Abstract
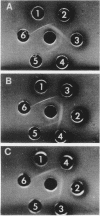
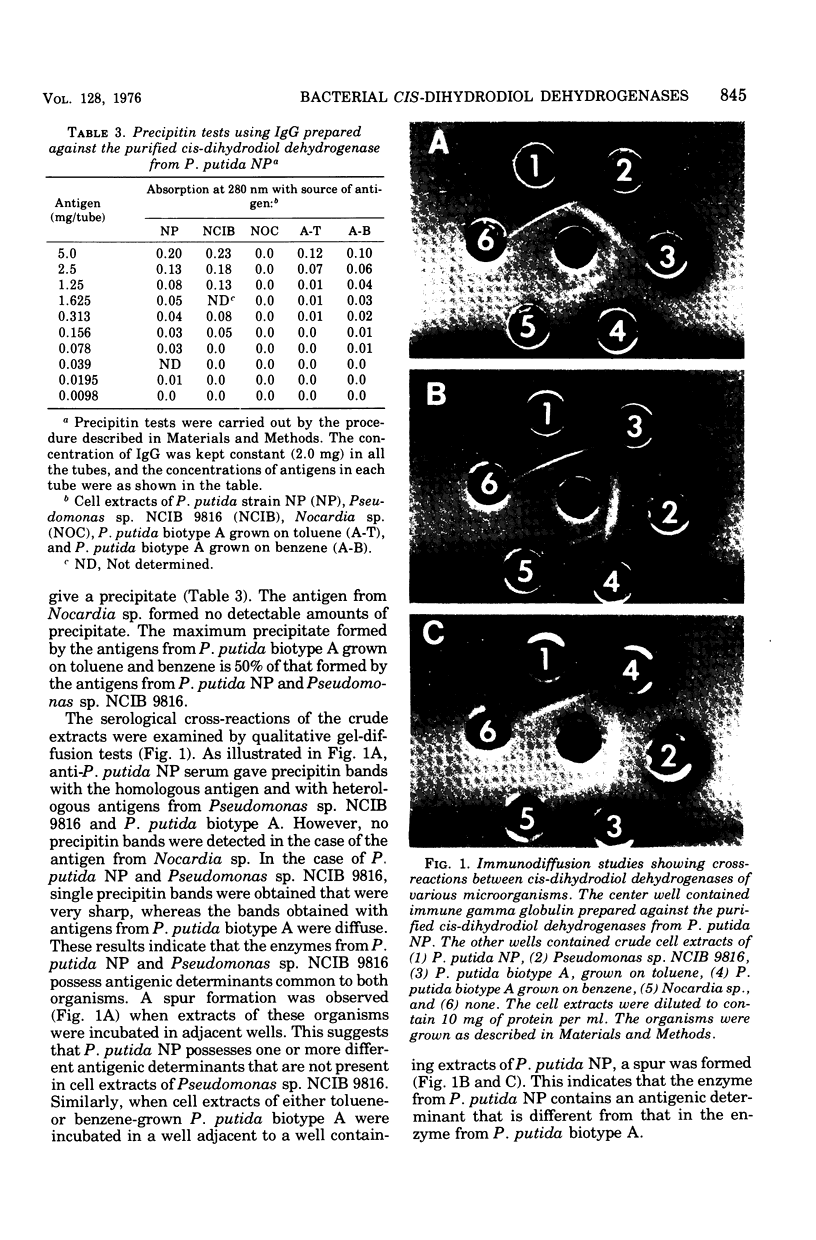
Cells of Pseudomonas putida NP, Pseudomonas species (NCIB 9816), and a Nocardia species, after growth on naphthalene as sole source of carbon and energy, contain a nicotinamide adenine dinucleotide (NAD+)-dependent enzyme that oxidizes cis-dihydrodiols of mono- and polycyclic aromatic compounds. Similarly, cells of a strain of P. putida biotype A, when grown either on toluene or benzene vapors, were found to contain a dehydrogenase that oxidized dihydrodiols of aromatic hydrocarbons with cis stereochemistry and required NAD+ as an electron acceptor. In all these cases, no enzymatic activity was detected when trans-naphthalene dihydrodiol was used as a substrate. Purified cis-naphthalene dihydrodiol dehydrogenase was injected into rabbits to obtain antibodies. Physiocochemical and immunological properties of cis-dihydrodiol:NAD+ oxidoreductases from four different organisms were examined. Kinetic analysis showed that, in all the cases, enzymes exhibited higher affinity for cis-dihydrodiols than for NAD+ and had pH optima between 8.8 and 9.0. except in the case of the enzyme from Nocarida sp., which showed maximum activity at pH 8.4. Molecular-weight determination of the dehydrogenases from the four different organisms by gel filtration on a Sephadex G-200 column gave values ranging from 92,000 for the enzyme from Nocardia sp. to 160,000 for that from P. putida biotype A. All the dehydrogenases, except the one from Nocardia sp., exhibited immunological cross-reaction with the antibodies prepared against the enzyme purified from P. putida NP.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYENGAR P. K., HAYAISHI O., NAKAJIMA M., TOMIDA I. Enzymic aromatization of 3,5-cyclohexadiene-1,2-diol. Biochim Biophys Acta. 1959 May;33(1):111–119. doi: 10.1016/0006-3002(59)90504-9. [DOI] [PubMed] [Google Scholar]
- Akhtar M. N., Boyd D. R., Thompson N. J., Koreeda M., Gibson D. T., Mahadevan V., Jerina D. M. Absolute sterochemistry of the dihydroanthracene-cis- and -trans-1,2-diols produced from anthracene by mammals and bacteria. J Chem Soc Perkin 1. 1975;(23):2506–2511. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. The metabolism of benzene by bacteria. Purification and some properties of the enzyme cis-1,2-dihydroxycyclohexa-3,5-diene (nicotinamide adenine dinucleotide) oxidoreductase (cis-benzene glycol dehydrogenase). Biochem J. 1973 Dec;136(4):927–934. doi: 10.1042/bj1360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M., Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia. 1972 Oct 15;28(10):1129–1149. doi: 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Gschwendt B., Yeh W. K., Kobal V. M. Initial reactions in the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry. 1973 Apr 10;12(8):1520–1528. doi: 10.1021/bi00732a008. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Schuld C. L., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry. 1968 Nov;7(11):3795–3802. doi: 10.1021/bi00851a003. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Högn T., Jaenicke L. Benzene metabolism of Moraxella species. Eur J Biochem. 1972 Oct;30(2):369–375. doi: 10.1111/j.1432-1033.1972.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Yeh H. J., Jerina D. M., Patel T. R., Davey J. F., Gibson D. T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975 Feb 11;14(3):575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. 3. Formation of 1,2-naphthalene oxide from naphthalene by liver microsomes. J Am Chem Soc. 1968 Nov 6;90(23):6525–6527. doi: 10.1021/ja01025a058. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal V. M., Gibson D. T., Davis R. E., Garza A. X-ray determination of the absolute stereochemistry of the initial oxidation product formed from toluene by Pseudomonas puida 39-D. J Am Chem Soc. 1973 Jun 27;95(13):4420–4421. doi: 10.1021/ja00794a048. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oesch F., Daly J. Conversion of naphthalene to trans-naphthalene dihydrodiol: evidence for the presence of a coupled aryl monooxygenase-epoxide hydrase system in hepatic microsomes. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1713–1720. doi: 10.1016/0006-291x(72)90807-8. [DOI] [PubMed] [Google Scholar]
- Oesch F., Jerina D. M., Daly J. W., Lu A. Y., Kuntzman R., Conney A. H. A reconstituted microsomal enzyme system that converts naphthalene to trans-1,2-dihydroxy-1,2-dihydronaphthalene via naphthalene-1,2-oxide: presence of epoxide hydrase in cytochrome P-450 and P-448 fractions. Arch Biochem Biophys. 1972 Nov;153(1):62–67. doi: 10.1016/0003-9861(72)90420-1. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of benzoic acid by bacteria: 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid is an intermediate in the formation of catechol. J Bacteriol. 1971 Oct;108(1):89–94. doi: 10.1128/jb.108.1.89-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Yamauchi T., Fujisawa H. Studies on mechanism of double hydroxylation. I. Evidence for participation of NADH-cytochrome c reductase in the reaction of benzoate 1,2-dioxygenase (benzoate hydroxylase). Biochem Biophys Res Commun. 1975 Nov 3;67(1):264–271. doi: 10.1016/0006-291x(75)90311-3. [DOI] [PubMed] [Google Scholar]
- Ziffer H., Jerina D. M., Gibson D. T., Kobal V. M. Absolute stereochemistry of the (+)-cis-1,2-dihydroxy-3-methylcyclohexa-3,5-diene produced from toluene by Pseudomonas putida. J Am Chem Soc. 1973 Jun 13;95(12):4048–4049. doi: 10.1021/ja00793a036. [DOI] [PubMed] [Google Scholar]