Abstract
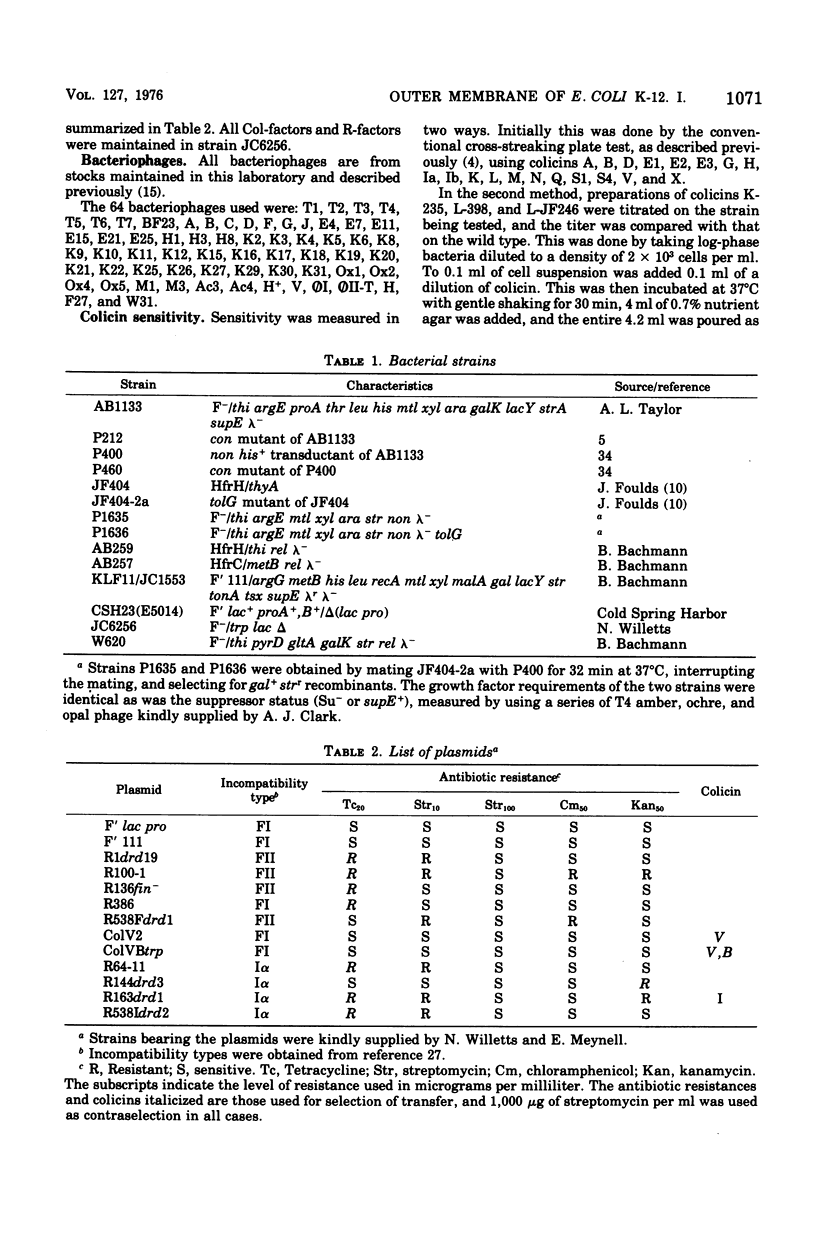
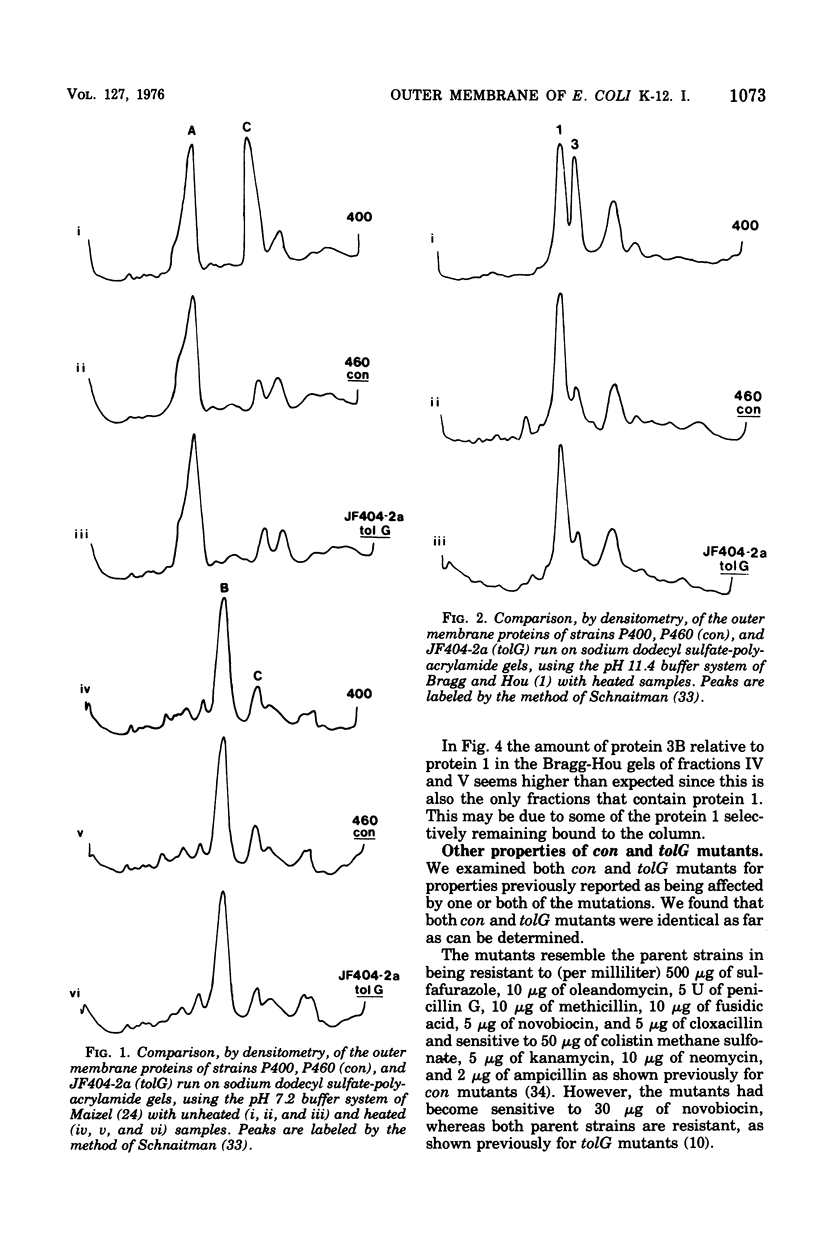
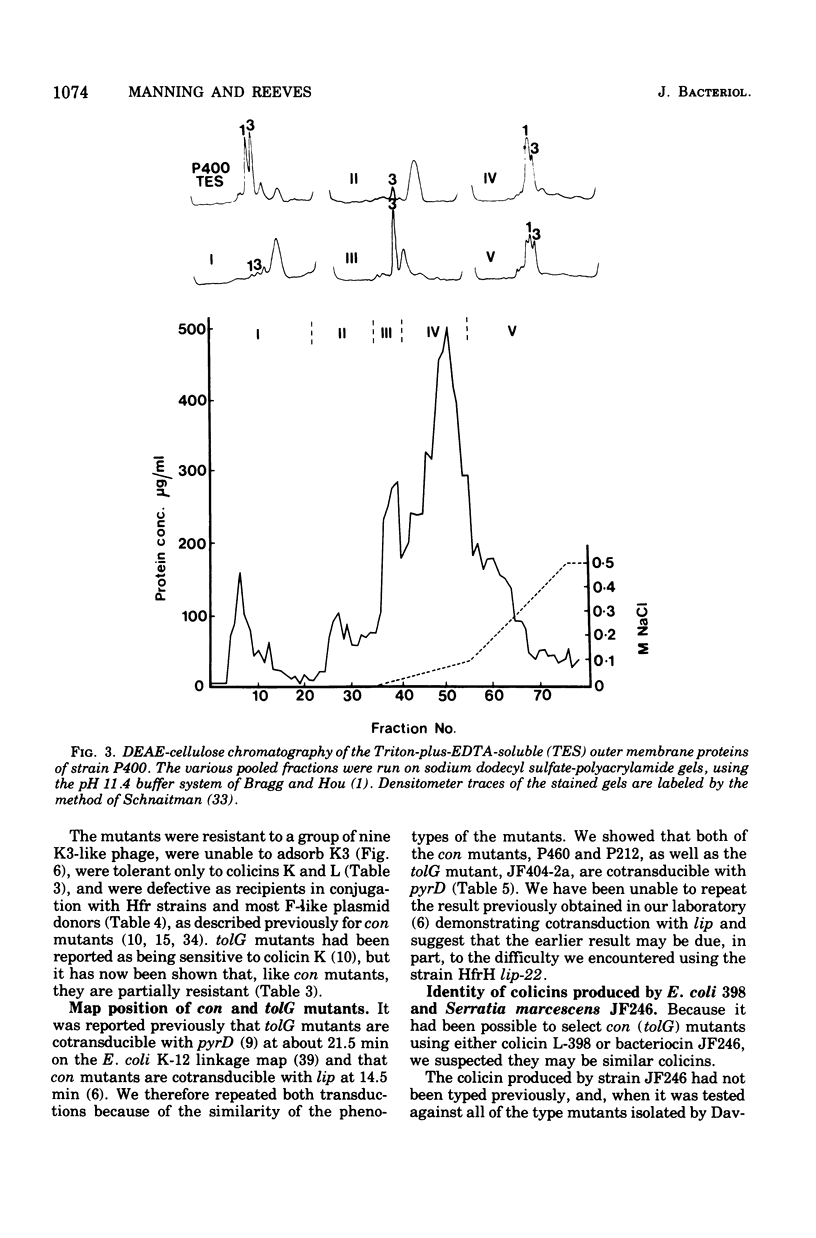
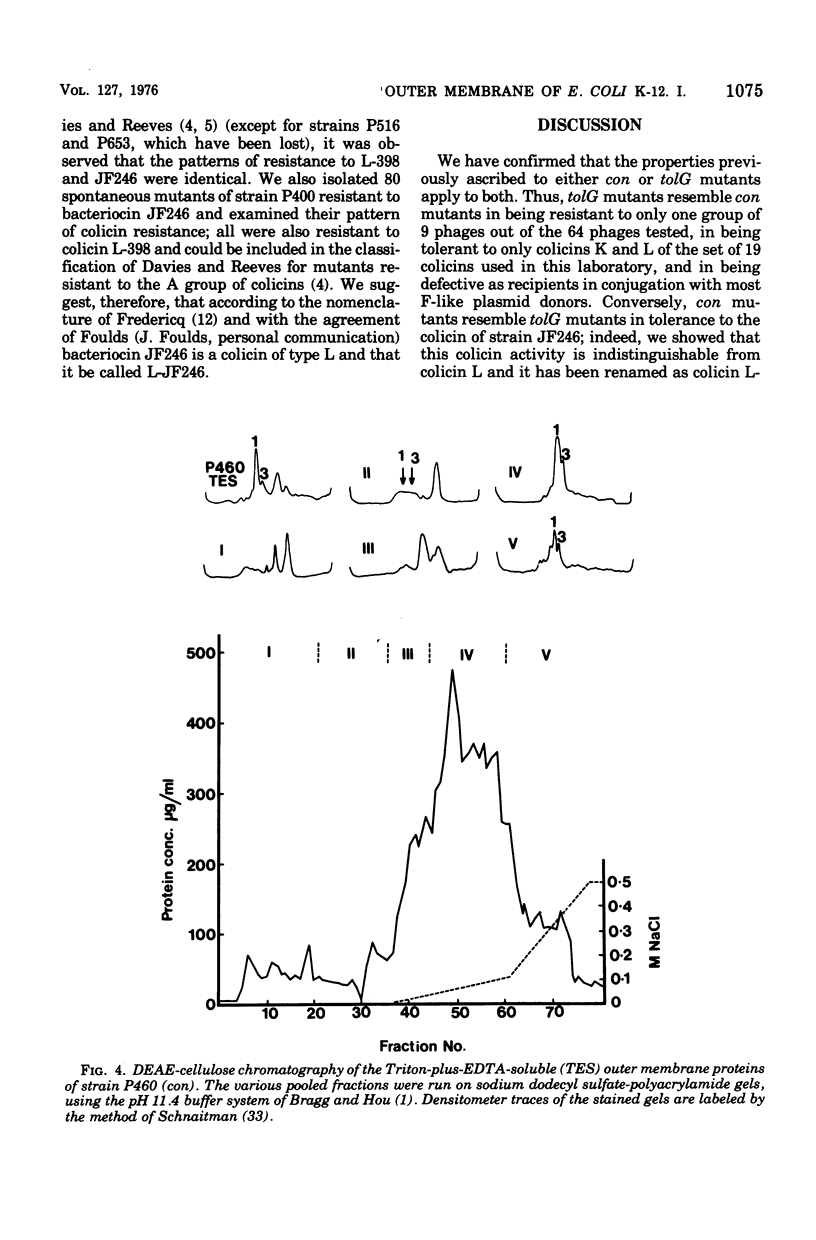
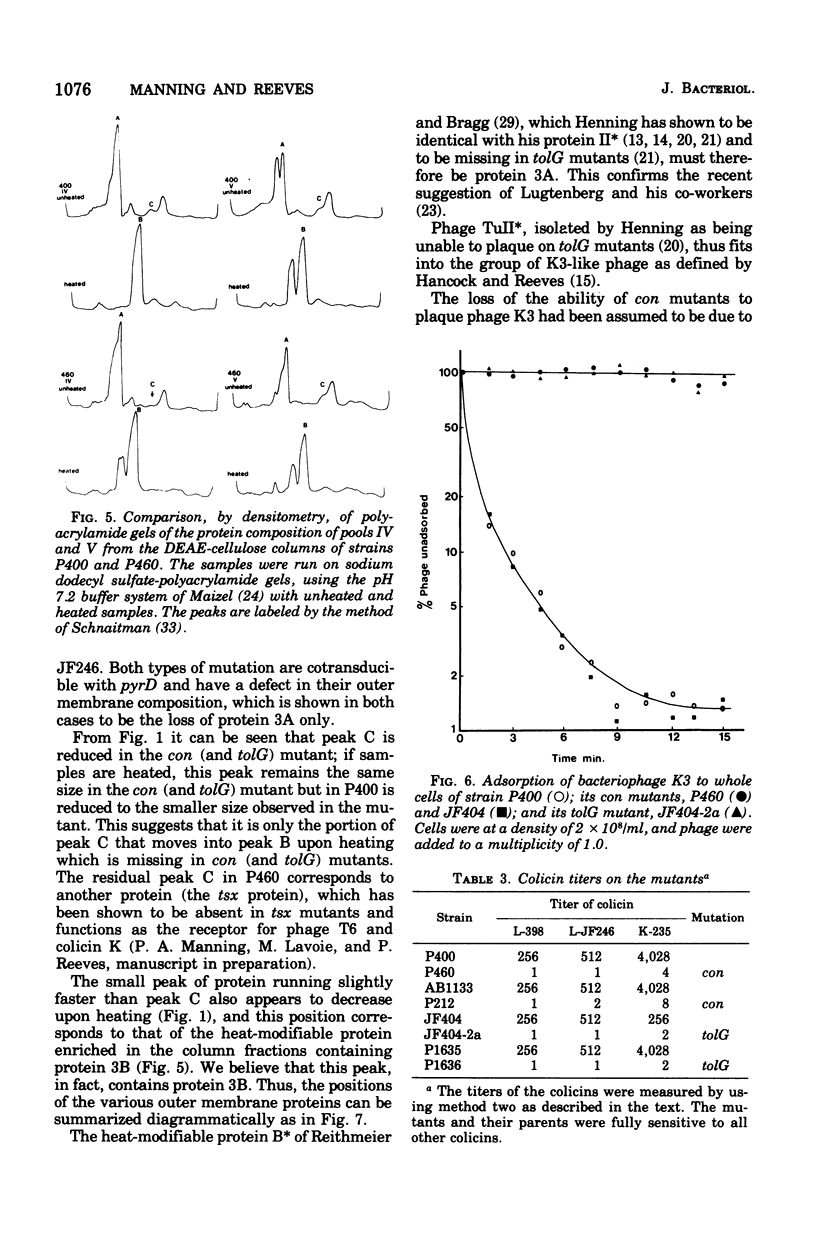
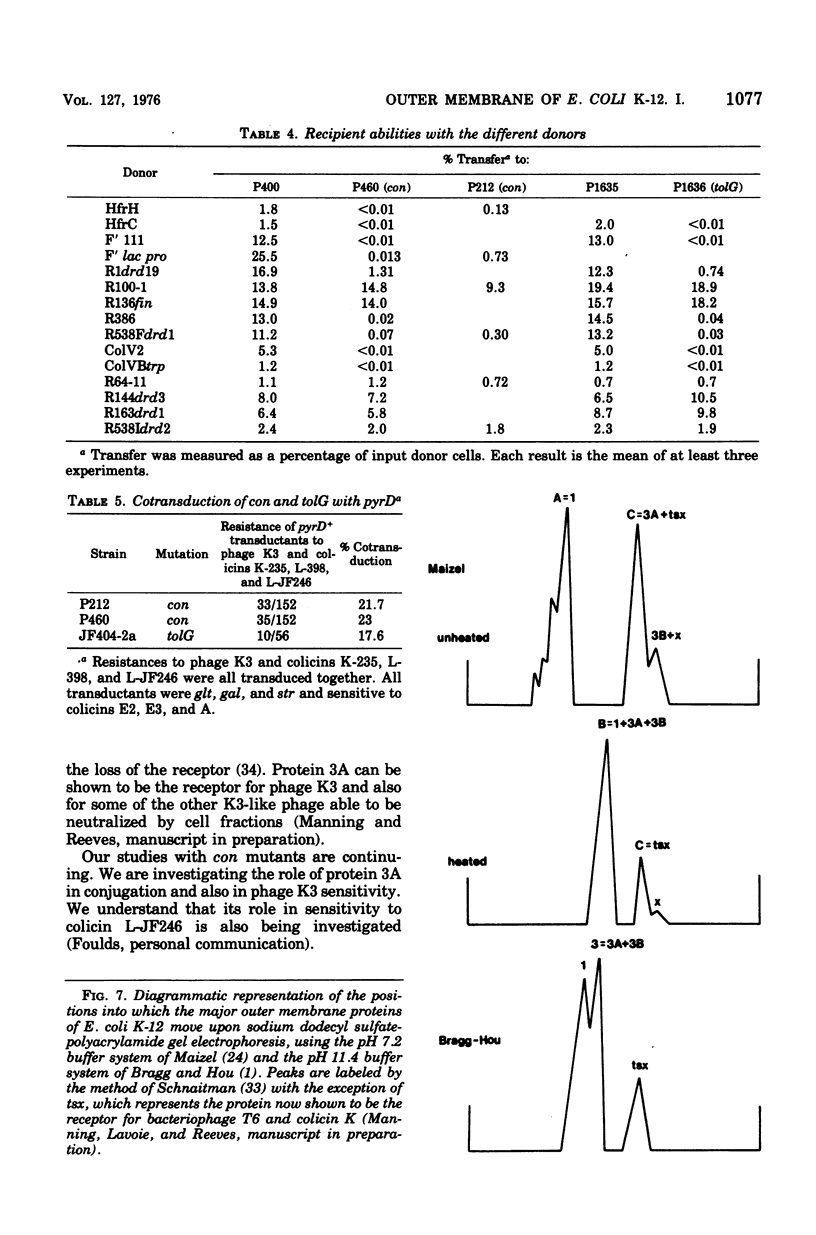
Two classes of mutants, con and tolG, that appeared to be very similar in a number of respects have been shown to be identical and cotransducible with pyrD. By diethylaminoethyl-cellulose chromatography of the outer membranes, we have shown that the mutants are missing only protein 3A and retain protein 3B. Using con mutants, we were thus able to identify protein 3B on the pH 7.2 gel system of Maizel where it runs separately from protein 3A if unheated samples are used. tolG mutants were shown to be identical to con mutants in being conjugation defective with most F-like plasmid donors but not with I-like plasmid donors, and in their resistance pattern to bacteriophages and colicins. During the course of this study, it was observed that the bacteriocin produced by Serratia marcescenc JF246 was identical in its activity spectrum to colicin L-398 and is now considered to be a colicin of type L.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Colicin tolerance and map location of conjugation-deficient mutants. J Bacteriol. 1975 Jul;123(1):372–373. doi: 10.1128/jb.123.1.372-373.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicines et bactériophages. Ann Inst Pasteur (Paris) 1953 Jan;84(1):294–312. [PubMed] [Google Scholar]
- FREDERICQ P. Spectres d'activité differents des souches productrices de colicine E ou K et des bactériophages des groupes II ou III. C R Seances Soc Biol Fil. 1951 Sep;145(17-18):1433–1436. [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Chromosomal location of the tolG locus for tolerance to bacteriocin JF246 in Escherichia coli K-12. J Bacteriol. 1974 Mar;117(3):1354–1355. doi: 10.1128/jb.117.3.1354-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Purification and partial characterization of a bacteriocin from Serratia marcescens. J Bacteriol. 1972 Jun;110(3):1001–1009. doi: 10.1128/jb.110.3.1001-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Henning U. Cell envelope and shape of Escherichia coli K12. Isolation and preliminary characterization of the major ghost-membrane proteins. Eur J Biochem. 1974 Sep 1;47(2):343–352. doi: 10.1111/j.1432-1033.1974.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- HAUDUROY P., PAPAVASSILIOU J. Identification of a new type of colicine (colicine L). Nature. 1962 Aug 18;195:730–732. doi: 10.1038/195730a0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. Maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Apr;122(1):206–214. doi: 10.1128/jb.122.1.206-214.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L. Role of the receptor for bacteriophage lambda in the functioning of the maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Oct;124(1):119–126. doi: 10.1128/jb.124.1.119-126.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Henning U., Hindennach I., Haller I. The major proteins of the Escherichia coli outer cell envelope membrane: evidence for the structural gene of protein II. FEBS Lett. 1976 Jan 1;61(1):46–48. doi: 10.1016/0014-5793(76)80168-8. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Puspurs A., Reeves P. Outer membrane of Escherichia coli K-12: isolation of mutants with altered protein 3A by using host range mutants of bacteriophage K3. J Bacteriol. 1976 Sep;127(3):1080–1084. doi: 10.1128/jb.127.3.1080-1084.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Reeves P. Recipient ability of bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1975 Oct;124(1):576–577. doi: 10.1128/jb.124.1.576-577.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Purification and characterization of heat-modifiable protein from the outer membrane of Escherichia coli. FEBS Lett. 1974 May 1;41(2):195–198. doi: 10.1016/0014-5793(74)81210-x. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Reeves P. Characterization of lethal zygosis associated with conjugation in Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):58–70. doi: 10.1128/jb.113.1.58-70.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Reeves P. Physiology of Escherichia coli K-12 during conjugation: altered recipient cell functions associated with lethal zygosis. J Bacteriol. 1973 Apr;114(1):11–17. doi: 10.1128/jb.114.1.11-17.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


