Abstract
Human hematopoiesis originates in a population of stem cells with transplantable lympho-myeloid reconstituting potential, but a method for quantitating such cells has not been available. We now describe a simple assay that meets this need. It is based on the ability of sublethally irradiated immunodeficient nonobese diabetic–scid/scid (NOD/SCID) mice to be engrafted by intravenously injected human hematopoietic cells and uses limiting dilution analysis to measure the frequency of human cells that produce both CD34−CD19+ (B-lymphoid) and CD34+ (myeloid) colony-forming cell progeny in the marrow of such recipients 6 to 8 weeks post-transplant. Human cord blood (CB) contains ≈5 of these competitive repopulating units (CRU) per ml that have a similar distribution between the CD38− and CD38+ subsets of CD34+ CB cells as long-term culture-initiating cells (LTC-IC) (4:1 vs. 2:1). Incubation of purified CD34+CD38− human CB cells in serum-free medium containing flt-3 ligand, Steel factor, interleukin 3, interleukin 6, and granulocyte colony-stimulating factor for 5–8 days resulted in a 100-fold expansion of colony-forming cells, a 4-fold expansion of LTC-IC, and a 2-fold (but significant, P < 0.02) increase in CRU. The culture-derived CRU, like the original CB CRU, generated pluripotent, erythroid, granulopoietic, megakaryopoietic, and pre-B cell progeny upon transplantation into NOD/SCID mice. These findings demonstrate an equivalent phenotypic heterogeneity amongst human CB cells detectable as CRU and LTC-IC. In addition, their similarly modest response to stimulation by a combination of cytokines that extensively amplify LTC-IC from normal adult marrow underscores the importance of ontogeny-dependent changes in human hematopoietic stem cell proliferation and self-renewal.
Keywords: hematopoietic stem cells, transplantation, in vitro cell expansion
Identification of conditions that support the self-renewal and expansion of human hematopoietic stem cells remains a major goal of experimental and clinical hematology. Analysis of various endpoints of hematopoietic reconstitution from defined transplants has provided extensive information about these cells in several species including humans. In mice, this approach has been further developed to allow both adult and fetal hematopoietic stem cells to be specifically identified and quantitated using an assay that measures the frequency of cells in a given test cell suspension that at limiting dilutions can be seen to individually, competitively, and durably repopulate both the lymphoid and myeloid systems of histocompatible but genetically distinguishable recipients (1–4). To ensure maximal efficiency of detection of these so-called competitive repopulating units (CRU), the recipient mice are pretreated with a myeloablative conditioning regimen and then transplanted with sufficient additional cells to allow their survival independent of the stem cell content of the test transplant. Alternatively, the recipients may be given a sublethal dose of radiation (5). Clinical studies with purified subpopulations of human cells (6–9) have suggested some of the properties of transplantable human hematopoietic cells, but an experimental method for their enumeration has not been available. In fact, until recently, attention has focused primarily on the identification of properties of hematopoietic cells that might prove useful as surrogate endpoints of stem cell potential. The ability of a rare subset of hematopoietic cells [referred to as long-term culture-initiating cells (LTC-IC)] to generate myeloid colony-forming cell (CFC) progeny for at least 4 weeks in fibroblast-containing cocultures is one such endpoint (10). The fact that LTC-IC and CRU in both the fetal liver and adult bone marrow of mice are phenotypically similar, exist at similar frequencies, and copurify (in contrast to all other known progenitor types detectable either in vitro or in vivo) (11–13) has suggested that LTC-IC and CRU may be the same cells—i.e., that the functions required for cells to be detectable in these two assays are coordinately regulated during normal hematopoietic cell development. This concept is further supported by the demonstration that at least some CRU proliferate and undergo self-renewal divisions under the same culture conditions as are used to stimulate LTC-IC proliferation and differentiation into CFC (2). However, differences in the factors required to elicit and sustain murine CRU and LTC-IC activity in vitro and in vivo have also been identified (13, 14). Thus CRU and LTC-IC do not depend on the same molecular functions for their detection and, under certain circumstances, these functions can be dissociated.
Recently, a variety of immunodeficient xenogeneic recipients have been found to support the growth of transplanted human hematopoietic cells (15–17) with higher overall levels of human hematopoiesis obtained in sublethally irradiated nonobese diabetic–scid/scid (NOD/SCID) mice than in any other strain thus far tested (18, 19). The amplification, multilineage composition, durability, continuing proliferation, and retransplantability of the human hematopoietic cell populations regenerated in these mice all suggest their origin from a transplantable human cell type with extensive proliferative and differentiation potential (19, 20). In this report, we describe a simple method for quantitating human cord blood (CB) cells that produce both lymphoid and myeloid progeny in sublethally irradiated NOD/SCID mice injected i.v. with limiting dilutions of test cells (without exogenously administered cytokines). The assay can be applied to highly purified populations and has been used to demonstrate human CRU expansion in 5- to 8-day cultures of CD34+CD38− CB cells in serum-free cultures containing flt3-ligand (FL), Steel factor (SF), interleukin 3 (IL-3), IL-6, and granulocyte colony-stimulating factor (G-CSF).
MATERIALS AND METHODS
CB Cell Preparation.
CB from normal, full-term infants delivered by cesarean section were collected in tubes containing heparin according to protocols approved by the University of British Columbia Clinical Screening Committee for Research Involving Human Subjects. In most experiments, low density (<1.077 g/cm3) cells were obtained by centrifugation of the initial cell sample on Ficoll-Hypaque (Pharmacia LKB). In three experiments, red blood cells were removed either by lysis at 4°C in 0.83% ammonium chloride with 0.1% sodium bicarbonate (pH 7.0) or by hydroxyethyl starch (DuPont)-assisted sedimentation. The enriched white blood cell fraction was then used either without further manipulation, or after being enriched for CD34+ cells using a high gradient magnetic cell separation procedure in which cells expressing markers of mature human hematopoietic cells were removed on a StemSep column (StemCell Technologies, Vancouver) according to the manufacturer’s directions. Aliquots of cells were stained before and after this separation with a fluorescein isothiocyanate (FITC)-conjugated anti-CD34 antibody (8G12) (kindly provided by P. Lansdorp, Terry Fox Laboratory) to calculate the recovery, enrichment, and purity of the CD34+ cells isolated. The average (± SEM) CD34+ cell content of the starting cell suspension was 0.4 ± 0.1%, and after depletion of the lineage-marker+ cells this increased to 43 ± 5%. The corresponding CD34+ cell recovery and enrichment values for these lin− CB preparations were 120 ± 20% and 160 ± 30-fold, respectively (n = 8). To isolate the CD34+CD38− and CD34+CD38+ subpopulations from these lin− cells, they were first suspended in Hank’s balanced salt solution with 2% fetal calf serum and 0.02% sodium azide (HFN) supplemented with 5% human serum, then incubated on ice for 10 min, followed by staining with anti-CD34 FITC and anti-CD38 phycoerythrin (PE) (Becton Dickinson). This was followed by two washes in HFN, in the last case, in the presence of 2 μg/ml propidium iodide (PI; Sigma) to allow exclusion of nonviable (PI+) cells. Throughout the staining procedure, the cells were maintained at 4°C. Cells were analyzed and sorted on a FACStar+ (Becton Dickinson) equipped with a 5 W argon laser and a 30 mW helium neon laser. Additional aliquots of cells were stained with irrelevant isotype-matched control antibodies labeled with FITC and PE to establish gates for identifying positively stained cells (fluorescence greater than that exhibited by 99.9% of cells in the corresponding controls).
Progenitor Assays.
CFC and LTC-IC assays were performed as described (21).
Animals.
A colony of NOD/LtSZ–scid/scid (NOD/SCID) mice was established in the animal facility of the British Columbia Cancer Research Center from breeders originally provided by L. Schultz (The Jackson Laboratories). All NOD/SCID mice were kept under sterile conditions in microisolator cages and were provided exclusively with autoclaved food and water. Just before, and for 2 months after, total-body irradiation the mice received acidified H2O (pH = 3).
Competitive Repopulation Assay.
Mice were given 350 cGy of total body 137Cs γ-irradiation (≈1 cGy/min) and were then injected i.v. with varying numbers of test cells (as indicated) plus 106 irradiated (1,500 cGy) normal human bone marrow cells (obtained as cadaveric samples from the Northwest Tissue Center, Seattle) as carrier cells (for test grafts of ≤106 cells). Mice were killed 6–8 weeks posttransplant for assessment of the number and types of human cells detectable in both femurs and both tibias. CRU frequencies were determined by the method of maximum likelihood from the proportions of negative recipients measured in groups of mice injected with different numbers of test cells as described (1). In the present study, negative recipients were defined as mice that did not contain detectable numbers of both human B-lymphoid cells—i.e., ≤5 positive CD34−CD19+ cells per 5,000 cells analyzed and human CD34+ myeloid progenitors [colony-forming unit-granulocyte, macrophage (CFU-GM) and/or burst-forming unit-erythroid (BFU-E) and/or colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM)].
Serum-Free Expansion Cultures.
CD34+CD38− cells were incubated at ≈103–104 cells/ml (0.1–10 ml per culture) in Iscove’s medium supplemented with 10 mg/ml BSA, 10 μg/ml human insulin, 200 μg/ml human transferrin (StemCell Technologies), 10−4 M 2-mercaptoethanol (Sigma), plus 40 μg/ml low density lipoproteins (Sigma) and the following recombinant human growth factors: 20 ng/ml IL-3 (Sandoz), 20 ng/ml IL-6 (Cangene, Mississauga, ON, Canada), 20 ng/ml G-CSF and 100 ng/ml SF (Amgen), and 100 ng/ml FL (Immunex). These suspension cultures were incubated unperturbed, usually for 5 or 6 days (n = 5), and in one experiment for 8 days at 37°C. At the end of this time, all cells were harvested and counted, and aliquots were assayed in vitro for CFC, LTC-IC and in NOD/SCID mice for CRU. In two additional experiments CD34+CD38− CB cells were cultured as single cells [deposited using the fluorescence-activated cell sorter (FACS) cloning attachment] in the individual wells of 96-well plates, each of which had been preloaded with 100 μl of the same medium. At the end of 6 days, 70 of the clones produced were harvested individually and injected into 70 sublethally irradiated NOD/SCID mice (one clone per recipient).
Analysis of Human Cells in NOD/SCID Mice.
Femurs and tibias were flushed with HFN and cell counts performed. Human Fc receptors were blocked by a first incubation of the cells in 5% normal human serum and murine Fc receptors by a second incubation of the cells in 2.4G2 [an anti-mouse Fc receptor mAb (22)]. To quantitate the total number of human cells present, a small aliquot was stained with anti-CD45–FITC (HLel, Becton Dickinson) and anti-CD71–FITC (OKT9). This aliquot was also stained with anti-CD19–PE (Leu-12, Becton Dickinson) and cyanine-5-succinimidyl-labeled anti-CD34 (8G12) to allow the exclusive detection of human pre-B (CD34−CD19+) cells (23). The majority of the cells were stained with anti-CD34-PE and anti-gpIIb/IIIa CD41–FITC (3H2) (24) antibodies to allow quantitation of cells with these markers and isolation of human CD34+ cells by FACS for subsequent plating in CFC and LTC-IC assays. All cells were also stained with PI (see above) in the final wash to allow exclusion of dead (PI+) cells from these analyses and collections. Aliquots of the same original cell suspension were stained with both FITC-conjugated and PE-conjugated mouse Ig as negative controls. Positive cells were defined as those demonstrating greater fluorescence than that exhibited by ≥99.9% of these negative control cells. Incubation of normal NOD/SCID marrow cells with all anti-human antibodies used showed ≤0.1% of murine myeloid cells to be stained nonspecifically.
RESULTS
Both Human Lymphoid and Human Myeloid Cells Are Found in NOD/SCID Mice Transplanted with Limiting Numbers of Human CB Cells.
Groups of sublethally irradiated NOD/SCID mice were injected with decreasing doses of light-density CB cells or highly enriched (>99.9% pure) CD34+CD38− or CD34+CD38+ CB cells. Six to 8 weeks later their marrows were assessed for the presence of various types of human hematopoietic cells as described. In a total of 115 mice in which human cells were detected, 91% contained both lymphoid (CD34−CD19+) and myeloid (CD34+ CFC) elements (Fig. 1). Analysis of mice injected with limiting numbers of transplantable human CB cells (i.e., cell doses that, on average, should have resulted in <15% of the mice being engrafted with human cells) showed that examples of mice containing human pre-B cells in the absence of human myeloid cells, or vice versa, were rare regardless of whether the mice had been transplanted with unseparated light-density CB cells, or highly purified CD34+CD38− or CD34+CD38+ cells (Table 1). Thus, no evidence of either lymphoid- or myeloid-restricted repopulating cells in human CB was obtained using a 6- to 8-week readout in NOD/SCID mice and, in fact, essentially all of the regenerating activity detected using this endpoint could be attributed to transplantable CB cells with lympho-myeloid potential. In addition, 40% of these clonally repopulated mice showed evidence of human erythroid as well as granulopoietic cell differentiation (i.e., both BFU-E and CFU-GM were detected).
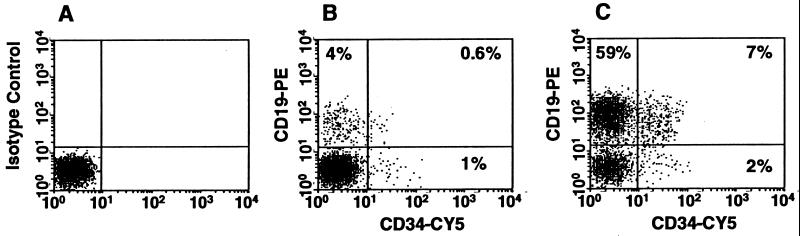
Figure 1.
Comparison of FACS profiles of marrow cells from NOD/SCID mice transplanted 6 and 8 weeks previously with 300 CD34+CD38− cells (A and B) (i.e., at limiting dilution) and 3 × 103 CD34+CD38− cells (C) from the same original CB sample. In A the cells were stained with an irrelevant (control) isotype-matched mouse IgG. In B and C, the cells were stained with anti-human CD34–CY5 (cyanine-5-succinimidyl) and anti-human CD19–PE. Evidence of human cells of all three phenotypes examined in these analyses is seen in both mice (B and C).
Table 1.
NOD/SCID mice transplanted with limiting numbers of human CB repopulating cells usually contain both lymphoid and myeloid cells of human origin
| Progeny combinations | Proportion of positive mice transplanted with
|
||
|---|---|---|---|
| Light-density cells, % (<4 × 105/mouse) | CD34+CD38− cells, % (<800/mouse) | CD34+CD38+ cells, % (<104/mouse) | |
| M+ and/or L+ | 67 (12/18) | 36 (20/55) | 43 (7/16) |
| M+ only | 6 (1/18) | 2 (1/55) | 6 (1/16) |
| L+ only | 0 (0/18) | 4 (2/55) | 6 (1/16) |
| Expected L+ M+ (due to coincidence) | 0 | 0.1 | 0.4 |
| Observed L+ M+ | 61 (11/18) | 32 (17/55) | 31 (5/16) |
M+, myeloid; L+, lymphoid. Only mice injected with doses of cells expected to contain <1 repopulating cell of any kind (based on limiting dilution analysis of the entire data set in that group) were considered in this analysis—i.e., <15% of these mice would have received >1 repopulating human cell (same experiments as shown in Table 2, but for the analysis shown here, mice were considered negative only if they contained neither lymphoid nor myeloid cells). Note also that these cell doses represent the calculated numbers expected to contain <1 such repopulating cell but, as can be seen, did not always give 37% negative mice.
Frequency and Characterization of CRU in Human CB.
Limiting dilution analysis was then used to determine the frequency of these transplantable lympho-myeloid cells in various subpopulations of human CB (Table 2). Individual mice were scored as positive only when the number of human lymphoid (CD34−CD19+) cells present 6–8 weeks posttransplant constituted ≥0.1% of the total marrow population and human (CD34+) granulopoietic progenitors, with or without erythroid clonogenic progenitors, were also demonstrable in the cells removed from two femurs and two tibias. Because these four bones contain ≈25% of the total marrow volume of a mouse (25), the minimal output required for assignment of human CRU activity was thus ≈2 × 105 pre-B cells and ≈200 CFC at 6–8 weeks posttransplant. Any recipient who did not fulfill both of these criteria was scored as negative. The average frequency of CRU in the light density fraction of CB cells calculated from the results of these experiments was ≈1 per 6 × 105 cells (range defined by ± SE = 1 per 5 × 105 to 1 per 8 × 105 cells, Table 3), or 4.5 (3.4–5.4) CRU per ml of CB (n = 3).
Table 2.
Frequency of “negative” NOD/SCID mice 6–8 weeks after transplantation with varying numbers of freshly isolated or cultured CB cells
| Type of cell | No. of experiments | Total No. of CB assessed | Cells/mouse | Proportion of negative mice |
|---|---|---|---|---|
| Light density | 3 | 7 | 3 × 106 | 2/10 |
| 1 × 106 | 2/8 | |||
| 8 × 105 | 0/6 | |||
| 3 × 105 | 3/8 | |||
| 2 × 105 | 1/6 | |||
| 5 × 104 | 3/4 | |||
| CD34+CD38+ | 2 | 4 | 5 × 104 | 0/5 |
| 1.5 × 104 | 5/6 | |||
| 7,500 | 2/6 | |||
| 3,000 | 5/5 | |||
| 600 | 4/5 | |||
| CD34+CD38− | 7 | 16 | 4,400 | 1/2 |
| 3,000 | 2/11 | |||
| 2,000 | 5/5 | |||
| 1,000 | 5/17 | |||
| 500 | 5/12 | |||
| 300 | 1/4 | |||
| 200 | 10/14 | |||
| 50 | 21/25 | |||
| Cultured CD34+CD38− | 6 | 14 | 4,400 | 0/3 |
| 1,000 | 2/11 | |||
| 500 | 3/5 | |||
| 200 | 9/12 | |||
| 100 | 3/3 | |||
| 50 | 5/6 | |||
| 40 | 3/7 |
Negative mice were defined as those that did not contain detectable levels of both human CD34−CD19+ (B-lymphoid) and CD34+ CFC (myeloid) cells. Pooled data from the number of experiments shown (2 to 3 CB samples pooled per experiment).
Table 3.
Comparison of the frequencies of different types of progenitors in the light-density, purified, and cultured human CB populations studied
| Progenitor | Light density (per 106 cells) | CD34+CD38+ (per 103 cells) | CD34+CD38− (per 103 cells) | Cultured CD34+CD38− (per initial 103 cells) |
|---|---|---|---|---|
| CFC | 4,000 ± 2,300 | 500 | 310 ± 130 | 30,000 ± 12,000 |
| LTC-IC | 52 ± 46 | 52 | 560 ± 230 | 2,100 ± 900 |
| CRU | 1.7 (1.3–2) | 0.06 (0.04–0.08) | 1.1 (0.9–1.3) | 2 (1.6–2.5) |
| 3 | 2 | 7 | 6 |
Values shown for CFC and LTC-IC are the mean ± SEM and for CRU are the mean with the range defined by ± SE shown in parentheses. The total cell expansion in the cultures of CD34+CD38− cells was 78 ± 33-fold.
Several lines of evidence, including the finding that clinical transplants of CD34+ cell-enriched bone marrow transplants, give timely hematopoietic reconstitution of patients treated with myeloablative conditioning therapy (6–8) suggest that human cells with repopulating ability are CD34+. However, human CD34+ hematopoietic cells are now known to be heterogeneous both functionally and in terms of other markers they may express (23, 26). One such marker, whose absence on a minor subset of human hematopoietic CD34+ cells (including human CB cells) has proven useful for isolating a population that is highly enriched in cells with properties of primitive progenitors is CD38 (27–34). To investigate the phenotype of human CB cells capable of regenerating lympho-myelopoiesis in NOD/SCID mice, the CD34+ light density CB cells were subdivided into a CD38− or CD38lo and a CD38+ fraction and then limiting dilution CRU assays were performed on these subsets. The average frequency of CRU in the CD34+CD38lo population determined from the pooled results of seven experiments was found to be 1 per 900 cells (range defined by ± SE = 1 per 750 to 1 per 1,100 cells, Table 3). This represents a 600-fold enrichment of CRU relative to their frequency in the light density fraction of CB cells. In two experiments, CRU assays were performed on the CD38+ and CD38− fractions of the same CB samples. From these assays, values of 1 CRU per 18,000 CD34+CD38+ CB cells and 1 CRU per 400 CD34+CD38− CB cells were obtained. Thus some CRU could be detected in the CD34+CD38+ fraction and at >33-fold higher frequencies than in the original light density fraction of CB cells, but at a 45-fold lower frequency than in the corresponding CD34+CD38− fraction. However, because the majority of the CD34+ cells are also CD38+, absolute numbers of CD38+ CRU are much higher than predicted by a comparison of their frequencies. The ratio of total CD34+CD38− CRU to CD34+CD38+ CRU in CB was calculated to be 4:1. LTC-IC and CFC assays of these same fractions showed that both of these assays detect cells at much higher frequencies than CRU (several hundred-fold) and in fact account for the majority of all the CD34+ cells (Table 3). However, the distribution of LTC-IC between the CD38− subset and CD38+ subsets (64% and 36%, respectively) proved to be similar to that calculated for CRU, whereas the corresponding values for CFC were 20% and 80%.
Quantitation of CRU and Other Progenitors in Short-Term Cultures of CD34+CD38− Human CB Cells.
On the basis of previous studies indicating FL, SF, and IL-3 to be important stimulators of bone marrow LTC-IC amplification (21), and SF and IL-6 to be effective stimulators of primitive cells in both marrow and CB (35–38), we set up experiments to determine whether CRU activity would be amplified, maintained, or lost when CD34+CD38lo CB cells were incubated in serum-free medium supplemented with FL and SF (both at 100 ng/ml) and IL-3, IL-6, and G-CSF (all at 20 ng/ml). The results of the CRU assays performed on aliquots of the cells used to initiate these cultures as well as on aliquots of the cells harvested from them 5 to 8 days later (six experiments) are shown in Tables 2 and 3. The frequency of CRU in the cultured cells was 1 per 500 input CD34+CD38− CB cells (range defined by ± SE = 1 per 400 to 1 per 600). This represents a small (2-fold) but significant (P < 0.02, Student’s two-tailed t test) increase over the input values of these experiments.
In two additional experiments, 120 CD34+CD38− CB cells were cultured as single cells under the same conditions. At the end of 5 days, 33 of the original 120 wells (i.e., 28%) did not appear to contain any viable cells. In each of the remaining 87 wells, between 2 and 270 viable (refractile) cells were seen (with no wells containing only one viable cell). The following day (day 6), the first 70 clones were injected individually into 70 sublethally irradiated NOD/SCID mice (1 clone per mouse). Six to 8 weeks later, 68 of these recipients showed no evidence of engraftment with human cells. In the other two mice, a small proportion (0.2%) of all the cells present in the marrow appeared to be positive human CD34+ cells, but no CD19+ cells were detected and when the CD34+ cells were isolated (972 and 115 cells, respectively) and assayed, none of these were found to be CFC.
In addition to evaluating CRU, we also measured the frequency, and hence total number of CFC and LTC-IC present in the starting light density population, the CD34+CD38+ cells, the CD34+CD38lo cells, and in the cell populations generated from CD34+CD38lo cells in the 5- to 8-day cultures. As previously demonstrated (31), LTC-IC were detectable in both the CD34+CD38lo and CD34+CD38+ fractions of CB (Table 3). In the cultured population, the total number of cells increased 78 ± 33-fold, the number of CFC 98-fold and the number of LTC-IC 4-fold (Table 3).
Comparison of the Cellular Output of Different Sources of Human CRU.
Table 4 compares the average output of different types of human hematopoietic cells generated in NOD/SCID mice, per injected CRU, for each type of CB population transplanted—i.e., light-density cells, CD34+CD38+ cells, CD34+CD38lo cells, and the cells produced in 5- to 8 day-old cultures of CD34+CD38lo cells. In all cases, the predominant types of progeny present 6–8 weeks posttransplant were human pre-B (CD34−CD19+) cells (≈106–107 per mouse per injected CRU). However, the output of very primitive human myeloid cells—i.e., LTC-IC and CFU-GEMM, was substantial (≈50–1,300 per mouse per injected CRU), with intermediate numbers of progeny indicative of differentiation along the erythroid, megakaryocyte, and granulopoietic lineages (103 to 5 × 105 per mouse per injected CRU). These values were similar for the CD34+CD38+ and CD34+CD38− CRU in fresh CB but were slightly lower for the culture-derived CRU.
Table 4.
Comparison of the numbers and types of human progeny present after 6–8 weeks in NOD/SCID recipients of various subsets of fresh or cultured human CB cells expressed per injected CRU
| Endpoint | Light-Density | CD34+CD38+ | CD34+CD38− | Cultured Cells |
|---|---|---|---|---|
| CD34−CD19+ | 0.9 × 107 | 0.8 × 107 | 1.0 × 107 | 0.1 × 107 |
| CD34+ | 2.2 × 106 | 2.5 × 106 | 2.2 × 106 | 0.4 × 106 |
| CD41+ | 4.0 × 105 | 2.0 × 105 | 5.0 × 105 | 2.0 × 105 |
| BFU-E | 2,800 | 7,000 | 3,500 | 1,300 |
| CFU-GM | 27,000 | 28,000 | 28,000 | 9,000 |
| CFU-GEMM | 150 | 130 | 520 | 180 |
| LTC-IC | 200 | 1,300 | 240 | 50 |
DISCUSSION
In this report we describe a quantitative in vivo assay for transplantable normal human cells with lympho-myeloid differentiation potential. Quantitation of these cells is achieved by limiting dilution analysis of the frequency of cells that are individually able to regenerate detectable numbers of both lymphoid (CD34−CD19+) and myeloid (CD34+ erythroid and/or granulopoietic progenitors) within the marrow of immunodeficient (NOD/SCID) mice transplanted 6–8 weeks previously. The mice are pretreated with a close to lethal dose of radiation, sufficient to provide a potent stimulus for i.v. transplanted human stem cells to proliferate and differentiate, but insufficient to kill more than a small proportion of the mice even in the absence of any protection provided by the injected cells (19). Strong evidence that this assay detects single human cells with lymphoid and multilineage myeloid potential was provided by the demonstration that both human pre-B cells and human CFU-GM were usually seen in individual mice (and in almost half of these human BFU-E were also demonstrable), even when unseparated human CB cells were injected at doses that were insufficient to engraft more than two thirds of the recipients. This assay thus successfully incorporates the same principles as the murine CRU assay (1) and we propose the same term (CRU) be used for the human cells it detects.
The average frequency of human CB CRU measured using this assay is 1 per 6 × 105 light density cells or 5 CRU per ml of CB. These values are much lower than the numbers of cells detectable as LTC-IC; however, both were found predominantly, although not exclusively, in the CD34+CD38− subpopulation. The presence of CD34+CD38+ as well as CD34+CD38− cells in adult human marrow that can engraft fetal sheep has recently been reported (34) as has the ability of NOD/SCID mice to be engrafted with CD34+CD38− human CB cells (39). Notable differences between CD38+ and CD38− subpopulations of CD34+ cells have been demonstrated even for functionally similar cells (30, 31, 40, 41), including those with in vivo repopulating activity (34). In the present study, no difference was seen in the types or numbers of 6- to 8-week progeny generated in NOD/SCID recipients of human CD34+CD38+ and CD34+CD38− CB CRU in contrast to the culture-derived CB CRU which produced the same spectrum of progeny types but at somewhat reduced levels. Recent studies have indicated that NOD/SCID mice engrafted with light-density human CB cells can also regenerate progeny CRU (20). It will, therefore, be of interest in future work to determine whether a greater self-renewal capacity in the NOD/SCID system is associated with a lack of CD38 expression by the original CD34+ hematopoietic cells transplanted, as suggested by studies in the sheep model (34). In addition, the NOD/SCID recipient could offer other opportunities to investigate potential molecular determinants of human totipotent stem cell self-renewal (42, 43).
Human CB has recently attracted attention as a source of hematopoietic stem cells both for transplantation and gene therapy applications. However, concern that a single CB collection may not be sufficient to guarantee engraftment of adult allogeneic recipients has also stimulated considerable interest in developing methods for expanding CB stem cell numbers in vitro. Similarly, gene transfer using retroviral vectors requires that the target stem cells be proliferating under conditions where stem cell functions are retained. In related studies of the responses of CD34+CD38− cells isolated from human marrow to various cytokine combinations, we have found that LTC-IC function can be maintained or lost according to the relative or absolute concentrations of FL, SF, and IL-3 to which the cells are exposed, without significant effects on their viability or mitotic activation (44). As a first application of the CRU assay, we therefore asked whether conditions that expand the LTC-IC population from the CD34+CD38− subset of cells in adult human marrow 20- to 30-fold (21, 45) would similarly expand the CRU population in cultures of CD34+CD38− CB cells. The results show that, in spite of the large increases in total cells and CFC (80- and 100-fold, respectively) anticipated from earlier studies (35), LTC-IC and CRU numbers were increased only 4-fold and 2-fold, respectively. Similar results for CB LTC-IC expansion under these conditions have recently been reported by others (46). It thus appears that CD34+CD38− CB and adult marrow cells may differ in their cytokine requirements for promoting self-renewal divisions. Subsequent multifactorial design experiments have confirmed this (47). Nevertheless, the fact that a net increase in CRU numbers can be obtained under conditions that yield only 4-fold expansions of LTC-IC is encouraging and supports the concept that the modified LTC-IC assay used in the present studies (48) is highly predictive of changes that may occur in CRU numbers. Thus, even though the cells identified by these two assays may not necessarily represent identical cell populations, it can be anticipated that conditions able to more effectively stimulate CB LTC-IC expansion may also stimulate greater increases in CB CRU.
Acknowledgments
We thank Jessyca Maltman and Maya Sinclaire for technical assistance, Gayle Thornbury for FACS operation, Bernadine Fox for help in preparing the manuscript, Dr. Peter Lansdorp (Terry Fox Laboratory), and StemCell, Sandoz (now Novartis), Immunex, Cangene, and Amgen for generous gifts of reagents. Grant support from the National Institutes of Health (HL 55435), Sandoz International (Novartis), the E. Shrödinger Foundation (No. J1034-MED to A.P.), and the National Cancer Institute of Canada with funds from the Terry Fox Run is also gratefully acknowledged. E.C. held an National Cancer Institute of Canada Physician-Scientist Fellowship and C. Eaves is an National Cancer Institute of Canada Terry Fox Cancer Research Scientist.
ABBREVIATIONS
- NOD/SCID
nonobese diabetic–scid/scid
- CRU
competitive repopulating unit
- LTC-IC
long-term culture-initiating cell
- CFC
colony-forming cell
- CB
cord blood
- SF
Steel factor
- FL
flt3-ligand
- IL
interleukin
- CSF
colony-stimulating factor
- CFU-GM
colony-forming unit-granulocyte, macrophage
- CFU-GEMM
colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte
- BFU-E
burst-forming unit-erythroid
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- PI
propidium iodide
- FACS
fluorescence-activated cell sorter
References
- 1.Szilvassy S J, Humphries R K, Lansdorp P M, Eaves A C, Eaves C J. Proc Natl Acad Sci USA. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser C C, Szilvassy S J, Eaves C J, Humphries R K. Proc Natl Acad Sci USA. 1992;89:1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebel V I, Dragowska W, Eaves C J, Humphries R K, Lansdorp P M. Blood. 1994;83:128–136. [PubMed] [Google Scholar]
- 4.Harrison D E, Jordan C T, Zhong R K, Astle C M. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 5.Trevisan M, Yan X, Iscove N N. Blood. 1996;88:4149–4158. [PubMed] [Google Scholar]
- 6.Berenson R J, Bensinger W I, Hill R S, Andrews R G, Garcia-Lopez J, Kalamasz D F, Still B J, Spitzer G, Buckner C D, Bernstein I D, Thomas E D. Blood. 1991;77:1717–1722. [PubMed] [Google Scholar]
- 7.Shpall E J, Jones R B, Bearman S I, Franklin W A, Archer P G, Curiel T, Bitter M, Claman H N, Stemmer S M, Purdy M, Myers S E, Hami L, Taffs S, Heimfeld S, Hallagan J, Berenson R J. J Clin Oncol. 1994;12:28–36. doi: 10.1200/JCO.1994.12.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Korbling M, Przepiorka D, Huh Y O, Engel H, van Besien K, Giralt S, Andersson B, Kleine H D, Seong D, Deisseroth A B, Andreeff M, Champlin R. Blood. 1995;85:1659–1665. [PubMed] [Google Scholar]
- 9.Dunbar C E, Cottler-Fox M, O’Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M, Leitman S F, Wilson W H, Cowan K, Young N S, Nienhuis A W. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 10.Sutherland H J, Lansdorp P M, Henkelman D H, Eaves A C, Eaves C J. Proc Natl Acad Sci USA. 1990;87:3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ploemacher R E, Van Der Sluijs J P, van Beurden C A J, Baert M R M, Chan P L. Blood. 1991;78:2527–2533. [PubMed] [Google Scholar]
- 12.Lemieux M E, Rebel V I, Lansdorp P M, Eaves C J. Blood. 1995;86:1339–1347. [PubMed] [Google Scholar]
- 13.Miller C L, Rebel V I, Helgason C D, Lansdorp P M, Eaves C J. Blood. 1997;89:1214–1223. [PubMed] [Google Scholar]
- 14.Lemieux M E, Eaves C J. Blood. 1996;88:1639–1648. [PubMed] [Google Scholar]
- 15.Zanjani E D, Flake A W, Rice H, Hedrick M, Tavassoli M. J Clin Invest. 1994;93:1051–1055. doi: 10.1172/JCI117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dick J E. Semin Immunol. 1996;8:197–206. doi: 10.1006/smim.1996.0025. [DOI] [PubMed] [Google Scholar]
- 17.Nolta J A, Dao M A, Wells S, Smogorzewska M, Kohn D B. Proc Natl Acad Sci USA. 1996;93:2414–2419. doi: 10.1073/pnas.93.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pflumio F, Izac B, Katz A, Shultz L D, Vainchenker W, Coulombel L. Blood. 1996;88:3731–3740. [PubMed] [Google Scholar]
- 19.Cashman J D, Lapidot T, Wang J C Y, Doedens M, Shultz L D, Lansdorp P, Dick J E, Eaves C J. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- 20.Cashman, J., Bockhold, K., Hogge, D. E., Eaves, A. C. & Eaves, C. J. (1997) Br. J. Haematol., in press. [DOI] [PubMed]
- 21.Petzer A L, Zandstra P W, Piret J M, Eaves C J. J Exp Med. 1996;183:2551–2558. doi: 10.1084/jem.183.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unkeless J C. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjonnfjord G E, Steen R, Veiby O P, Egeland T. Exp Hematol. 1996;24:875–882. [PubMed] [Google Scholar]
- 24.Hogge D, Fanning S, Bockhold K, Petzer A, Lambie K, Lansdorp P, Eaves A, Eaves C. Br J Haematol. 1997;96:790–800. doi: 10.1046/j.1365-2141.1997.d01-2092.x. [DOI] [PubMed] [Google Scholar]
- 25.Boggs D R. Am J Hematol. 1984;16:277–286. doi: 10.1002/ajh.2830160309. [DOI] [PubMed] [Google Scholar]
- 26.Krause D S, Fackler M J, Civin C I, May W S. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 27.Terstappen L W M M, Huang S, Safford M, Lansdorp P M, Loken M R. Blood. 1991;77:1218–1227. [PubMed] [Google Scholar]
- 28.Rusten L S, Jacobsen S E W, Kaalhus O, Veiby O P, Funderud S, Smeland E B. Blood. 1994;84:1473–1481. [PubMed] [Google Scholar]
- 29.Murray L, Chen B, Galy A, Chen S, Tushinski R, Uchida N, Negrin R, Tricot G, Jagannath S, Vesole D, Barlogie B, Hoffman R, Tsukamoto A. Blood. 1995;85:368–378. [PubMed] [Google Scholar]
- 30.Issaad C, Croisille L, Katz A, Vainchenker W, Coulombel L. Blood. 1993;81:2916–2924. [PubMed] [Google Scholar]
- 31.Hao Q H, Shah A J, Thiemann F T, Smogorzewska E M, Crooks G M. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 32.Hao Q L, Thiemann F T, Petersen D, Smogorzewska E M, Crooks G M. Blood. 1996;88:3306–3313. [PubMed] [Google Scholar]
- 33.Rawlings D J, Quan S, Hao Q, Thiemann F T, Smogorzewska M, Witte O N, Crooks G M. Exp Hematol. 1997;25:66–72. [PubMed] [Google Scholar]
- 34.Civin C I, Almeida-Porada G, Lee M, Olweus J, Terstappen L W M M, Zanjani E D. Blood. 1996;88:4102–4109. [PubMed] [Google Scholar]
- 35.Lansdorp P M, Dragowska W, Mayani H. J Exp Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sui X, Tsuji K, Tanaka R, Tajima S, Muraoka K, Ebihara Y, Ikebuchi K, Yasukawa K, Taga T, Kishimoto T, Nakahata T. Proc Natl Acad Sci USA. 1995;92:2859–2863. doi: 10.1073/pnas.92.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traycoff C M, Kosak S T, Grigsby S, Srour E F. Blood. 1995;85:2059–2068. [PubMed] [Google Scholar]
- 38.DiGiusto D L, Lee R, Moon J, Moss K, O’Toole T, Voytovich A, Webster D, Mule J J. Blood. 1996;87:1261–1271. [PubMed] [Google Scholar]
- 39.Larochelle A, Vormoor J, Hanenberg H, Wang J C Y, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, Williams D A, Dick J E. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 40.Sauvageau G, Lansdorp P M, Eaves C J, Hogge D E, Dragowska W H, Reid D S, Largman C, Lawrence H J, Humphries R K. Proc Natl Acad Sci USA. 1994;91:12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prosper F, Stroncek D, Verfaillie C M. Blood. 1996;88:2033–2042. [PubMed] [Google Scholar]
- 42.Sauvageau G, Thorsteinsdottir U, Eaves C J, Lawrence H J, Largman C, Lansdorp P M, Humphries R K. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- 43.Yonemura Y, Ku H, Hirayama F, Souza L M, Ogawa M. Proc Natl Acad Sci USA. 1996;93:4040–4044. doi: 10.1073/pnas.93.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zandstra P W, Conneally E, Petzer A L, Piret J M, Eaves C J. Proc Natl Acad Sci USA. 1997;94:4698–4703. doi: 10.1073/pnas.94.9.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petzer A L, Hogge D E, Lansdorp P M, Reid D S, Eaves C J. Proc Natl Acad Sci USA. 1996;93:1470–1474. doi: 10.1073/pnas.93.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kogler G, Callejas J, Hakenberg P, Enczmann J, Adams O, Daubener W, Krempe C, Gobel U, Somville T, Wernet P. J Hematother. 1996;5:105–116. doi: 10.1089/scd.1.1996.5.105. [DOI] [PubMed] [Google Scholar]
- 47.Zandstra, P. W., Conneally, E., Piret, J. M. & Eaves, C. J. (1997) Exp. Hematol., in press.
- 48.Hogge D E, Lansdorp P M, Reid D, Gerhard B, Eaves C J. Blood. 1996;88:3765–3773. [PubMed] [Google Scholar]