Abstract
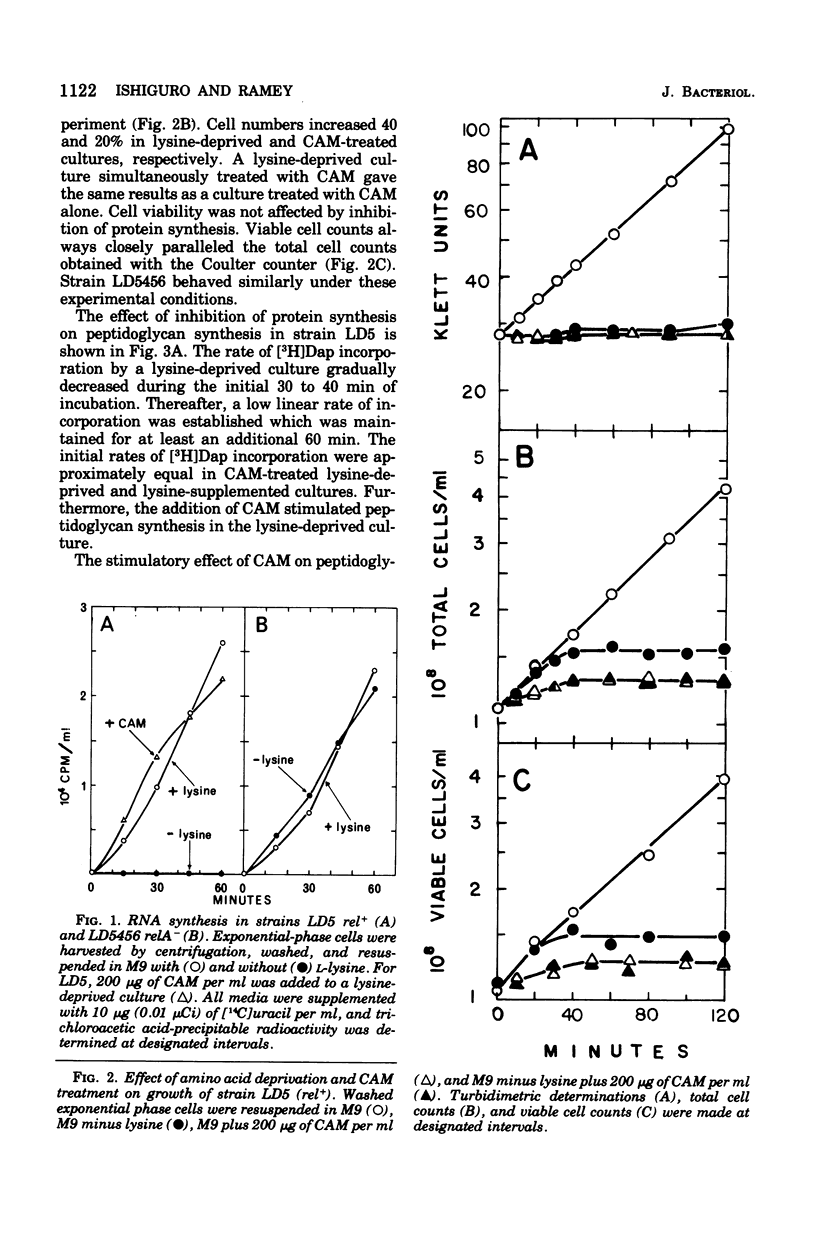
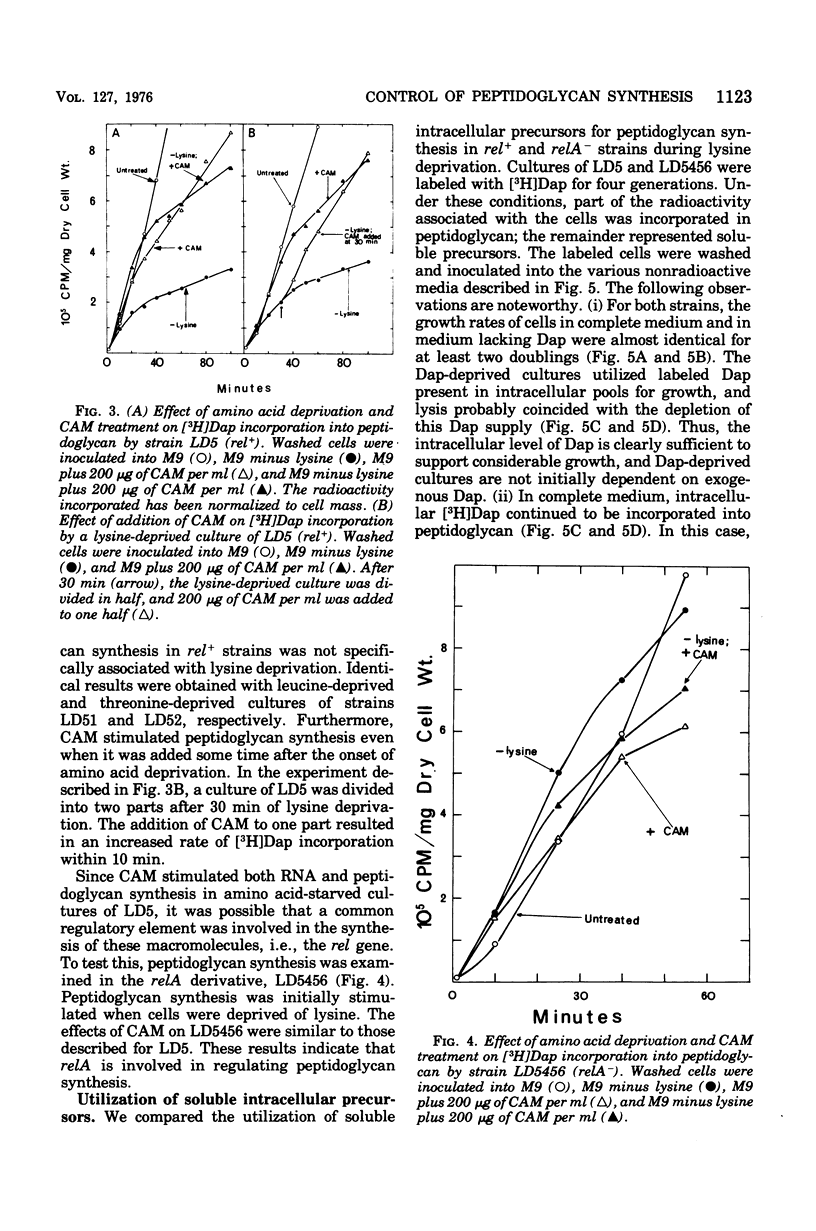
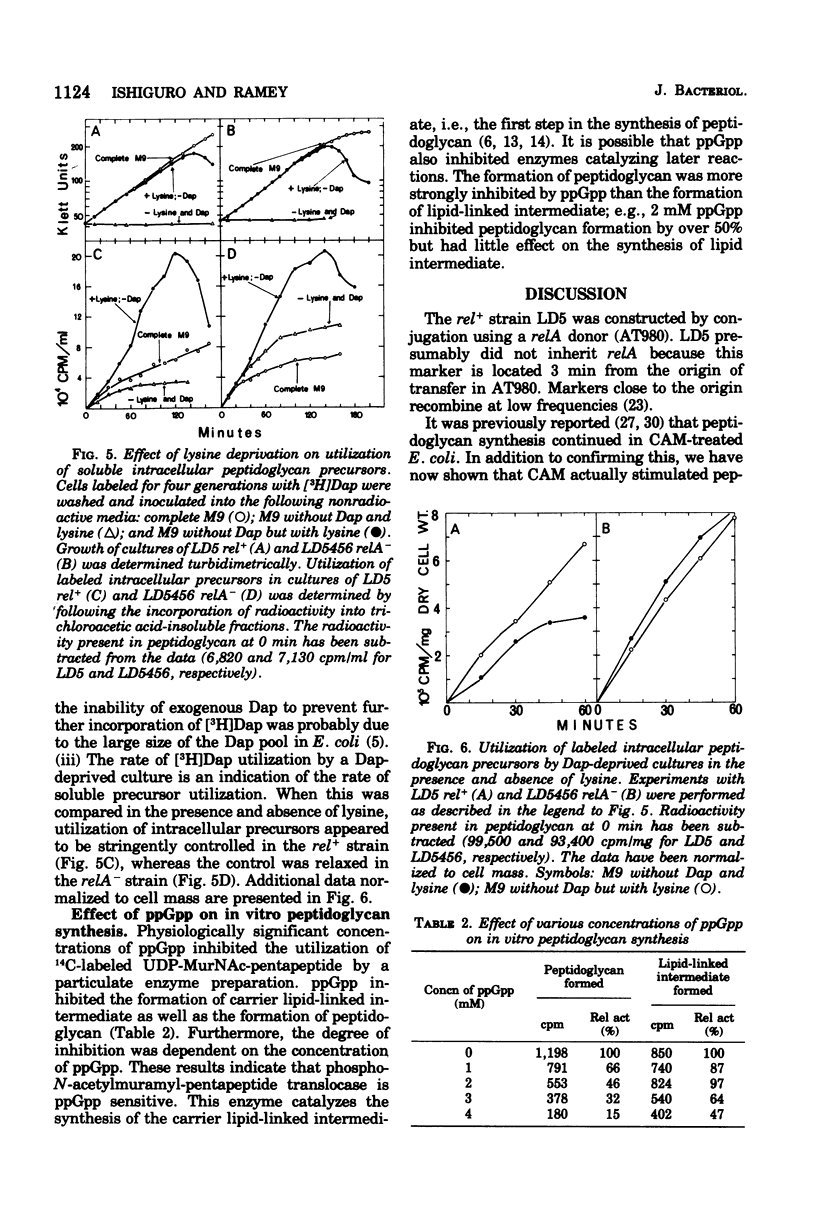
[3H]Diaminopimelic acid (Dap) was incorporated exclusively into peptidoglycan by Escherichia coli strains auxotrophic for both lysine and Dap. The rate of [3H]Dap incorporation by stringent (rel+) strains was significantly decreased when cells were deprived of required amino acids. The addition of chloramphenicol to amino acid-starved rel+ cultured stimulated both peptidoglycan and ribonucleic acid synthesis. In contrast, a relaxed (relA) derivative incorporated [3H]Dap at comparable rates in the presence or absence of required amino acids. Physiologically significant concentrations of guanosine 5'-diphosphate 3'-diphosphate (ppGpp) inhibited the in vitro synthesis of both carrier lipid-linked intermediate and peptidoglycan catalyzed by a particulate enzyme system. The degree of inhibition was dependent on the concentration of ppGpp in the reaction mixture. Thus, the results of in vivo and in vitro studies indicate that peptidoglycan synthesis is stringently controlled in E. coli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I., SPIEGELMAN S. On the nature of the ribonucleic acid synthesized in the presence of chloramphenicol. Biochim Biophys Acta. 1961 Oct 14;53:84–95. doi: 10.1016/0006-3002(61)90796-x. [DOI] [PubMed] [Google Scholar]
- ARONSON A. I., SPIEGELMAN S. Protein and ribonucleic acid synthesis in a chloramphenicol-inhibited system. Biochim Biophys Acta. 1961 Oct 14;53:70–84. doi: 10.1016/0006-3002(61)90795-8. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block R., Haseltine W. A. Thermolability of the stringent factor in rel mutants of Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):625–629. doi: 10.1016/0022-2836(73)90228-3. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43(0):89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- COMB D. G. The enzymatic addition of D-alanyl-D-alanine to a uridine nucleotide-peptide. J Biol Chem. 1962 May;237:1601–1604. [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Chung K. L. Autoradiographic studies of bacterial cell wall replication. I. Cell wall growth of Bacillus cereus in the presence of chloramphenicol. Can J Microbiol. 1967 Apr;13(4):341–350. doi: 10.1139/m67-046. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Greenberg G. R. Effect of the folic acid analogue, trimethoprim, on growth, macromolecular synthesis, and incorporation of exogenous thymine in Escherichia coli. J Bacteriol. 1972 Jun;110(3):905–916. doi: 10.1128/jb.110.3.905-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L. Bacterial cell surface polysaccharides. Annu Rev Biochem. 1973;42:91–112. doi: 10.1146/annurev.bi.42.070173.000515. [DOI] [PubMed] [Google Scholar]
- Golden N. G., Powell G. L. Stringent and relaxed control of phospholipid metabolism in Escherichia coli. J Biol Chem. 1972 Oct 25;247(20):6651–6658. [PubMed] [Google Scholar]
- Good C. M., Tipper D. J. Conditional mutants of Staphylococcus aureus defective in cell wall precursor synthesis. J Bacteriol. 1972 Jul;111(1):231–241. doi: 10.1128/jb.111.1.231-241.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. On the mechanism of action of vancomycin: inhibition of peptidoglycan synthesis in Gaffkya homari. Antimicrob Agents Chemother. 1974 Dec;6(6):722–728. doi: 10.1128/aac.6.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. The regulation of polyamine synthesis during the stringent control in Escherichia coli. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1104–1111. doi: 10.1016/s0006-291x(74)80092-6. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavallé R. Nouveaux mutants de régulation de la synthèse de l'ARN. Bull Soc Chim Biol (Paris) 1965;47(8):1567–1570. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Leutgeb W., Schwarz U. Zur Biosynthese des formgebenden Elements der Bakterienzellwand. I. Abbau des Mureins als erster Schritt beim Wachstum des Sacculus. Z Naturforsch B. 1967 May;22(5):545–549. [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Goldfine H. The involvement of guanosine 5-diphosphate-3-diphosphate in the regulation of phospholipid biosynthesis in Escherichia coli. Lack of ppGpp inhibition of acyltransfer from acyl-ACP to sn-glycerol 3-phosphate. J Biol Chem. 1975 Jul 10;250(13):4911–4917. [PubMed] [Google Scholar]
- Lugtenberg E. J., De Haas-Menger L., Ruyters W. H. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J Bacteriol. 1972 Jan;109(1):326–335. doi: 10.1128/jb.109.1.326-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J. Studies on Escherichia coli enzymes involved in the synthesis of uridine diphosphate-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1972 Apr;110(1):26–34. doi: 10.1128/jb.110.1.26-34.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A., van Bellegem T. H. In vivo and in vitro action of new antibiotics interfering with the utilization of N-acetyl-glucosamine-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1971 Oct;108(1):20–29. doi: 10.1128/jb.108.1.20-29.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Pizer L. I. Regulation of phospholipid synthesis in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1973 Oct;116(1):355–366. doi: 10.1128/jb.116.1.355-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Meade L. A., Tropp B. E. Lipid synthesis in stringent Escherichia coli: an artifact in acetate labeling of phospholipids during a shiftdown in growth rate. J Bacteriol. 1975 Jan;121(1):396–399. doi: 10.1128/jb.121.1.396-399.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiness G., Yang H. L., Zubay G., Cashel M. Effects of guanosine tetraphosphate on cell-free synthesis of Escherichia coli ribosomal RNA and other gene products. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2881–2885. doi: 10.1073/pnas.72.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


