Abstract
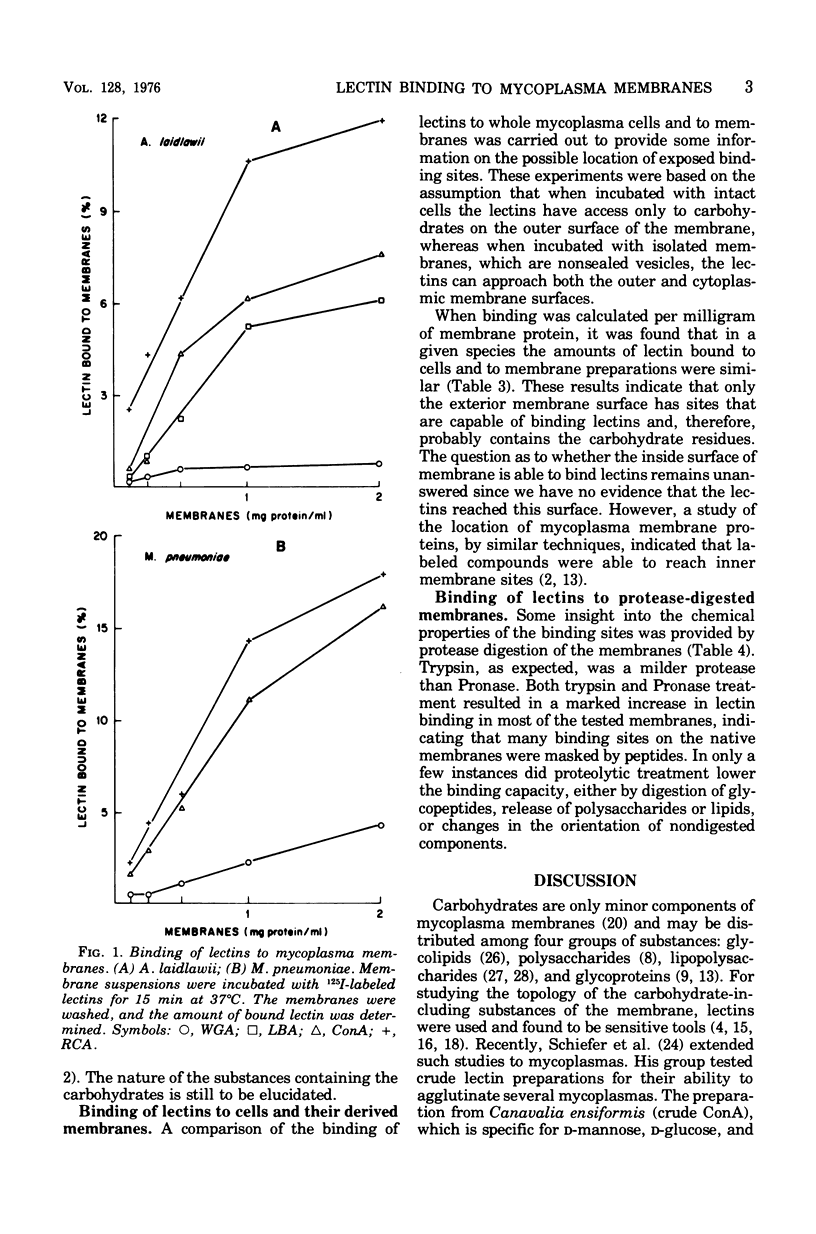
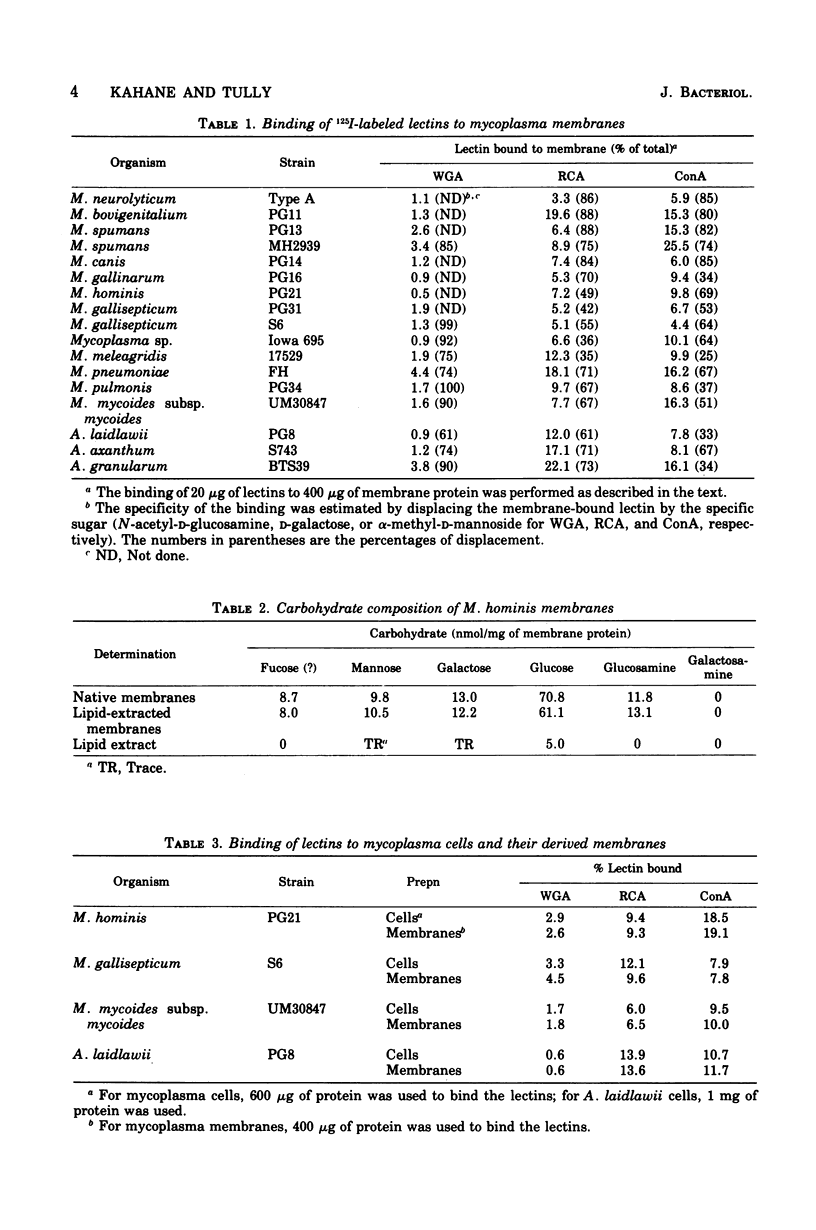
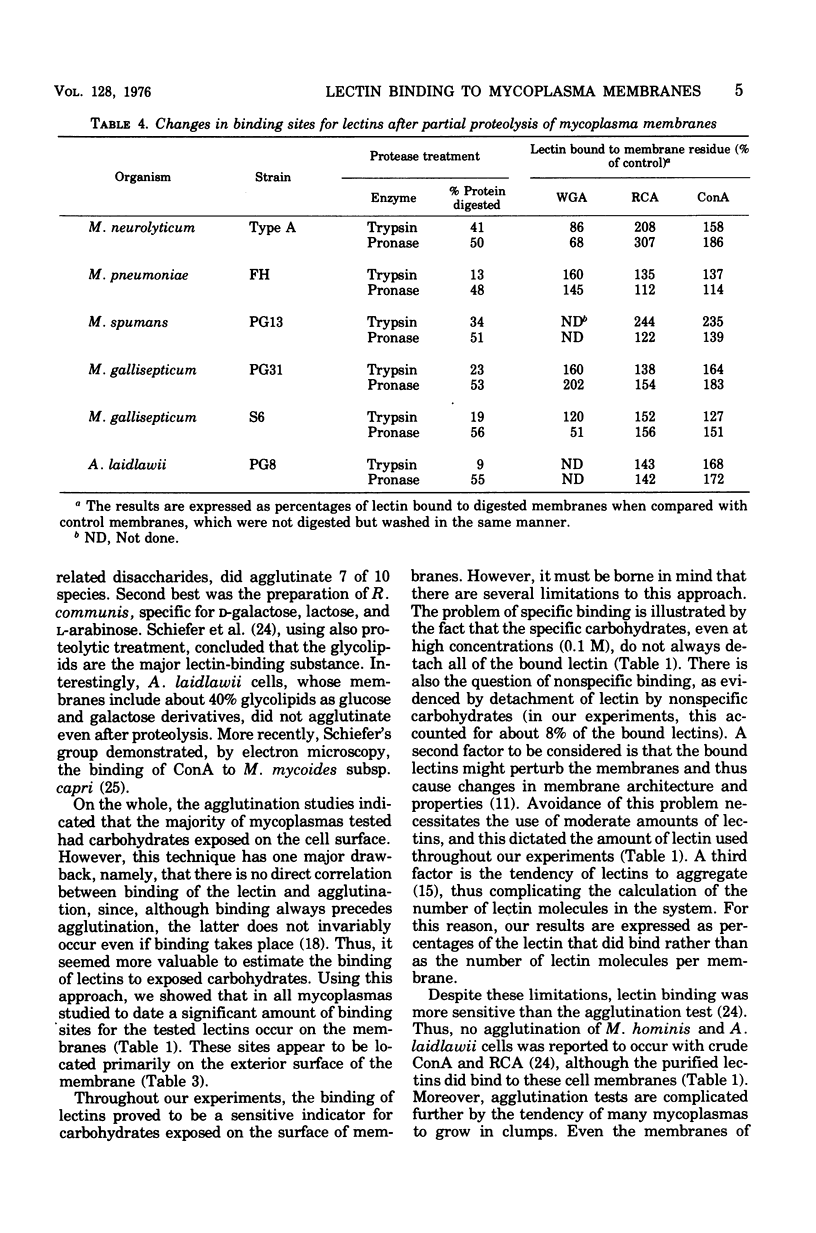
The binding of iodinated wheat germ agglutinin, Ricinus communis agglutinin, and concanavalin A to mycoplasma cells and membranes was examined. All mycoplasmas studied specifically bound concanavalin A or R. communis agglutinin and, to a lesser degree, wheat germ agglutinin. The binding of lectins to whole cells was similar to that recorded for membranes, suggesting that significant binding only occurred on the outer surface of the mycoplasma membrane. Proteolysis of the membrane almost always increased the capacity to bind lectins, which indicates that additional carbohydrate groups on the mycoplasma membrane are masked by a protein layer or protein complexes on the membrane. The observation that carbohydrates are apparently exposed on the surface of mycoplasma membranes should stimulate more concentrated study on the isolation and chemical characterization of these substances since it is quite likely that they are responsible for a variety of reactions between mycoplasmas and host cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Amar A., Rottem S., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. VI. Composition and disposition of proteins in membranes from aging Mycoplasma hominis cultures. Biochim Biophys Acta. 1976 Mar 5;426(2):258–270. doi: 10.1016/0005-2736(76)90336-9. [DOI] [PubMed] [Google Scholar]
- Beckman B. L., Kenny G. E. Immunochemical analysis of serologically active lipids of Mycoplasma pneumoniae. J Bacteriol. 1968 Oct;96(4):1171–1180. doi: 10.1128/jb.96.4.1171-1180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Brunner H., James W. D., Horswood R. L., Chanock R. M. Measurement of Mycoplasma pneumoniae mycoplasmacidal antibody in human serum. J Immunol. 1972 Jun;108(6):1491–1498. [PubMed] [Google Scholar]
- Clamp J. R. Analysis of glycoproteins. Biochem Soc Symp. 1974;(40):3–16. [PubMed] [Google Scholar]
- Galbraith W., Goldstein I. J. Phytohemagglutinins: A new class of metalloproteins. Isolation, purification, and some properties of the lectin from Phaseolus lunatus. FEBS Lett. 1970 Aug 17;9(4):197–201. doi: 10.1016/0014-5793(70)80354-4. [DOI] [PubMed] [Google Scholar]
- Gilliam J. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. IX. Isolation and characterization of the membrane polyhexosamine. Biochim Biophys Acta. 1972 Aug 9;274(2):353–363. doi: 10.1016/0005-2736(72)90183-6. [DOI] [PubMed] [Google Scholar]
- Goel M. C., Lemcke R. M. Dissociation of Mycoplasma gallisepticum membranes with lithium diiodosalicylate and isolation of glycoprotein. Ann Microbiol (Paris) 1975 Oct-Nov;126(3):299–312. [PubMed] [Google Scholar]
- Howard C. J., Gourlay R. N., Collins J. Serological comparison and haemagglutinating activity of Mycoplasma dispar. J Hyg (Lond) 1974 Dec;73(3):457–466. doi: 10.1017/s0022172400042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H., Nicolson G. L. Lectin binding and perturbation of the outer surface of the cell membrane induces a transmembrane organizational alteration at the inner surface. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2212–2216. doi: 10.1073/pnas.71.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Furthmayr H., Marchesi V. T. Isolation of membrane glycoproteins by affinity chromatography in the presence of detergents. Biochim Biophys Acta. 1976 Mar 19;426(3):464–476. doi: 10.1016/0005-2736(76)90391-6. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Immunological analysis of Mycoplasma membranes. J Bacteriol. 1969 Oct;100(1):187–194. doi: 10.1128/jb.100.1.187-194.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Lotan R., Sharon N., Mirelman D. Interaction of wheat-germ agglutinin with bacterial cells and cell-wall polymers. Eur J Biochem. 1975 Jun 16;55(1):257–262. doi: 10.1111/j.1432-1033.1975.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Maruyama H. B. Agglutination of bacterial spheroplast. 3. Relationship of labelled concanavalin A binding to the agglutinability. J Biochem. 1974 Jan;75(1):165–170. doi: 10.1093/oxfordjournals.jbchem.a130370. [DOI] [PubMed] [Google Scholar]
- Plackett P., Marmion B. P., Shaw E. J., Lemcke R. M. Immunochemical analysis of Mycoplasma pneumoniae. 3. Separation and chemical identification of serologically active lipids. Aust J Exp Biol Med Sci. 1969 Apr;47(2):171–195. doi: 10.1038/icb.1969.19. [DOI] [PubMed] [Google Scholar]
- Razin S., Prescott B., Caldes G., James W. D., Chanock R. M. Role of Glycolipids and Phosphatidylglycerol in the Serological Activity of Mycoplasma pneumoniae. Infect Immun. 1970 Apr;1(4):408–416. doi: 10.1128/iai.1.4.408-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Tully J. G. Cholesterol requirement of mycoplasmas. J Bacteriol. 1970 May;102(2):306–310. doi: 10.1128/jb.102.2.306-310.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Membrane lipids of Mycoplasma hominis. J Bacteriol. 1973 Feb;113(2):565–571. doi: 10.1128/jb.113.2.565-571.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer H. G., Gerhardt U., Brunner H., Krüpe M. Studies with lectins on the surface carbohydrate structures of mycoplasma membranes. J Bacteriol. 1974 Oct;120(1):81–88. doi: 10.1128/jb.120.1.81-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer H. G., Krauss H., Brunner H., Gerhardt U. Ultrastructural visualization of surface carbohydrate structures on mycoplasma membranes by concanavalin A. J Bacteriol. 1975 Dec;124(3):1598–1600. doi: 10.1128/jb.124.3.1598-1600.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Mayberry W. R. Distribution and composition of lipopolysaccharides from mycoplasmas. J Bacteriol. 1976 Mar;125(3):916–922. doi: 10.1128/jb.125.3.916-922.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Smith P. F., Langworthy T. A., Mayberry W. R. Immunological analysis of glycolipids and lipopolysaccharides derived from various mycoplasmas. Infect Immun. 1974 Dec;10(6):1273–1279. doi: 10.1128/iai.10.6.1273-1279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Kurokawa T., Onozaki K., Ichiki N., Osawa T., Ukita T. Purification of galactose-binding phytoagglutinins and phytotoxin by affinity column chromatography using sepharose. Experientia. 1972 Jan 15;28(1):84–85. doi: 10.1007/BF01928278. [DOI] [PubMed] [Google Scholar]


