Abstract
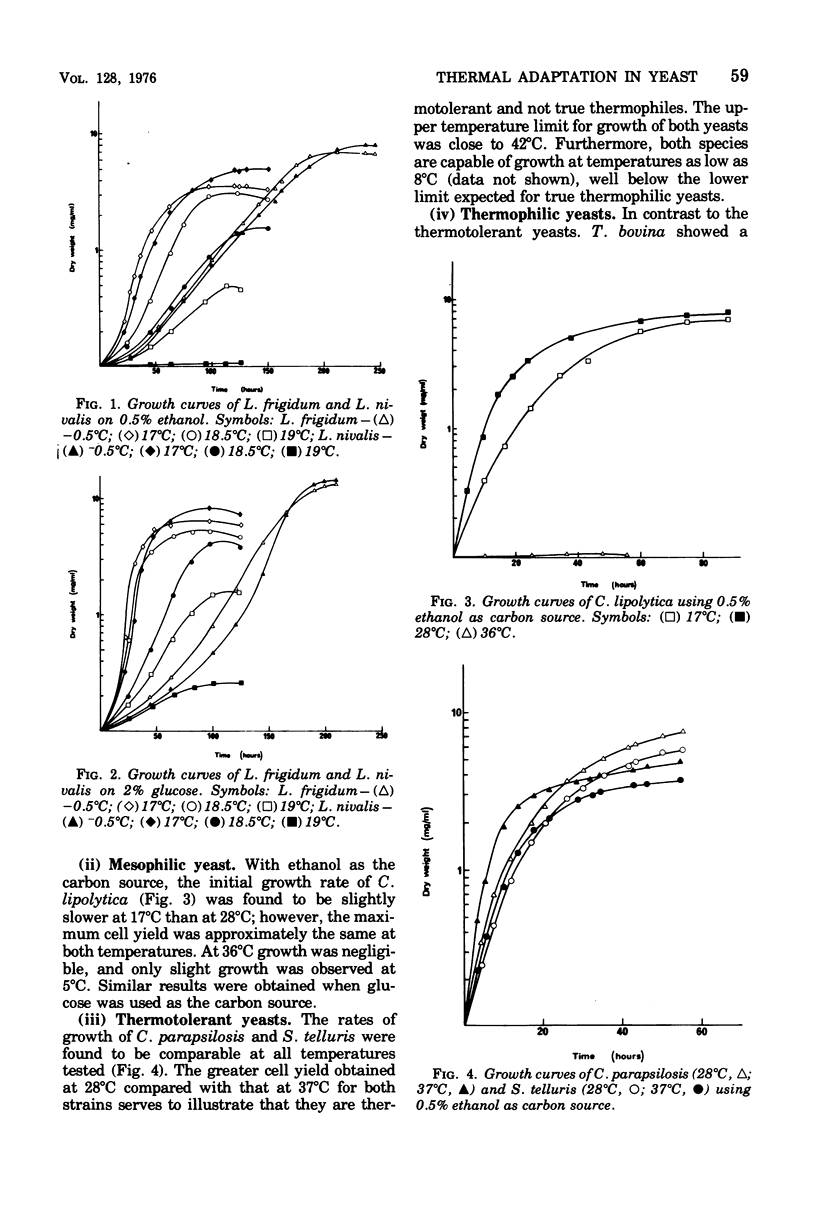
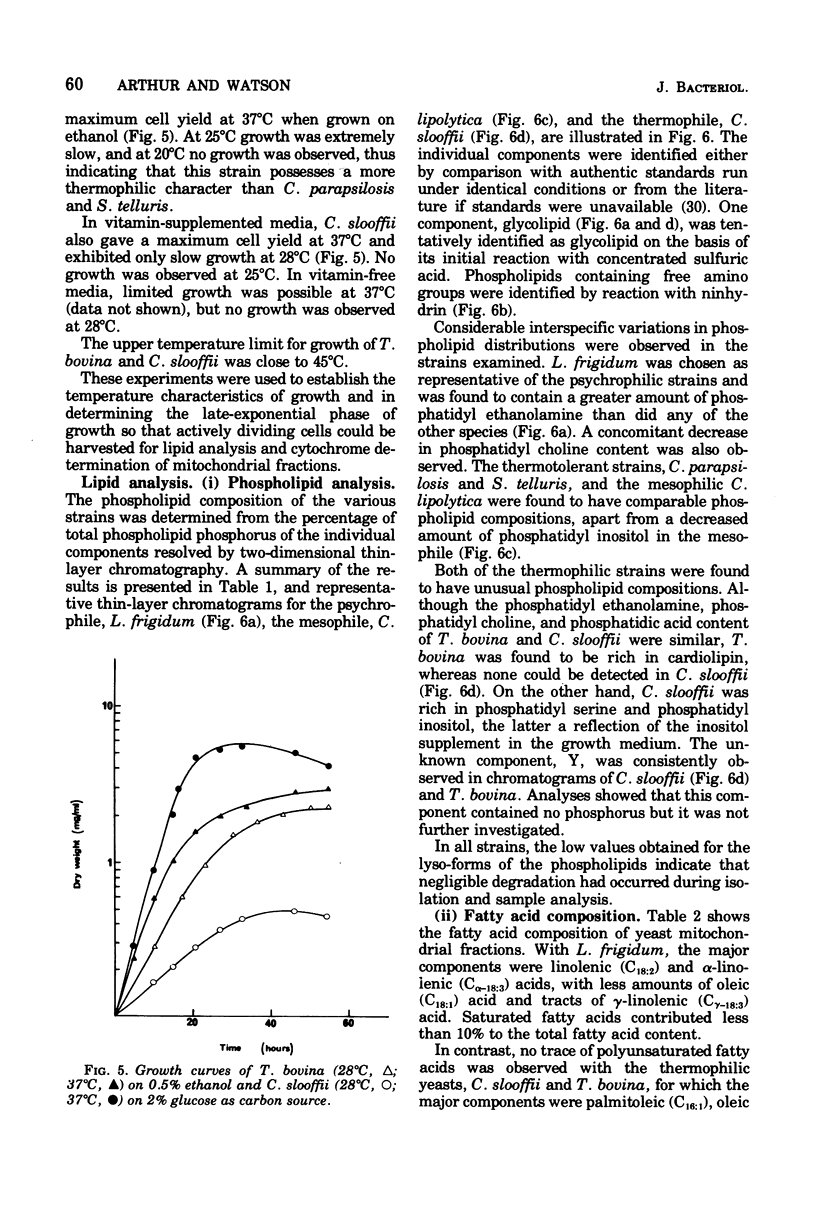
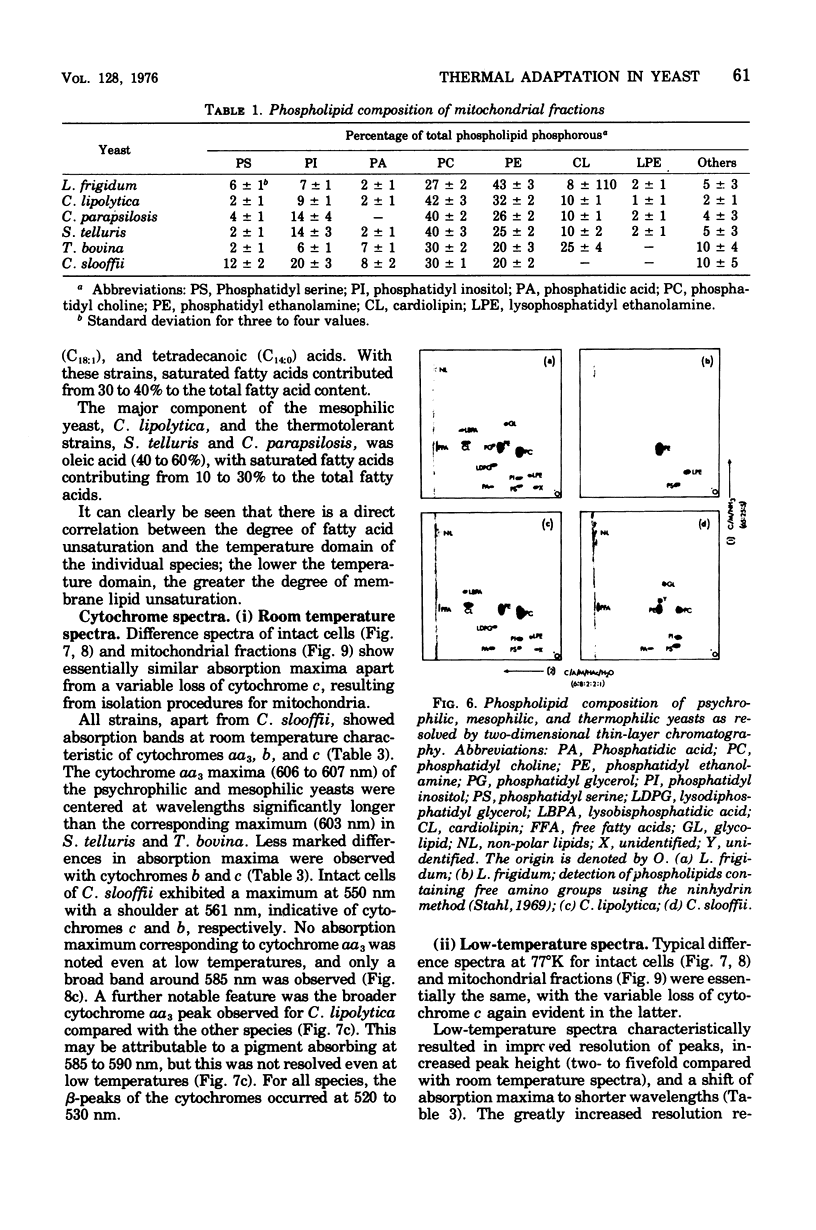
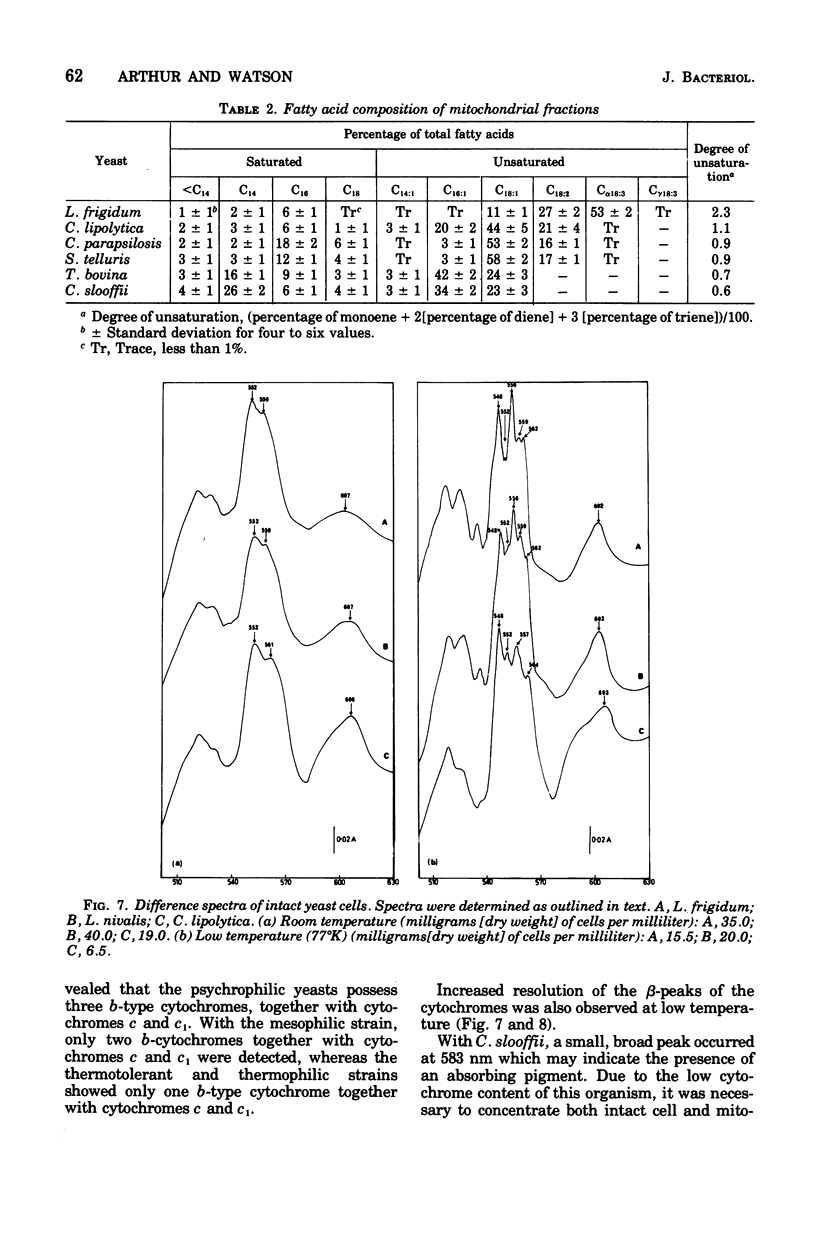
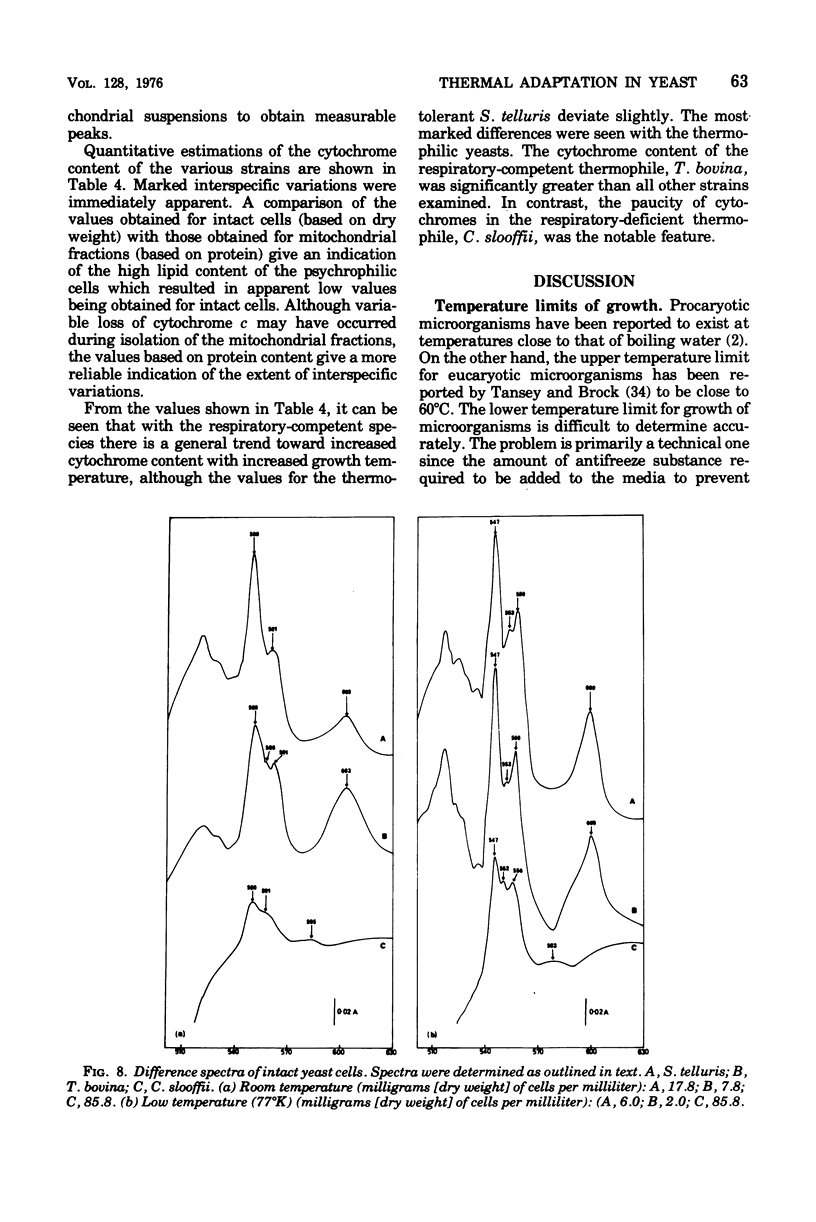
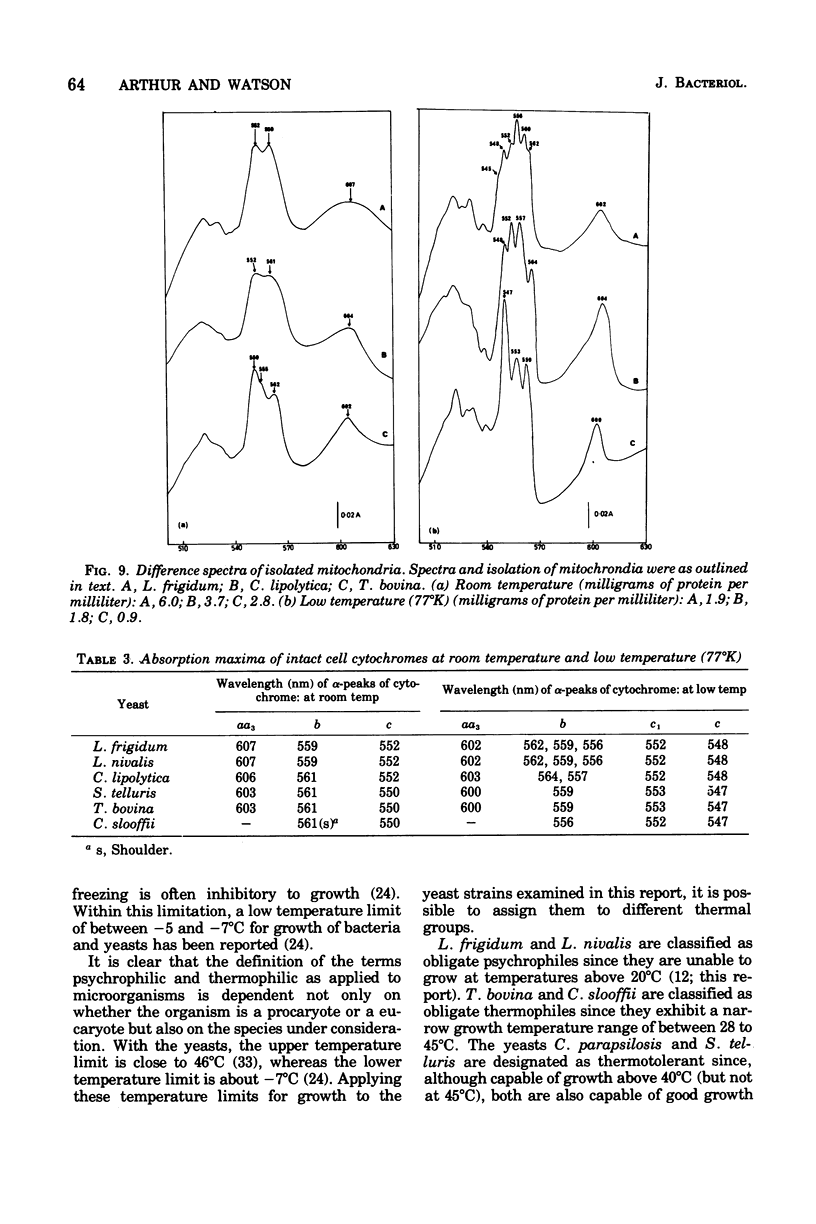
The temperature limits of growth of a number of yeast species were examined, and on this basis the organisms were classified into different thermal categories. The following species were examined: Leucosporidium frigidum and Leucosporidium nivalis, psychrophilic, temperature limits of growth, -2 to 20 degrees C; Canadian lipolytica mesophilic, temperature limits of growth, 5 to 35 degrees Candida parapsilosis and Saccharomyces telluris, thermotolerant, temperature limits of growth, 8 to 42 degrees C; Torulopsis bovina and Candida slooffi, thermophilic, temperature limits of growth, 25 to 45 degrees C and 28 to 45 degrees C, respectively. The membrane lipid and cytochrome composition of mitochrondrial fractions isolated from these yeasts were compared. There was a direct correlation between the growth temperature and the degree of membrane of lipid unsaturation; the lower the temperature, the greater the degree of lipid unsaturation. The membrane lipid composition of the thermophilic yeasts were distinguished by the high percentage (30 to 40%) of saturated fatty acid, as compared with the mesophilic and psychrophilic yeasts. The latter contained approximately 90% unsaturated fatty acid, 55% of which was linolenic acid, C alpha-18:3. Changes in phospholipid composition in relation to temperature were also noted. The respiratory-deficient thermophile, C. slooffi, was characterized by the absence of cardiolipin (sensitivity 0.1 mug of phosphorus) and cytochrome aa3. The absence of conventional mitochondrial structures in this thermophilic microorganism is tentatively suggested although low concentrations of cytochromes b, c, and c1 were detected by low-temperature spectroscopy. On the other hand, the respiratory-competent thermophile, T. bovina, was characterized by a high cardiolipin (25% of the total phospholipid) and cytochrome aa3 content (1 nmol/mg of mitochrondrial protein). Low-temperature spectra showed the presence of one b-type cytochrome in the thermophilic yeasts, two b-type cytochromes in the mesophilic yeasts, and three b-type cytochromes in the psychrophilic yeasts. It was concluded that a knowledge of the properties of the biological membrane is fundamental to an understanding of the ability of a microorganism to grow and reproduce in different temperature environments.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULDER C. J. INDUCTION OF PETITE MUTATION AND INHIBITION OF SYNTHESIS OF RESPIRATORY ENZYMES IN VARIOUS YEASTS. Antonie Van Leeuwenhoek. 1964;30:1–9. doi: 10.1007/BF02046695. [DOI] [PubMed] [Google Scholar]
- Bott T. L., Brock T. D. Bacterial growth rates above 90 degrees C in Yellowstone hot springs. Science. 1969 Jun 20;164(3886):1411–1412. doi: 10.1126/science.164.3886.1411. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Fatty-acid composition of Candida utilis as affected by growth temperature and dissolved-oxygen tension. J Bacteriol. 1969 Aug;99(2):371–378. doi: 10.1128/jb.99.2.371-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Subcellular fractionation by zonal centrifugation of glucose-repressed anaerobically grown Saccharomyces carlsbergensis. Biochem J. 1972 May;127(4):693–703. doi: 10.1042/bj1270693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claisse M. L., Pajot P. F. Presence of cytochrome c1 in cytoplasmic "petite" mutants of Saccharomyces cerevisiae. Eur J Biochem. 1974 Nov 1;49(1):49–59. doi: 10.1111/j.1432-1033.1974.tb03810.x. [DOI] [PubMed] [Google Scholar]
- DeVries A. L., Komatsu S. K., Feeney R. E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J Biol Chem. 1970 Jun 10;245(11):2901–2908. [PubMed] [Google Scholar]
- Di Menna M. E. Three new yeasts from Antarctic soils: Candida nivalis, Candida gelida and Candida frigida spp.n. Antonie Van Leeuwenhoek. 1966;32(1):25–28. doi: 10.1007/BF02097442. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fell J. W., Statzell A. C., Hunter I. L., Phaff H. J. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie Van Leeuwenhoek. 1969;35(4):433–462. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- KREGER-VAN RIJ N. J. The relationship between Saccharomyces tellustris and Candida bovina. Antonie Van Leeuwenhoek. 1958;24(2):137–144. doi: 10.1007/BF02548441. [DOI] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Larkin J. M., Stokes J. L. Growth of psychrophilic microphilic microorganisms at subzero temperatures. Can J Microbiol. 1968 Feb;14(2):97–101. doi: 10.1139/m68-017. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash C. H., Grant D. W., Sinclair N. A. Thermolability of protein synthesis in a cell-free system from the obligately psychrophilic yeast Candida gelida. Can J Microbiol. 1969 Apr;15(4):339–343. doi: 10.1139/m69-063. [DOI] [PubMed] [Google Scholar]
- Nash C. H., Sinclair N. A. Thermal injury and death in an obligately psychrophilic yeast, Candida nivalis. Can J Microbiol. 1968 Jun;14(6):691–697. doi: 10.1139/m68-115. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Characterization of a membrane-bound phospholipid desaturase system of candida lipolytica. Biochim Biophys Acta. 1975 Mar 24;380(3):442–453. doi: 10.1016/0005-2760(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sato N., Onishi T., Chance B. Spectral study of b cytochromes in yeast mitochondria and intact cells. Biochim Biophys Acta. 1972 Sep 20;275(3):288–297. doi: 10.1016/0005-2728(72)90209-5. [DOI] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey M. R., Brock T. D. The upper temperature limit for eukaryotic organisms. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2426–2428. doi: 10.1073/pnas.69.9.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos L. R., Cury R. Thermophilic enteric yeasts. Annu Rev Microbiol. 1971;25:49–74. doi: 10.1146/annurev.mi.25.100171.000405. [DOI] [PubMed] [Google Scholar]
- VAN DER WALT J. P. Three new sporogenous yeasts from soil. Antonie Van Leeuwenhoek. 1957;23(1):23–29. doi: 10.1007/BF02545856. [DOI] [PubMed] [Google Scholar]
- VAN UDEN N., DO SOUSA L. C., FARINHA M. On the intestinal yeast flora of horses, sheep, goats and swine. J Gen Microbiol. 1958 Dec;19(3):435–445. doi: 10.1099/00221287-19-3-435. [DOI] [PubMed] [Google Scholar]
- VAN UDEN N., DO SOUSA L. C. Yeasts from the bovine caecum. J Gen Microbiol. 1957 Apr;16(2):385–395. doi: 10.1099/00221287-16-2-385. [DOI] [PubMed] [Google Scholar]
- Volland C., Chaix P. Etude de la chaîne respiratoire de mitochondries de levures Candida lipolytica cultivées sur hexadécane. Bull Soc Chim Biol (Paris) 1970 Jun;52(5):581–584. [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Wikström M. K. The different cytochrome b components in the respiratory chain of animal mitochondria and their role in electron transport and energy conservation. Biochim Biophys Acta. 1973 Dec 7;301(2):155–193. doi: 10.1016/0304-4173(73)90003-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Epel D. The cytochrome system of sea urchin sperm. Arch Biochem Biophys. 1968 Jul;126(1):83–90. doi: 10.1016/0003-9861(68)90562-6. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]


