagonist idazoxan 20 min either before or immediately after training. Retention was tested 48 h later. Idazoxan induced a dose-dependent enhancement of retention performance and was more effective when administered post-training. In Experiment 2, animals received pre- or post-training intra-BLA infusions of a selective α2-adrenoceptor agonist UK 14,304. The agonist induced a dose-dependent impairment of retention performance and, as with the antagonist treatments, post-training infusions were more effective. These results provide additional evidence that consolidation of inhibitory avoidance memory depends critically on prolonged activation of the noradrenergic system in the BLA and indicate that this modulatory influence is mediated, in part, by pre-synaptic α2-adrenoceptors.
Emotionally arousing experiences generally create strong, long-lasting memories (Christianson 1992; McGaugh 2004). Extensive evidence supports the hypothesis that the hormonal systems activated by emotional arousal strengthen memory by modulating the neurobiological processes underlying memory consolidation (Gold and van Buskirk 1975; McGaugh 1989, 2000; McGaugh and Roozendaal 2002).
Several kinds of evidence indicate that adrenal stress hormones affect memory storage via an interaction with noradrenergic mechanisms in the amygdala (McGaugh et al. 1996 for review). Norepinephrine (NE) is released in the amygdala by stressful or arousing stimulation of the kind used in inhibitory avoidance (IA) training (Galvez et al. 1996; Quirarte et al. 1998) and retention performance varies directly with the amount of NE released in the amygdala by the training experience (McIntyre et al. 2002). There is extensive evidence that post-training intra-amygdala administration of NE or β-adrenoceptor agonists enhances memory consolidation and that β-adrenoceptor antagonists impair memory (Gallagher et al. 1977; Liang et al. 1986, 1990; Introini-Collison et al. 1991). Further, the memory-modulating effects of stress hormones or drugs affecting adrenergic, gamma-aminobutyric acid (GABA), opioid peptidergic, and glucocorticoid systems are known to be mediated by the activation of β-adrenergic system within the amygdala (Liang et al. 1986; McGaugh et al. 1988; Introini-Collison et al. 1989, 1995; McGaugh et al. 1996; Quirarte et al. 1997). These findings, considered together with the evidence high density of β-adrenoceptor subtypes within the amygdala (Alexander et al. 1975; Bylund and Snyder 1976), provide strong evidence that noradrenergic effects on memory consolidation are mediated, at least in part, by an activation of β-adrenoceptors in the amygdala.
There is also extensive evidence that the memory-modulatory effects of the β-adrenoceptor system are mediated selectively by the basolateral nucleus of the amygdala (BLA). Post-training infusions of NE or the β-adrenoceptor agonist clenbuterol administered selectively into the BLA enhance retention of IA and water-maze training, as well as contextual fear conditioning and extinction (Ferry and McGaugh, 1999; Hatfield and McGaugh 1999; LaLumiere et al. 2003; Berlau and McGaugh 2006). Further, infusions of β-adrenoceptor antagonists administered selectively into the BLA block the memory-enhancing effects of post-training systemic injections of glucocorticoids (Quirarte et al. 1997).
The amygdala also contains two types of α-adrenoceptors (U’Prichard et al. 1980; Unnerstal et al. 1984; Zilles et al. 1993). Whereas the α1-adrenoceptor subtype is located post-synaptically (for review, see Hardman et al. 1996), the α2-adrenoceptor subtype is predominantly located on presynaptic noradrenergic terminals and its activation inhibits NE release (Langer 1974; Starke 1979; Talley et al. 1996). The findings of studies of the effects of nonselective α-adrenoceptor agonists or antagonists on memory consolidation have provided conflicting evidence concerning whether the α1- and α2-adrenoceptor subtypes play different roles in the processes underlying memory storage. Post-training infusions of the non-selective α-adrenoceptor antagonist, phentolamine, into the amygdala were reported to induce dose-dependent enhancement of IA retention (Gallagher and Kapp 1981) whereas administration of the selective α1-adrenoceptor antagonist, prazosin, did not induce significant effects (McGaugh et al. 1988; Liang et al. 1995). Post-training activation of BLA α-adrenoceptors with phenylephrine induced a complex pattern of effects (Ferry et al. 1999a). Although phenylephrine has been reported to be a selective α1-adrenoceptor agonist (Hardman et al. 1996), phenylephrine also stimulates pre-junctional α2-adrenoceptors (Wikberg 1973; Flavahan and McGrath 1981; van Meel et al. 1981); thus, the combined activation of α1- and α2-adrenoceptors could have conflicting effects on memory storage.
In previous experiments investigating the involvement of BLA α1-adrenoceptors in memory consolidation, we found that post-training infusions of the selective α1-adrenoceptor antagonist prazosin administered into the BLA impaired IA retention, whereas selective activation of α1-adrenoceptors enhanced retention (Ferry et al. 1999a). Furthermore, the memory-modulatory effects of NE in the BLA appear to be mediated by an interaction between α1- and β-adrenoceptors as post-training infusions of the β-adrenoceptor antagonist atenolol into the BLA blocked the memory enhancement induced by selective α1-adrenoceptor activation (Ferry et al. 1999a).
Anatomical findings indicate that there is a higher density of α2-adrenoceptors than α1-adrenoceptors in the amygdala (Unnerstal et al. 1984; Zilles et al. 1993). Although there is extensive evidence that β- and α1-adrenoceptor subtypes are involved in memory modulation, studies have not, as yet, examined the involvement of α2-adrenoceptors in the BLA in memory consolidation. These receptors are known to be involved in regulating NE release in the central nervous system (Dennis et al. 1987) and more precisely in the amygdala (Fendt et al. 1994). Moreover, the amount of NE released in the amygdala directly varies with the intensity of a footshock like that used in IA training (Galvez et al. 1996; Quirarte et al. 1998) and significantly correlates with retention latencies (McIntyre et al. 2002). Therefore, selective activation or blocking of these receptors should be expected to modulate memory storage. To investigate this issue, the present experiments investigated the effects of post-training activation or blockade of α2-adrenoceptors in the BLA on retention of IA training. Experiment 1 examined the effects of pre- or post-training α2-adrenoceptor activation in the BLA on IA retention whereas Experiment 2 examined the effect of pre- or post-training blockade of BLA α2-adrenoceptors.
Results
Experiment 1: Effects of pre- or post-training BLA α2-adrenoceptor activation
Six animals did not survive surgery. Histological examination revealed that 83 animals had correct bilateral cannulae placements in the BLA. A representative cannula placement in the BLA is shown in Figure 1. The data of 30 animals with cannulae tips located outside the BLA were excluded from the analyses. Final groups were constituted as follows. Groups injected pre-training: Vehicle: n = 9; idazoxan 0.2 μg: n = 11; idazoxan 0.3 μg: n = 9; idazoxan 0.4 μg: n = 8. Groups injected post-training: Vehicle: n = 211; idazoxan 0.2 μg: n = 13; idazoxan 0.3 μg: n = 12; idazoxan 0.4 μg; n = 10.
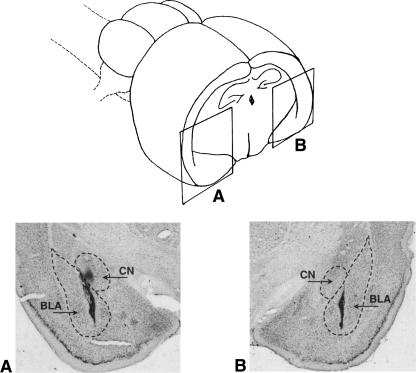
Figure 1.
(Top) Schematic representation of the amygdaloid complex. The solid lines indicate the position of the photomicrograph (bottom) representing the cannula (upper arrow) and the injection tip (lower arrow) placement. BLA, basolateral nucleus of the amygdala; CN, central nucleus of the amygdala.
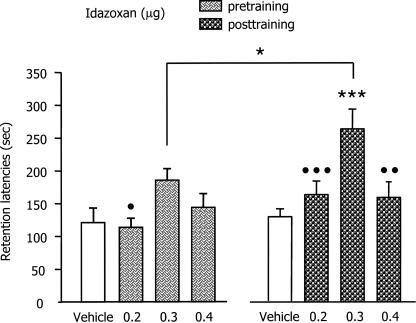
The retention test latencies are shown in Figure 2. The mean (±SEM) retention latencies of the pre- and post-training vehicle injected groups were 118.66 ± 17.88 and 128.21 ± 13.29 sec, indicating that the 0.4 mA footshock induced retention of the IA training, as training latencies were ∼15 sec for both groups. A two-way ANOVA revealed a significant effect of idazoxan (F(3,75) = 8.83; P < 0.001); an effect of time of injection (F(1,75) = 6.24; P < 0.05) and no interaction between the two factors. In the pre-training condition, post-hoc between-group comparisons indicated that group infused with 0.3 μg idazoxan had significantly longer latencies than those infused with 0.2 μg idazoxan (P < 0.05) and approached significance when compared to the control group (P = 0.057). In the post-training condition, post-hoc between-groups comparisons indicated that the latencies of the group infused with 0.3 μg of idazoxan were significantly longer than those of the controls (P < 0.001) as well as those infused with either the 0.2 or 0.4 μg doses of the α2-adrenoceptor antagonist (P < 0.05 and 0.01 respectively). Post-hoc comparisons also indicated that the group infused with 0.3 μg of idazoxan post-training had longer retention latencies than those of the corresponding group infused pre-training (P < 0.05).
Figure 2.
The effects of pre- and post-training infusions of various doses of idazoxan (a selective α2-adrenoceptor antagonist) into the basolateral amygdala on inhibitory avoidance retention latencies. Error bars represent mean ± SEM latency (in seconds) to enter the dark compartment on the retention test. Pre-training groups were infused 20 min before training whereas post-training groups were infused immediately after the footshock administration. *P < 0.05 compared with the corresponding group of the other condition; ***P < 0.001 compared with vehicle-injected group; •P < 0.05; •••P < 0.001; ••P < 0.01 compared with 0.3 μg of idazoxan-injected group in the same condition. n = 9–13 per group.
Experiment 2: Effects of pre- and post-training BLA α2-adrenoceptor blockade
Three animals did not survive surgery. Histological examination revealed that 81 animals had correct bilateral cannulae placements in the BLA. Nineteen animals that had placements outside the BLA were excluded from the analyses. Final groups were constituted as follows. Groups injected pre-training: vehicle: n = 10 ; UK (UK 14,304) 0.3 ng: n = 9; UK 1.0 ng: n = 10; UK 3.0 ng: n = 9. Groups injected post-training: vehicle: n = 9 ; UK 0.3 ng: n = 10; UK 1.0 ng: n = 12; UK 3.0 ng: n = 12.
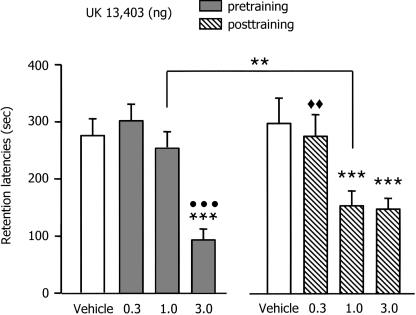
The retention test latencies are shown in Figure 3. The mean (±SEM) retention latencies of the vehicle groups injected pre- and post-training were 273.88 ± 26 and 296.47 ± 47.48 sec, respectively, indicating that the 0.5 mA footshock induced strong retention of the IA training. A two-way ANOVA revealed a significant effect of UK 14,304 (F(3,73) = 16.37; P < 0.001), no effect of time of injection (F(1,73) = 0.49; P = 0.48) and a significant interaction between these two factors (F(3,73) = 3.00; P < 0.05). In the pre-training condition, post-hoc between-group comparisons indicated that only the group infused with 3.0 ng UK 14,304 had significantly shorter latencies than those of the vehicle controls (P < 0.01), the 0.3 and 1.0 ng of UK 14,304 (P < 0.001). In the post-training condition, post-hoc between-group comparisons indicated that the animals infused with 1.0 and 3.0 ng of UK 14,304 had significant shorter latencies than those infused with the vehicle (P < 0.001) and the animals infused with the 0.3 ng of α2-adrenoceptor agonist. Moreover, comparisons indicated that the group infused pre-training with 1.0 ng of UK 14,304 had significant higher latencies than those of the corresponding post-training group (P < 0.01).
Figure 3.
The effects of immediate post-training infusions of various doses of UK 14,304 (a selective α2-adrenoceptor agonist) into the basolateral amygdala on inhibitory avoidance retention latencies. Error bars represent mean ± SEM latency (in seconds) to enter the dark compartment on the retention test. Pre-training groups were infused 20 min before training whereas post-training groups were infused immediately after the footshock administration. **P < 0.01 compared with the corresponding group of the other condition; ***P < 0.001 compared with vehicle-injected group; •••P < 0.001 compared with 0.1 and 1.0 ng of UK 14,304-injected group; ◆◆P < 0.01 compared with 1.0 and 3.0 ng of UK 14,304-injected group. n = 9–12 per group.
Discussion
The findings of these experiments provide evidence that pre-synaptic α2-adrenoceptors in the BLA are involved in modulating the consolidation of IA memory. Post-training intra-BLA infusions of the selective α2-adrenoceptor antagonist idazoxan induced a dose-dependent memory enhancement, whereas post-training infusions of the selective α2-adrenoceptor agonist UK 14,304 induced memory impairment. In experiment 1, post-training injection of 0.3 μg of idazoxan induced an enhancement of retention that was greater than that induced by injections administered 20 min before training. In the second experiment, pre-training intra-BLA infusions of only the highest dose (3.0 ng) of UK 14,304 impaired retention, whereas post-training infusions of both the 1.0 ng and the 3.0 ng doses impaired retention.
These results are consistent with those of studies in which systemic injection of selective α2-adrenergic drugs were found to disrupt and enhance consolidation of IA learning in the rat (Chopin et al. 2002). In addition, they fit with previous reports suggesting that the effects of peripheral administration of α2-adrenergic compounds on learning and memory performance are mediated through a direct action on central NE release (Abercrombie et al. 1988; Thomas and Holman 1991; Zarrindast et al. 2000; Chopin et al. 2002).
These findings are consistent with extensive prior evidence indicating a selective involvement of the BLA in adrenergic influences on memory storage (Roozendaal and McGaugh 1996, 1997; Quirarte et al. 1997). They are also consistent with previous results implicating amygdala α2-adrenoceptors in footshock based learning (Fendt et al. 1994; Schulz et al. 2002). Moreover, they clearly indicated that, in addition to involvement of α1- and β-adrenoceptors in the BLA (Ferry et al. 1999a, b), the α2-adrenoceptors participate in mediating the effects of training-induced or experimentally administered NE on memory storage. As pre-synaptic α2-negative feedback is known to regulate NE release (Starke 2001), including NE release in the amygdala (Langer 1974; Starke 1979; Fendt et al. 1994; Talley et al. 1996), the present findings provide additional evidence that memory consolidation is regulated by noradrenergic activation within the BLA.
Previous studies have found that IA memory, as well as memory for other kinds of training, is enhanced by post-training intra-BLA infusions of NE or the β-adrenoceptor agonist clenbuterol (Ferry and McGaugh 1999; Hatfield and McGaugh 1999). Further, the memory modulating effects of drugs affecting several modulatory influences, including those of the adrenal stress hormones epinephrine and corticosterone, GABAergic effects, and opioid peptidergic effects, are blocked by intra-amygdala infusions of the β-adrenoceptor antagonist propranolol (Liang et al. 1986; McGaugh et al. 1988; Introini-Collison et al. 1989; Introini-Collison et al. 1995; McGaugh et al. 1996; Quirarte et al. 1997). These findings are consistent with evidence from studies using in vivo microdialysis and high-performance liquid chromatography to assess NE release in the amygdala. Drugs that enhance memory, including epinephrine, the GABAergic antagonist picrotoxin, or the opioid antagonist naloxone enhance amygdala NE release after a footshock administration, whereas drugs that impair memory, including the GABAergic agonist muscimol and the opioid receptor agonist β-endorphin decrease the footshock-induced potentiation of NE release in the amygdala (Galvez et al. 1996; Quirarte et al. 1998; Williams et al. 1998; Hatfield et al. 1999). Additionally, footshock stimulation has been shown to increase NE levels in the amygdala and the amount released varies with the footshock intensity (Quirarte et al. 1998). Further, the level of NE release in the amygdala during IA training correlates highly with subsequent retention (McIntyre et al. 2002); i.e., better memory for IA training is displayed by animals that had higher levels of NE release in the BLA following training.
Several studies have shown that noradrenergic activation is critically important for physiological and behavioral responses to stressors (Redmond and Huang 1979; Bremner et al. 1996). The amygdala receives noradrenergic input from the locus coeruleus and the nucleus of the solitary tract (Fallon et al. 1978; Foote et al. 1983) which is activated by aversive stimuli such as footshock (Cedarbaum and Aghajanian 1978; Chiang and Aston-Jones 1993; Williams et al. 1998; Hassert et al. 2004). And, as noted above, increased amounts of NE are released in the amygdala by aversive footshock stimulation (Tanaka et al. 1991; Galvez et al. 1996; Quirarte et al. 1998; Williams et al. 1998). In addition, the α2-adrenoceptor antagonist yohimbine has been reported to amplify the immobilization stress-induced release of NE in the amygdala (Khoshbouei et al. 2002) with an amplitude comparable to that observed in the same brain region after a footshock administration (Quirarte et al. 1998; Hatfield et al. 1999), whereas clonidine, an α2-adrenoceptor agonist, has been reported to attenuate the footshock-induced NE release (Erb et al. 2000). Thus, the present findings are consistent with the hypothesis that the memory modulation induced by NE release in the amygdala result from the binding to α2-adrenergic autoreceptors that regulate the stress-induced release of NE in the BLA.
For example, because of the limits of the microdialysis technique cited, it is difficult to speculate when and for how long after a single infusion our adrenergic drugs induce their effects on NE release, since the minimal time interval between samples collection reported with this technique is about 15 min. However, the maximal effect of α2-adrenergic drugs on NE release has been observed 30 min after their infusion (Van Gaalen et al. 1997; Mateo and Meana 1999). In reference to the delay between administration of IA footshock during training and the peak of extracellular NE that is maximal after 30 min (McIntyre et al. 2002), it is likely that the effects of pre-training local injections of UK 14,304 and idazoxan into the BLA mainly resulted from the binding to α2-adrenergic autoreceptors that regulate the stress-induced release of NE. The fact that retention latencies were only influenced by the highest dose of UK 14,304 suggests that the first pool of footshock-induced NE release occurring seconds or minutes after shock administration is sufficient to enable the CS-US association. Additional studies using more sensitive measures to detect NE release with modern dosage techniques will probably help to confirm such a hypothesis.
Our finding that pre-training intra-BLA infusions of UK 14,304 and idazoxan induce effects on memory that were similar but of a smaller amplitude to those obtained in post-training groups suggests that the α2-adrenoceptor system in the BLA is more probably involved in a very fine memory-modulated tuning control of IA consolidation rather than in the encoding of CS-US association (see Schulz et al. 2002) during IA. The post-training effects are clearly consistent with the hypothesis that IA consolidation depends critically on the training-induced prolonged release of amygdala NE. As noted above, the evidence from microdialysis studies indicates that drugs infused into the BLA post-training affect the NE release at a maximal level around 30 min after their infusion (Van Gaalen et al. 1997; Mateo and Meana 1999). Moreover, this time interval has been described to be critical for the involvement of the amygdala during consolidation of IA (Bevilaqua et al. 1997; Izquierdo et al. 1997). Therefore, it is likely that the enhanced and inhibitory effects obtained by post-training injections of idazoxan and UK 14,304, respectively, on IA retention were due to the modulation of the prolonged training-induced increase in NE levels (McIntyre et al. 2002). Additionally, and importantly, our findings are consistent with the evidence of Pelletier et al. (2005) that a single footshock increases the firing of neurons in the basolateral amygdala and that the increase peaked after 30 min and subsided within 2 h.
In conclusion, our findings show that α2-induced modulation of NE release during post-trial consolidation significantly influences IA retention performance. Thus, they provide additional evidence of the memory-modulating role of NE release in the BLA. In addition, the findings are consistent with other evidence suggesting that α2-adrenoceptors in the BLA are a critical component in the modulating influence of NE on the IA memory consolidation and that the effect is probably due to prolonging the increase in training-induced levels of NE within the BLA.
Materials and Methods
Animals
Male Sprague Dawley rats (n = 222; body weight, 275–300 g at the time of surgery) obtained from Charles River Laboratories were used. After arrival, they were housed individually in a temperature-controlled (22°C) colony room and maintained on a standard 12-h light/dark cycle (light on at 07:00 h) with free access to food and water. All experiments were carried out during the light phase of the cycle between 10:00 and 14:00 h. The number of animals in each group is shown in the figure legends.
Surgery
One week after arrival, the animals were anesthetized with sodium pentobarbital (50 mg/kg body weight, i.p.) and given atropine sulphate (0.4 mg/kg, i.p.) to suppress salivation. The skull was fixed in a flat position to a stereotaxic frame (Kopf Instruments) and stainless steel guide cannulae (23 gauge, 15 mm long) were implanted bilaterally 2 mm dorsal to the BLA (coordinates: anteroposterior, −2.8 mm from Bregma; mediolateral, +5.0 mm from midline; dorsoventral, −6.7 mm from the skull surface) according to the atlas of Paxinos and Watson (1998). The cannulae and two anchoring screws were affixed to the skull with dental cement. Stylets (15-mm long 00 insect dissection pins) were inserted into each cannula to maintain patency and were removed only for the infusion of drugs. The rats were allowed to recover from surgery a minimum of 7 d before training was initiated.
Inhibitory avoidance (IA) apparatus and procedures
The IA apparatus consisted of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top, 6.4 cm wide at the floor) divided into two compartments separated by a sliding door that opened by retracting into the floor. The starting compartment (31 cm long) was illuminated and the shock compartment (60 cm long) was dark (McGaugh et al. 1988). The apparatus was located in a light- and sound-attenuated room.
The rat was placed in the starting compartment, with the door opened, and was allowed to enter the dark compartment. After the rat stepped completely into the dark compartment, the door was closed and a mild inescapable footshock with a duration of 1.0 sec was administered. Animals showing entrance latencies longer than 30 sec were eliminated from the study. The footshock intensity was adjusted for each experiment (0.4 mA for Experiment 1; 0.5 mA for Experiment 2). Bilateral microinfusions of adrenergic drugs were administered either 20 min before being placed in the dark alley (pre-training) or immediately after being removed from the dark alley (15 sec after termination of the footshock, post-training). On the 48-h retention test trial, the rat was placed in the starting compartment, as in the training session, and the latency to re-enter the dark compartment (maximum latency of 600 sec) was recorded and used as the measure of retention. Shock was not administered on the retention test trial.
Drugs
For Experiment 1, idazoxan hydrochloride (0.1, 0.2, 0.3, or 0.4 μg; kindly provided by the Center de Recherche Pierre Fabre, Castres, France), a selective α2-adrenoceptor antagonist (MacDonald and Scheinin 1995), was dissolved in 0.9% saline solution. Control animals received saline only. The doses of idazoxan were selected on the basis of previous behavioral experiments (Liang et al. 1995; Ferry et al. 1999a).
For Experiment 2, UK 14,304 [5-bromo-N-(4,5-dihydro-1-H-imidazol-2-yl)-6-quinoxalinamine] (0.3, 1.0, or 3.0 ng; Tocris Cookson Inc.), a selective α2-adrenoceptor agonist (Atkinson and Minneman 1991) was dissolved in 0.9% saline solution. Control animals received saline only. The doses of UK 14,304 were selected on the basis of previous behavioral experiments (McGaugh et al. 1988; Ferry and McGaugh 1999). Solutions of all drugs were prepared freshly before each experiment.
Infusion procedures
Bilateral pre- or post-training infusions of adrenergic agonists and antagonists into the BLA were administered through 30-gauge injection needles connected to a 10-μL Hamilton microsyringe by polyethylene tubing. The injection needles protruded 2 mm beyond the cannula tips to reach the BLA. A 0.2-μL injection volume per side was infused for 30 sec by an automated syringe pump (Sage Instruments). To allow diffusion of the drug, the injection needles were retained within the cannulae for an additional 50 sec after drug infusion. The injection volume was selected on the basis of previous experiments showing that selective neurotoxically induced lesions of the BLA are produced with an infusion volume of 0.2 μL (Ferry et al. 1995). Furthermore, drug infusions of this volume into either the BLA or the adjacent central amygdala induce differential effects on memory storage (Parent and McGaugh 1994).
Histology
After completion of behavioral testing, the rats were anesthetized with an overdose of sodium pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 0.9% saline (w/v) solution followed by 10% formaldehyde (v/v). At least 24 h before sectioning, the brains were placed in a 15% sucrose (w/v) solution for cryoprotection. Sections of 40 μm were made using a freezing microtome and stained with cresyl violet. The sections were examined under a light microscope and determination of the location of injection needle tips in the BLA was made according to the standardized atlas plates of Paxinos and Watson (1998).
Statistics
Retention data were analyzed with two-way ANOVAs with idazoxan (four levels) or UK 14,304 (four levels) both as between-subject variables, and time of injection (two levels). Further analysis used Fisher’s post hoc tests to determine the source of the detected significances in the ANOVAs. A probability level of <0.05 was accepted as statistically significant. The number of animals per group is indicated in the figure legends.
Acknowledgments
This research was supported by a fellowship from the Ralph and Leona Gerard Family Trust and financial support from the Agence Nationale de la Recherche ANR-05-PNRA-1.E7 AROMALIM (B.F.) and USPHS Grant MH12526 from NIMH (J.L.M.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.760908.
References
- Abercrombie E.D., Keller R.W., Zigmond M.J. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Alexander R.W., Davis J.N., Leflowitz R.J. Direct identification and characterization of beta-adrenergic receptors in rat brain. Nature. 1975;258:437–440. doi: 10.1038/258437a0. [DOI] [PubMed] [Google Scholar]
- Atkinson B.N., Minneman K.P. Multiple adrenergic receptor subtypes controlling cyclic AMP formation: Comparison of brain slices and primary neuronal and glial cultures. J. Neurochem. 1991;56:587–595. doi: 10.1111/j.1471-4159.1991.tb08190.x. [DOI] [PubMed] [Google Scholar]
- Berlau D.J., McGaugh J.L. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol. Learn. Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bevilaqua L., Ardenghi P., Schroder N., Bromberg E., Schmitz P.K., Schaeffer E., Quevedo J., Bianchin M., Walz R., Medina J.H., et al. Drugs acting upon the cyclic adenosine monophosphate/protein kinase A signaling pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav. Pharmacol. 1997;8:331–338. doi: 10.1097/00008877-199708000-00006. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Krystall J.H., Southwick S.M., Charney D.S. Noradrenergic mechanisms in stress and anxiety: I and II. Clinical studies. Synapse. 1996;23:28–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bylund D.B., Snyder S.H. Beta adrenergic receptor binding in membrane preparations from mammalian brain. Mol. Pharmacol. 1976;12:568–580. [PubMed] [Google Scholar]
- Cedarbaum J.M., Aghajanian G.K. Activation of locus coeruleus neurons by peripheral stimuli: Modulation by a collateral inhibitory mechanism. Life Sci. 1978;23:1383–1392. doi: 10.1016/0024-3205(78)90398-3. [DOI] [PubMed] [Google Scholar]
- Chiang C., Aston-Jones G. Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the ventral medulla. Neuroscience. 1993;53:705–715. doi: 10.1016/0306-4522(93)90618-p. [DOI] [PubMed] [Google Scholar]
- Chopin P., Colpaert F., Marien M. Effects of acute and subchronic administration of Dexefaroxan, an α2-adrenoceptor antagonist, on memory performance in young adult and aged rodents. J. Pharamacol. Exp. Ther. 2002;301:187–196. doi: 10.1124/jpet.301.1.187. [DOI] [PubMed] [Google Scholar]
- Christianson S.V. Remembering emotional events: Potential mechanisms. In: Christianson S.V., et al., editors. The handbook of emotion and memory: Research and theory. Lawrence Erlbaum Associates Inc; Hillsdale, NJ: 1992. pp. 307–340. [Google Scholar]
- Dennis T., l’Heureux R., Carter C., Scatton B. Presynaptic alpha2-adrenoceptors play a major role in the effects of Idazoxan on cortical noradrenaline release (as measured by in vivo dialysis) in the rat. J. Pharmacol. Exp. Ther. 1987;241:642–649. [PubMed] [Google Scholar]
- Erb S., Hitchcott P.K., Rajabi H., Mueller D., Shaham Y., Steward J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacol. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Fallon J.H., Koziell D.A., Moore R.Y. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J. Comp. Physiol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fendt M., Koch M., Schnitzler H.U. Amygdaloid noradrenaline is involved in the sensitization of the acoustic startle response in rats. Pharmacol. Biochem. Behav. 1994;48:307–314. doi: 10.1016/0091-3057(94)90532-0. [DOI] [PubMed] [Google Scholar]
- Ferry B., McGaugh J.L. Clenbuterol administration into the basolateral amygdala posttraining enhances retention in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B., Sandner G., Di Scala G. Neuroanatomical and functional specificity of basolateral amygdaloid nucleus in taste potentiated odor aversion. Neurobiol. Learn. Mem. 1995;64:169–180. doi: 10.1006/nlme.1995.1056. [DOI] [PubMed] [Google Scholar]
- Ferry B., Roozendaal B., McGaugh J.L. Involvement of the α1-adrenergic receptors in the basolateral amygdala in modulation of memory storage. Eur. J. Pharmacol. 1999a;372:9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Ferry B., Roozendaal B., McGaugh J.L. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α1-adrenoceptors. J. Neurosci. 1999b;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan N.A., McGrath J.C. Demonstration of simultaneous α1-, α2-, β1-, and β2-adrenoceptor mediated effects of phenylephrine in the cardiovascular system of the pithed rat. Br. J. Pharmacol. 1981;72:585–588. [Google Scholar]
- Foote S.L., Bloom F.E., Aston-Jones G. Nucleus of locus coeruleus: New evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Kapp B.S. Effect of phentolamine administration into the amygdala complex of rats on time-dependent memory processes. Behav. Neural Biol. 1981;31:90–95. doi: 10.1016/s0163-1047(81)91130-4. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Kapp B.S., Musty R.E., Driscoll P.A. Memory formation: Evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Galvez R., Mesches M., McGaugh J.L. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol. Learn. Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gold P.E., van Buskirk R.B. Facilitation of time-dependent memory processes with posttrial amygdala stimulation: Effect on memory varies with footshock level. Brain Res. 1975;86:509–513. doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- Hardman J.G., Limbird L.E., Molinoff P.B., Ruddon R.W., Gilman A.G. Catecholamines and sympathometic drugs: Endogeneous catecholamines. In: Goodman L.S., Gilman A., editors. The pharmacological basis of therapeutics. McGraw-Hill; New York: 1996. pp. 204–248. [Google Scholar]
- Hassert D.L., Miyashita T., Williams C.L. The effects of peripheral vagal nerve stimulation at a memory modulating intensity on norepinephrine output in the basolateral amygdala. Behav. Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- Hatfield T., McGaugh J.L. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol. Learn. Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Hatfield T., Spanis C., McGaugh J.L. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I.B., Nagahara A.H., McGaugh J.L. Memory-enhancement with intra-amygdala posttraining naloxone is blocked by concurrent administration of propranolol. Brain Res. 1989;476:94–101. doi: 10.1016/0006-8993(89)91540-0. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I.B., Miyazaki B., McGaugh J.L. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacol. 1991;104:541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I.B., Ford L., McGaugh J.L. Memory impairment induced by intra-amygdala β-endorphin is mediated by noradrenergic influences. Neurobiol. Learn. Mem. 1995;63:200–205. doi: 10.1006/nlme.1995.1021. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Quillfeldt J.A., Zanatta M.S., Quevedo J., Schaeffer E., Schmitz P.K., Medina J.H. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur. J. Neurosci. 1997;9:125–133. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H., Cecchi M., Dove S., Javors M., Morilak D.A. Behavioral reactivity to stress: Amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol. Biochem. Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- LaLumiere R.T., Buen T.V., McGaugh J.L. Posttraining intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J. Neurosci. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S.Z. Presynaptic regulation of catecholamine release. Biochem. Pharmacol. 1974;23:1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Liang K.C., Juler R., McGaugh J.L. Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liang K., McGaugh J.L., Yao H. Involvement of amygdala pathways in the influence of posttraining amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Liang K., Chen L., Huang T.E. The role of amygdala norepinephrine in memory formation: Involvement in the memory-enhancing effect of peripheral epinephrine. Chin. J. Physiol. 1995;38:81–91. [PubMed] [Google Scholar]
- MacDonald E., Scheinin M. Distribution and pharmacology of α2-adrenoceptors in the central nervous system. J. Physiol. Pharmacol. 1995;46:241–258. [PubMed] [Google Scholar]
- Mateo Y., Meana J.J. Determination of the somatodendritic α2-adrenoceptor subtype located in rat locus coeruleus that modulates cortical noradrenaline release in vivo. Eur. J. Pharmacol. 1999;379:53–57. doi: 10.1016/s0014-2999(99)00488-4. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu. Rev. Neurosci. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Memory—A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L., Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L., Introini-Collison I.B., Nagahara A.H. Memory-enhancing effects of posttraining naloxone: Involvement of β-adrenergic influences in the amygdaloid complex. Brain Res. 1988;446:37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L., Cahill L., Roozendaal B. Involvement of the amygdala in memory storage: Interaction with other brain systems. Proc. Natl. Acad. Sci. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C.K., Hatfield T., McGaugh J.L. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur. J. Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- Parent M.B., McGaugh J.L. Posttraining infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; New York: 1998. [Google Scholar]
- Pelletier J.G., Likchtik E., Filali M., Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: Implications for facilitated consolidation of emotional memories. Learn. Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte G.L., Roozendaal B., McGaugh J.L. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte G.L., Galvez R., Roozendaal B., McGaugh J.L. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Redmond D.E., Huang Y.H. Current concepts. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Schulz B., Fendt M., Schnitzler H.U. Clonidine injections into the lateral nucleus of the amygdala block acquisition and expression of fear-potentiated startle. Eur. J. Neurosci. 2002;15:151–157. doi: 10.1046/j.0953-816x.2001.01831.x. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic regulation of catecholamines release in the central nervous system. In: XX XX., Paton D.M., editors. The release of catecholamines from adrenergic neurons. Pergamon Press; New York: 1979. pp. 143–183. [Google Scholar]
- Starke K. Presynaptic autoreceptors in the third decade: Focus on alpha2-adrenoceptors. J. Neurochem. 2001;78:685–693. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Talley E.M., Rosin D.L., Lee A., Guyenet P.G., Lynch K.R. Distribution of α2-adrenergic receptor-like immunoreactivity in the rat central nervous system. J. Comp. Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yokoo H., Mizoguchi K., Yoshida M., Tsuda A., Tanaka M. Noradrenaline release in the rat amygdala is increased by stress: Studies with intracerebral microdialysis. Brain Res. 1991;544:174–176. doi: 10.1016/0006-8993(91)90902-8. [DOI] [PubMed] [Google Scholar]
- Thomas D.N., Holman R.B. A microdialysis study of the regulation of endogenous noradrenaline release in the rat hippocampus. J. Neurochem. 1991;56:1741–1746. doi: 10.1111/j.1471-4159.1991.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Unnerstal J.R., Kopajtic T.A., Kuhar M.J. Distribution of α2-agonist binding sites in the rat and human central nervous system: Analysis of some functional, anatomic correlates of the pharmacological effects of clonidine and related adrenergic agents. Brain Res. 1984;319:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- U’Prichard D.C., Reisine T.D., Mason S.T., Fibiger H.C., Yamamura H.I. Modulation of rat brain α- and β-adrenergic receptor populations by lesion of the dorsal noradrenergic bundle. Brain Res. 1980;187:143–154. doi: 10.1016/0006-8993(80)90500-4. [DOI] [PubMed] [Google Scholar]
- Van Gaalen M., Kawahara H., Westerink B.H.C. The locus coeruleus noradrenergic system in the rat brain studied by dual-probe microdialysis. Brain Res. 1997;763:56–62. doi: 10.1016/s0006-8993(97)00416-2. [DOI] [PubMed] [Google Scholar]
- van Meel J.C., De Jonge A., Timmermans P.B., Van Zwieten P.A. Selectivity of some alpha adrenoceptor agonists for peripheral alpha-1 and alpha-2 adrenoceptors in the normotensive rat. J. Pharmacol. Exp. Ther. 1981;219:760–767. [PubMed] [Google Scholar]
- Wikberg J. Localization of adrenergic receptors in guinea-pig ileum and rabbit jejenum to cholinergic neurons and to smooth muscle cells. Acta Physiologica Scandinavia. 1973;99:190–207. doi: 10.1111/j.1748-1716.1977.tb10370.x. [DOI] [PubMed] [Google Scholar]
- Williams C.L., Men D., Clayton E.C., Gold P.E. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: Contribution of the nucleus of the solitary tract. Behav. Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- Zarrindast M.R., Fazli-Tabei S., Semnanian S., Fathollahi Y., Yahyavi S.H. Effects of adrenoceptor agents on apomorphine-induced licking behavior in rats. Pharmacol. Biochem. Behav. 2000;65:275–279. doi: 10.1016/s0091-3057(99)00198-7. [DOI] [PubMed] [Google Scholar]
- Zilles K., Qu M., Schleicher A. Regional distribution and heterogeneity of α-adrenoceptors in the rat and human central nervous system. J. für Hirforshung. 1993;2:123–132. [PubMed] [Google Scholar]