Abstract
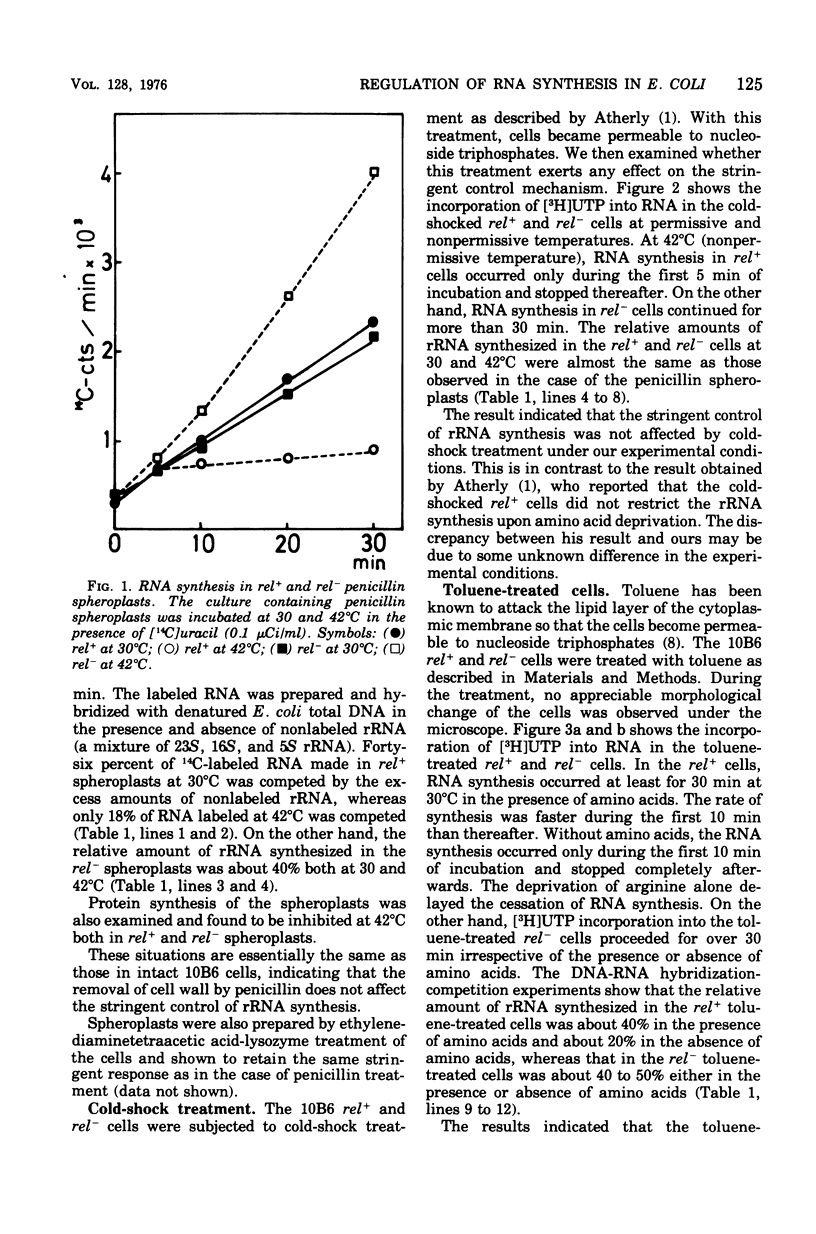
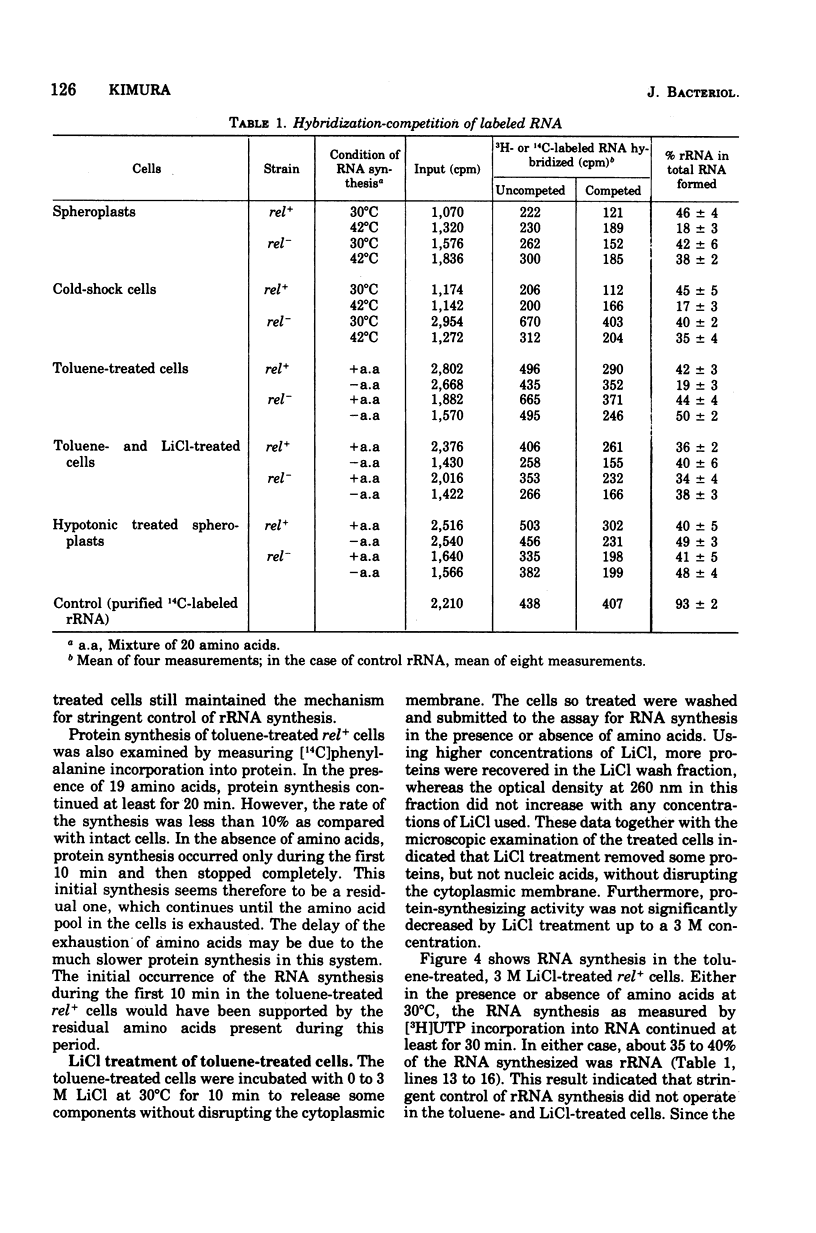
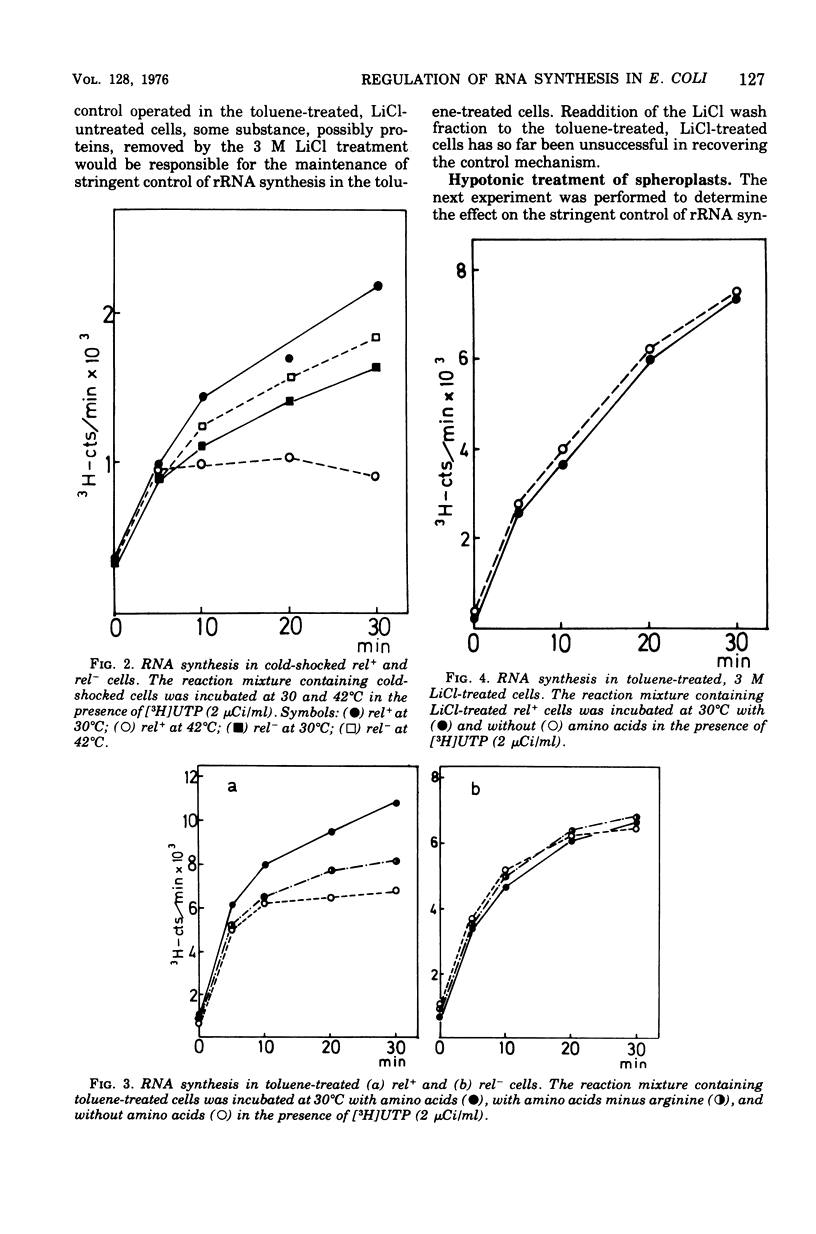
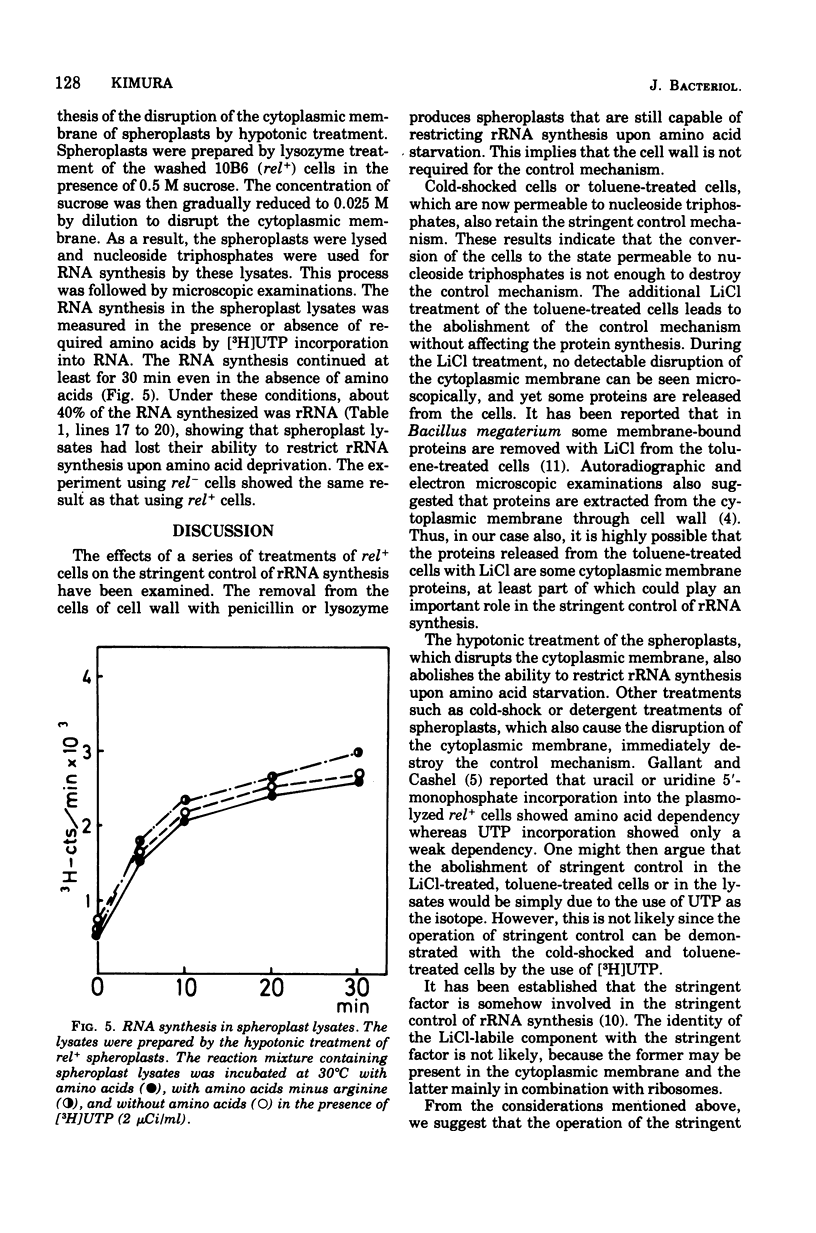
The effects on the stringent control of ribosomal ribonculeic acid synthesis of the removal of cell wall, cold-shock treatment of cells, LiCl treatment of toluene-treated cells, and hypotonic treatment of spheroplasts were examined using Escherichia coli rel+ cells. Neither the removal of cell wall with penicillin or lysozyme nor the cold-shock treatment of the cells had an effect on the stringent control. The control mechanism, however, disappeared after the LiCl treatment of the toluene-treated cells, with the release of some protein component(s), possibly from the cytoplasmic membrane. The hypotonic and other treatments of spheroplasts, which disrupt the cytoplasmic membrane, also led to the abolishment of the control mechanism. These results suggested that the operation of the stringent control of ribosomal ribonucleic acid synthesis requires the cytoplasmic membrane, in which some proteins labile with LiCl treatment are embedded.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G. Ribonucleic acid regulation in premeabilized cells of Escherichia coli capable of ribonucleic acid and protein synthesis. J Bacteriol. 1974 Jun;118(3):1186–1189. doi: 10.1128/jb.118.3.1186-1189.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Stent G. S. Nucleoside triphosphate pools and the regulation of RNA synthesis in E. coli. Proc Natl Acad Sci U S A. 1969 Feb;62(2):475–482. doi: 10.1073/pnas.62.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. R., Koch A. L. RNA synthesis in penicillin spheroplasts of Escherichia coli. Biochim Biophys Acta. 1968 Nov 20;169(1):44–57. doi: 10.1016/0005-2787(68)90007-5. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Gardner-Eckstrom G. L. Passage of a membrane protein through the walls of toluene-treated Bacillus megaterium cells. J Bacteriol. 1975 Aug;123(2):717–723. doi: 10.1128/jb.123.2.717-723.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Cashel M. On the mechanism of amino acid control of ribonucleic acid biosynthesis. J Mol Biol. 1967 May 14;25(3):545–553. doi: 10.1016/0022-2836(67)90205-7. [DOI] [PubMed] [Google Scholar]
- Kimura A., Muto A., Osawa S. Control of stable RNA synthesis in a temperature-sensitive mutant of elongation factor G of Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):203–214. doi: 10.1007/BF00268800. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. L., Radcliffe C. W., Pace N. R. Ribonucleic acid synthesis in bacteria treated with toluene. J Bacteriol. 1971 Aug;107(2):585–588. doi: 10.1128/jb.107.2.585-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taku A., Gardner H. L., Fan D. P. Reconstitution of cell wall synthesis in toluene- and LiCl-treated Bacillus megaterium cells by addition of a soluble protein extract. J Biol Chem. 1975 May 10;250(9):3375–3380. [PubMed] [Google Scholar]


