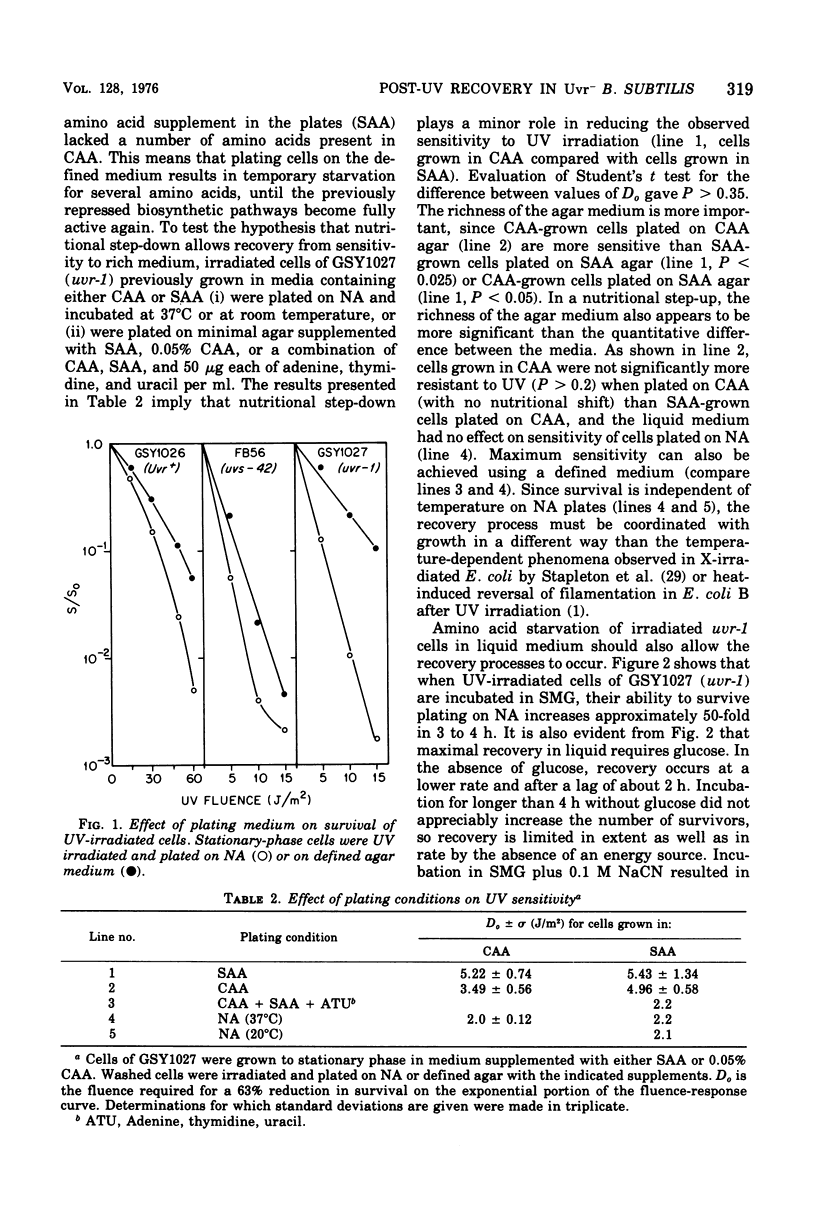
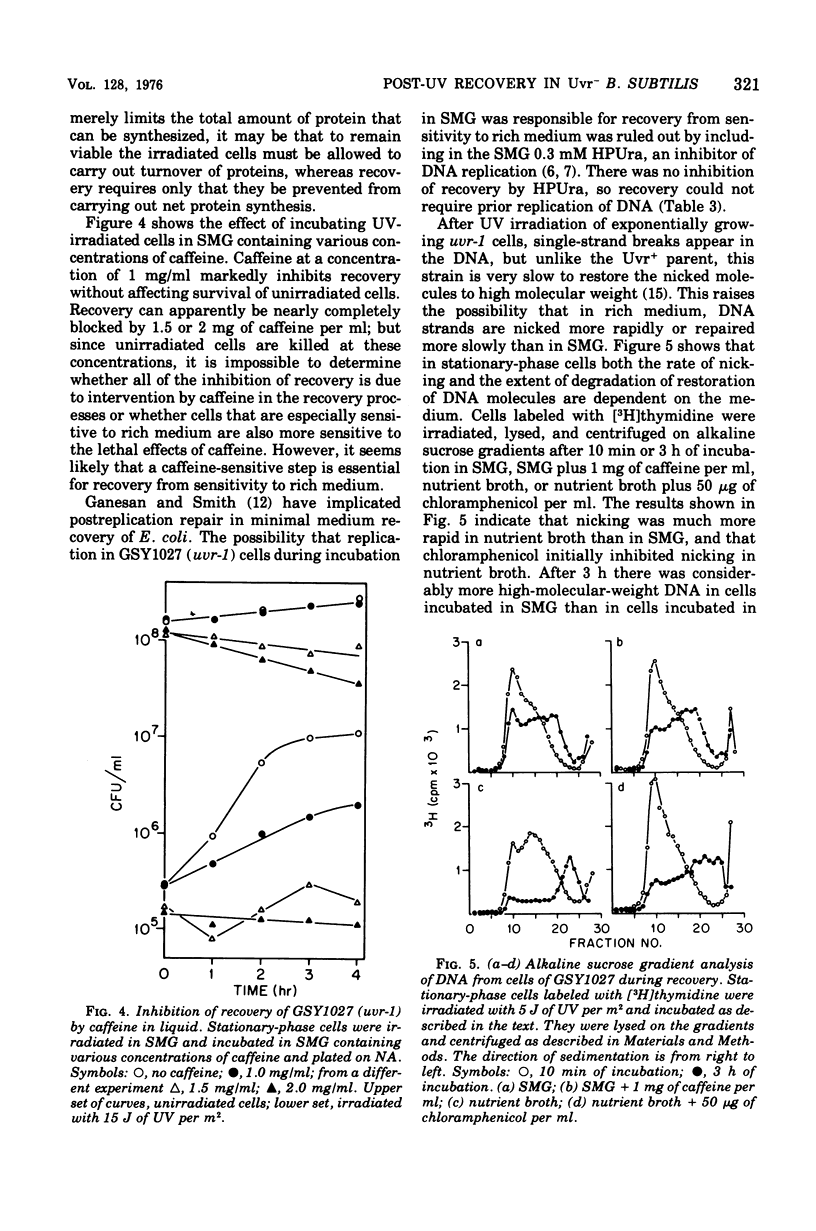
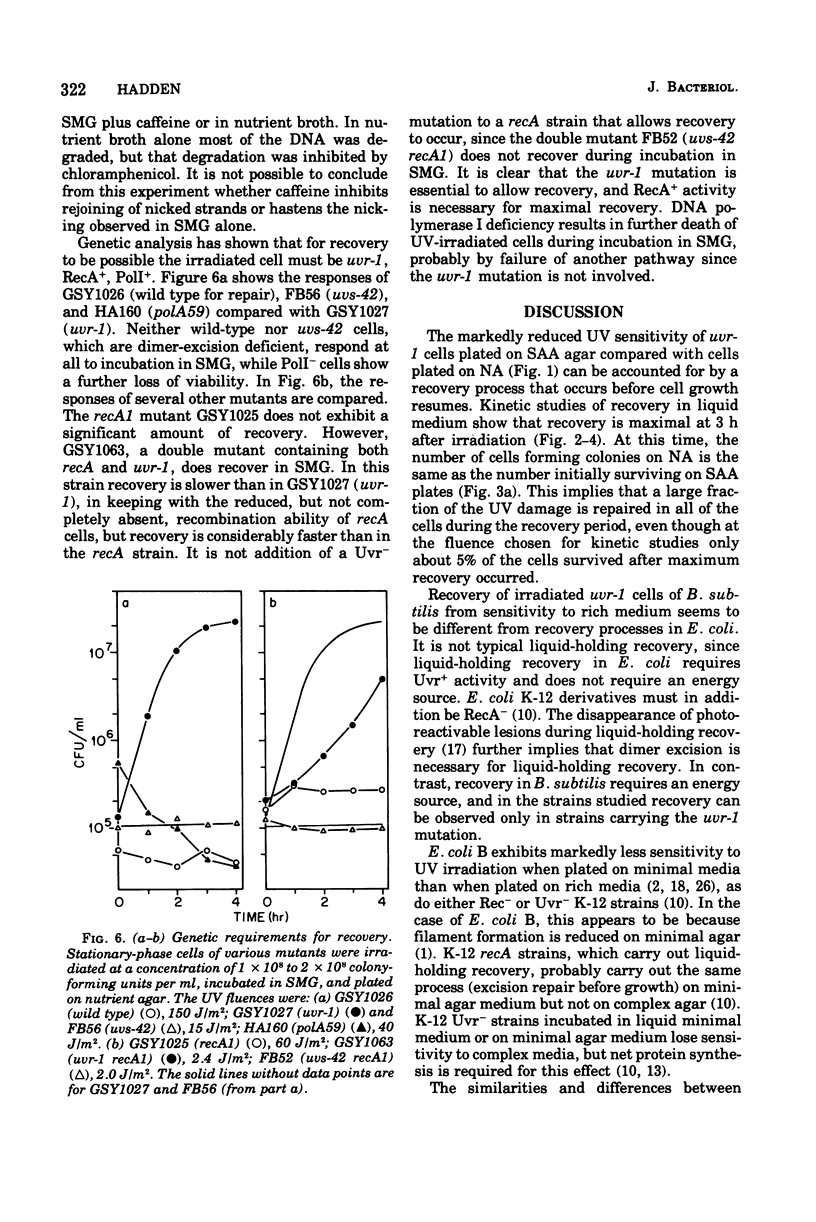
Abstract
A mutant (uvr-1) of Bacillus subtilis that is deficient in excision of ultraviolet (UV)-induced pyrimidine dimers from deoxyribonucleic acid (DNA) shows a marked increase in ability to survive UV irradiation when plated on amino acid-supplemented agar medium compared with its survival ability when plated on nutrient plating medium, the effect is considered to be one of growth-dependent lethality. Irradiated stationary phase uvr-1 cells, incubated in liquid medium lacking amino acids required for growth, recover from this sensitivity to rich medium within 3 to 4 h after irradiation. Recovery is greatly reduced in the absence of glucose oiminated. Exponentially growing cells have a limited ability to recover from sensitivity to rich medium. Growth-dependent lethality can also occur in liquid medium. In nutrient broth the ability of irradiated stationary-phase uvr-1 cells to form colonies on defined agar medium decreases during postirradiation incubation, but treatmeth with chloramphenicol inhibits the loss of colony-forming ability. Recovery from sensitivity to rich media is inhibited by caffeine but not by 6-(p-hydroxyphenylazo)-uracil, and inhibitor of DNA replication. Alkaline sucrose gradient profiles show that conditions allowing recovery also favor maintaining intact DNA strands, whereas DNA strand breakage or degradation is associated with loss of viability. Recovery from sensitivity to rich medium has not been observed in the Ur+ parent or in strains carrying the mutations uvs-42 (another deficiency in dimer excision), recA1, or polA59. A uvr-1 recA1 mutants shows a higher level of recovery than does the recA1 single mutant, but a much lower level than the uvr-1 single mutant. Apparently, both the uvr-1 defect and Rec+ and PoII+ functions are essential for recovery from sensitivity to rich medium. For optimal recovery, growth immediately after irradiation must be delayed. The process requires energy, apparently involves recombination, and probably results in rejoining of DNA strands in which incision but not excision has occurred.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ALPER T., GILLIES N. E. The relationship between growth and survival after irradiation of Escherichia coli strain B and two resistant mutants. J Gen Microbiol. 1960 Feb;22:113–128. doi: 10.1099/00221287-22-1-113. [DOI] [PubMed] [Google Scholar]
- BARNER H. D., COHEN S. S. The relation of growth to the lethal damage induced by ultraviolet irradiation in Escherichia coli. J Bacteriol. 1956 Feb;71(2):149–157. doi: 10.1128/jb.71.2.149-157.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Carreira L. B., Hadden C. T., Silverstein S. J. Evidence suggestive of compartmentalization of deoxyribonucleic acid-synthesizing systems in freeze-treated Bacillus subtilis. J Bacteriol. 1971 Dec;108(3):1250–1256. doi: 10.1128/jb.108.3.1250-1256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Hellerman G., Carreira L. Gene frequency analysis of chromosomal initiation sites in Bacillus subtilis after ultraviolet light or x-ray exposure. J Bacteriol. 1972 Jan;109(1):379–384. doi: 10.1128/jb.109.1.379-384.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark recovery processes in Escherichia coli irradiated with ultraviolet light. I. Effect of rec mutations on liquid holding recovery. J Bacteriol. 1968 Aug;96(2):365–373. doi: 10.1128/jb.96.2.365-373.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark-recovery processes in Escherichia coli irradiated with ultraviolet light. 3. Effect of rec mutations on recovery of excision-deficient mutants of Escherichia coli K-12. J Bacteriol. 1970 May;102(2):404–410. doi: 10.1128/jb.102.2.404-410.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Requirement for protein synthesis in rec-dependent repair of deoxyribonucleic acid in Escherichia coli after ultraviolet or X irradiation. J Bacteriol. 1972 Aug;111(2):575–585. doi: 10.1128/jb.111.2.575-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. The duration of recovery and DNA repair in excision deficient derivatives of Escherichia coli K-12 after ultraviolet irradiation. Mol Gen Genet. 1971;113(4):285–296. doi: 10.1007/BF00272328. [DOI] [PubMed] [Google Scholar]
- HILL R. F., SIMSON E. A study of radiosensitive and radioresistant mutants of Escherichia coli strain B. J Gen Microbiol. 1961 Jan;24:1–14. doi: 10.1099/00221287-24-1-1. [DOI] [PubMed] [Google Scholar]
- Hadden C. T., Billen D. Genetic analysis of repair of ultraviolet damage by competent and noncompetent cells of Bacillus subtilis. J Bacteriol. 1973 Jan;113(1):88–95. doi: 10.1128/jb.113.1.88-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm W. Dark repair of photorepairable UV lesions in Escherichia coli. Mutat Res. 1968 Jul-Aug;6(1):25–35. doi: 10.1016/0027-5107(68)90100-0. [DOI] [PubMed] [Google Scholar]
- Harm W. The role of host-cell repair in liquid-holding recovery of u.v.-irradiated Escherichia coli. Photochem Photobiol. 1966 Sep;5(9):747–760. doi: 10.1111/j.1751-1097.1966.tb05824.x. [DOI] [PubMed] [Google Scholar]
- Hill T., Prakash L., Strauss B. Mutagen stability of alkylation-sensitive mutants of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):47–55. doi: 10.1128/jb.110.1.47-55.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G., Eisenstark H. M., Serra M. C., Polsinelli M. Effect of caffeine on the recombination process of Bacillus subtilis. Mol Gen Genet. 1972;115(1):73–79. doi: 10.1007/BF00272219. [DOI] [PubMed] [Google Scholar]
- Munakata N., Ikeda Y. Inactivation of transforming DNA by ultraviolet irradiation: a study with ultraviot-sensitive mutants of Bacillus subtilis. Mutat Res. 1969 Mar-Apr;7(2):133–139. doi: 10.1016/0027-5107(69)90025-6. [DOI] [PubMed] [Google Scholar]
- PATRICK M. H., HAYNES R. H. DARK RECOVERY PHENOMENA IN YEAST. II. CONDITIONS THAT MODIFY THE RECOVERY PROCESS. Radiat Res. 1964 Dec;23:564–579. [PubMed] [Google Scholar]
- PATRICK M. H., HAYNES R. H., URETZ R. B. DARK RECOVERY PHENOMENA IN YEAST. 1. COMPARATIVE EFFECTS WITH VARIOUS INACTIVATING AGENTS. Radiat Res. 1964 Jan;21:144–163. [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- STAPLETON G. E., BILLEN D., HOLLAENDER A. Recovery of x-irradiated bacteria at suboptimal incubation temperatures. J Cell Physiol. 1953 Apr;41(2):345–357. doi: 10.1002/jcp.1030410211. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Survival, mutation and capacity to repair single-strand DNA breaks after gamma irradiation in different Exr - strains of Escherichia coli. Mol Gen Genet. 1972;119(2):93–102. doi: 10.1007/BF00269129. [DOI] [PubMed] [Google Scholar]
- WEATHERWAX R. S. Reactivation of ultraviolet-irradiated Escherichia coli. J Bacteriol. 1956 Sep;72(3):329–332. doi: 10.1128/jb.72.3.329-332.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., O'SULLIVAN A., SUEOKA N. SEQUENTIAL REPLICATION OF THE BACILLUS SUBTILIS CHROMOSOME. 3. REGULATION OF INITIATION. Proc Natl Acad Sci U S A. 1964 Oct;52:973–980. doi: 10.1073/pnas.52.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]


