Abstract
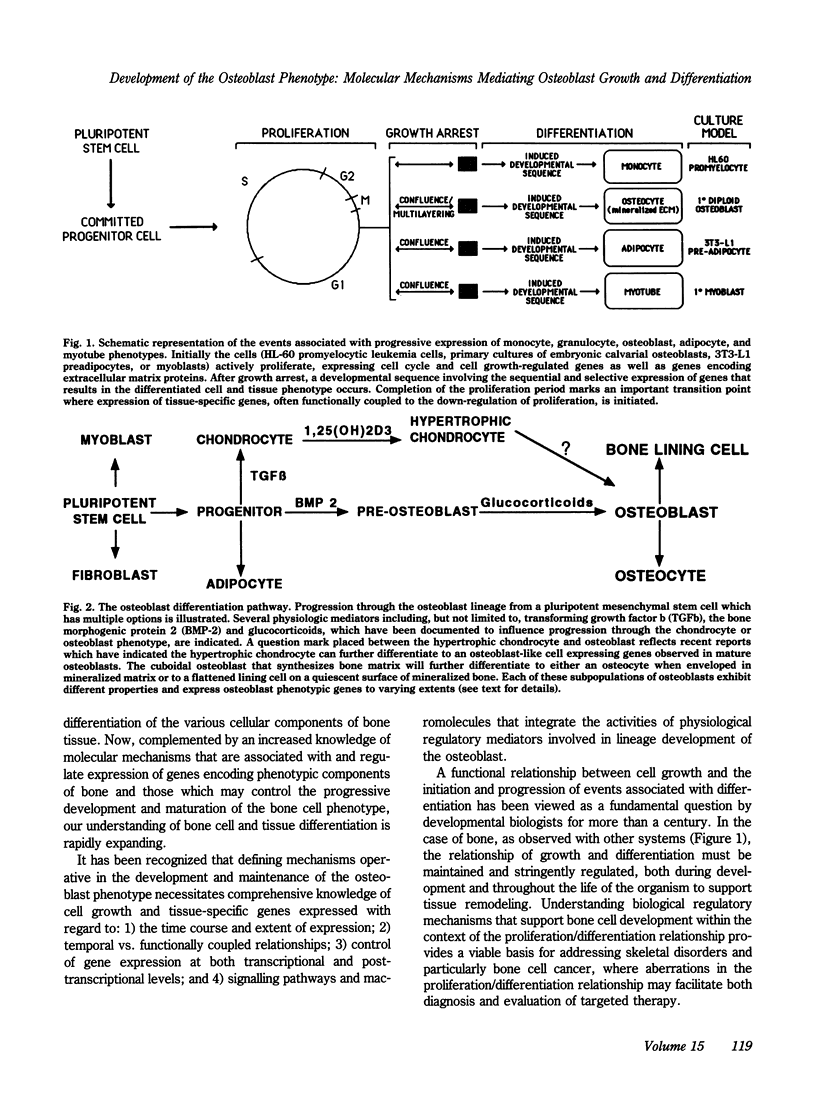
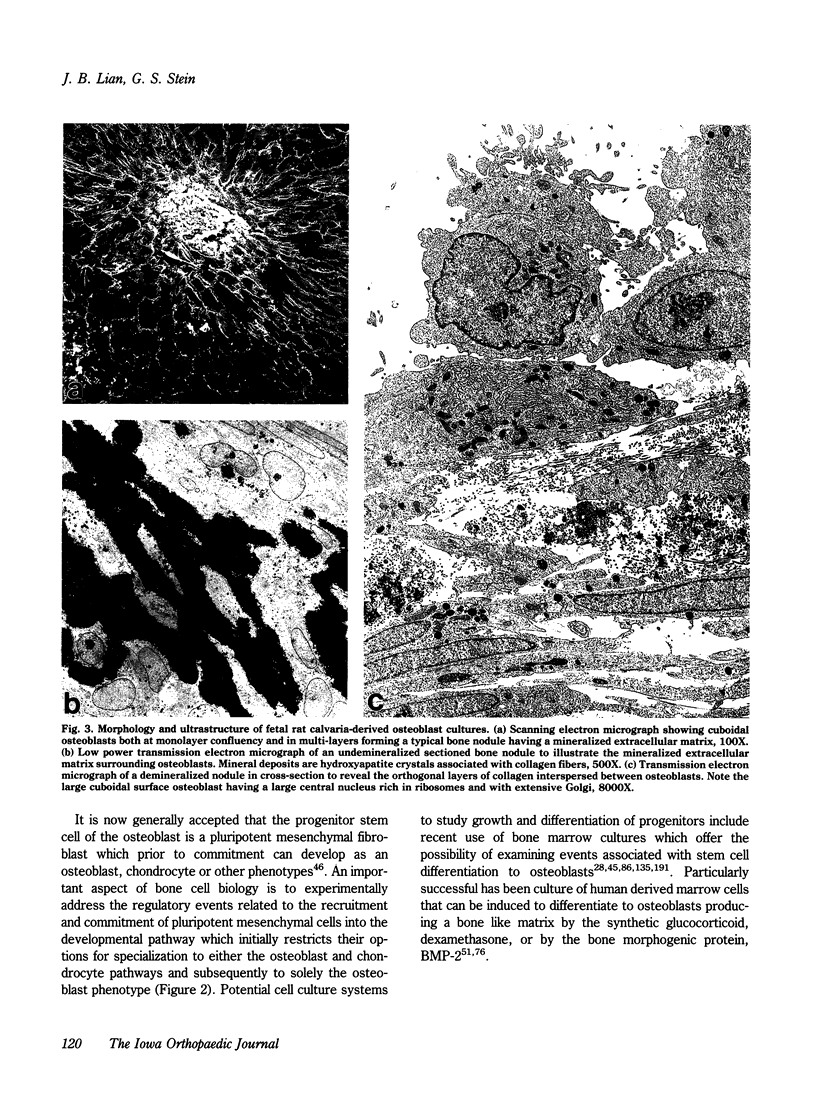
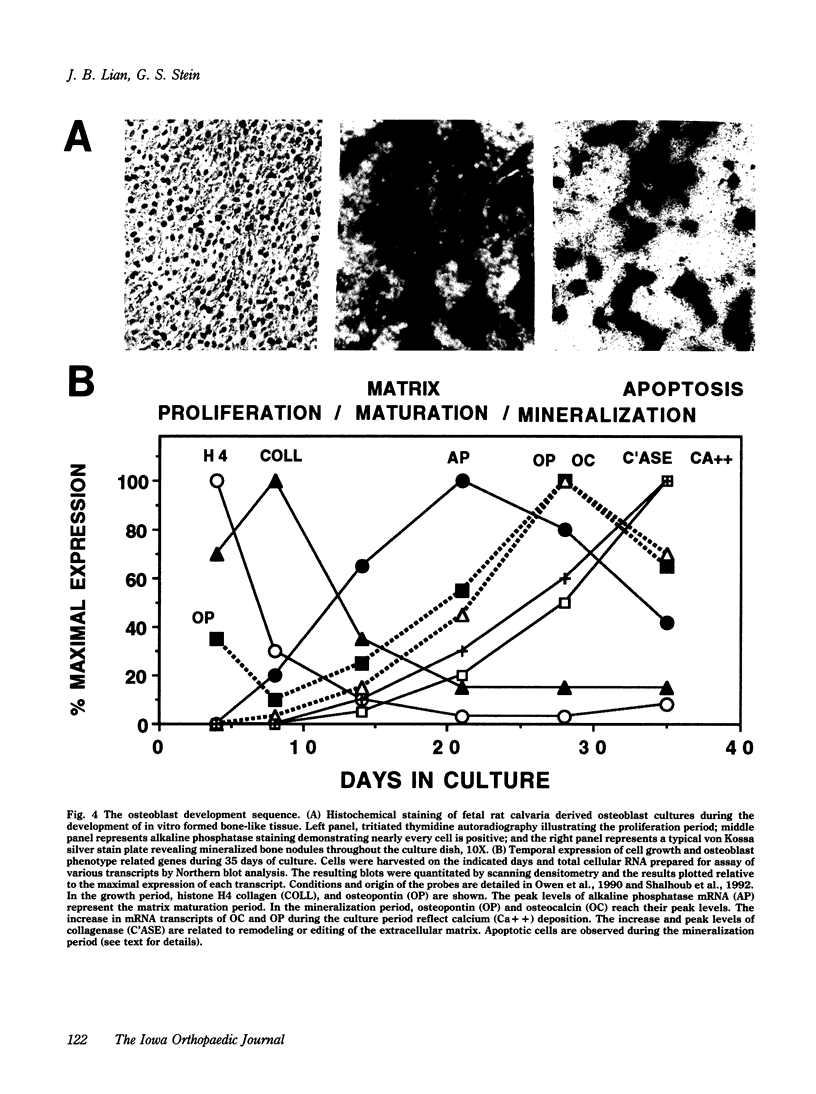
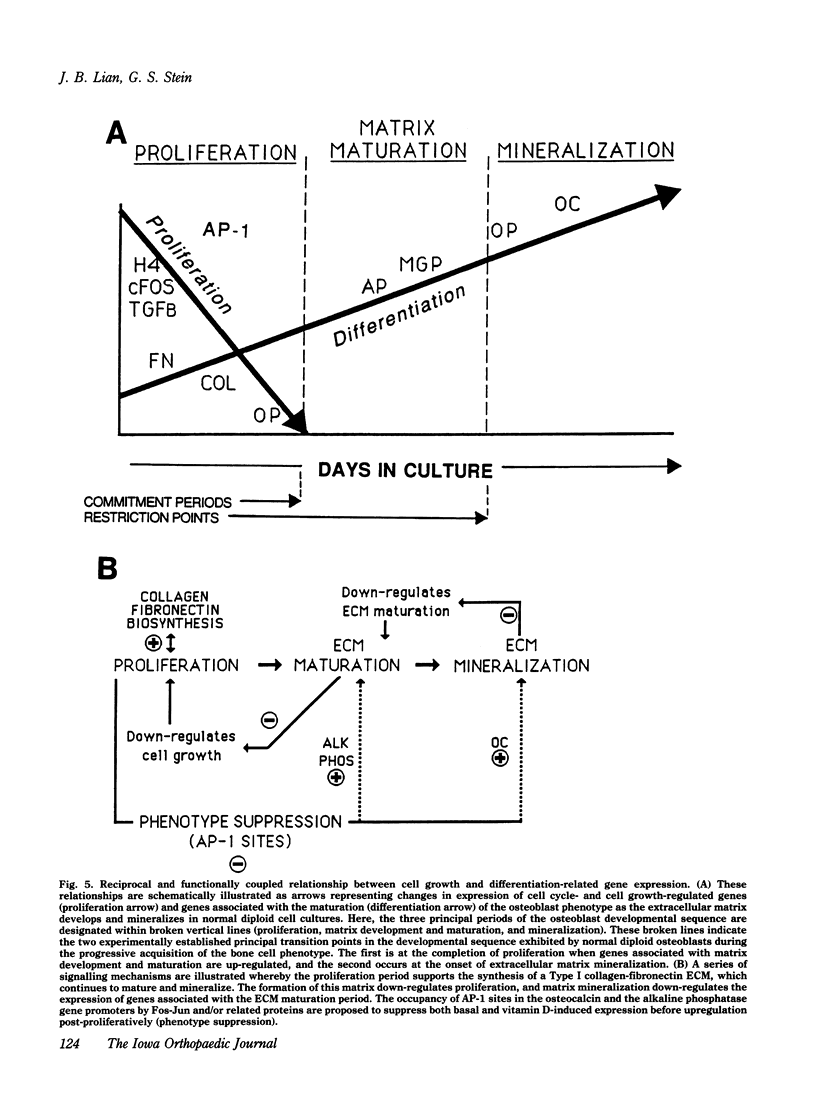
The formation of bone tissue involves multiple activities of the osteoblast. The combined application of molecular, biochemical, histochemical and ultrastructural approaches has defined stages in the development of the osteoblast phenotype with each subpopulation of cells exhibiting unique morphologic and functional properties in relation to the ordered deposition of the mineralized bone extracellular matrix (ECM). Peak levels of expressed genes reflect a maturational sequence of osteoblast growth and differentiation characterized by three principal periods: proliferation, ECM maturation and mineralization. A plethora of new information in the past several years provides the basis for insight into molecular mechanisms regulating the development and activities of differentiating osteoblasts. These new concepts will be discussed within the context of understanding cellular responses of bone tissue. To be considered are the following: 1) maturational stages of the osteoblast reflected by the selective expression of transcription factors (e.g., oncogenes, cyclins, homeodomain proteins) and phenotypic genes that provide signals for differentiation through the osteoblast lineage; 2) role of the extracellular matrix in mediating osteoblast growth and differentiation; 3) osteoblast stage specific responses to physiologic mediators (e.g., growth factors and hormones); 4) the developmentally regulated expression and selective responses of osteoblast phenotypic genes are supported by cooperative, synergistic and/or antagonistic activities at multiple basal and enhancer or suppressor sequences in gene promoters; and 5) deregulation of these control mechanisms in transformed osteoblasts and osteosarcoma cells.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. D., Birnbaum L. S. TCDD alters medial epithelial cell differentiation during palatogenesis. Toxicol Appl Pharmacol. 1989 Jun 15;99(2):276–286. doi: 10.1016/0041-008x(89)90010-0. [DOI] [PubMed] [Google Scholar]
- Andrianarivo A. G., Robinson J. A., Mann K. G., Tracy R. P. Growth on type I collagen promotes expression of the osteoblastic phenotype in human osteosarcoma MG-63 cells. J Cell Physiol. 1992 Nov;153(2):256–265. doi: 10.1002/jcp.1041530205. [DOI] [PubMed] [Google Scholar]
- Antosz M. E., Bellows C. G., Aubin J. E. Effects of transforming growth factor beta and epidermal growth factor on cell proliferation and the formation of bone nodules in isolated fetal rat calvaria cells. J Cell Physiol. 1989 Aug;140(2):386–395. doi: 10.1002/jcp.1041400225. [DOI] [PubMed] [Google Scholar]
- Aronow M. A., Gerstenfeld L. C., Owen T. A., Tassinari M. S., Stein G. S., Lian J. B. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990 May;143(2):213–221. doi: 10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Heersche J. N., Merrilees M. J., Sodek J. Isolation of bone cell clones with differences in growth, hormone responses, and extracellular matrix production. J Cell Biol. 1982 Feb;92(2):452–461. doi: 10.1083/jcb.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone L. M., Aronow M. A., Tassinari M. S., Conlon D., Canalis E., Stein G. S., Lian J. B. Differential effects of warfarin on mRNA levels of developmentally regulated vitamin K dependent proteins, osteocalcin, and matrix GLA protein in vitro. J Cell Physiol. 1994 Aug;160(2):255–264. doi: 10.1002/jcp.1041600207. [DOI] [PubMed] [Google Scholar]
- Barone L. M., Owen T. A., Tassinari M. S., Bortell R., Stein G. S., Lian J. B. Developmental expression and hormonal regulation of the rat matrix Gla protein (MGP) gene in chondrogenesis and osteogenesis. J Cell Biochem. 1991 Aug;46(4):351–365. doi: 10.1002/jcb.240460410. [DOI] [PubMed] [Google Scholar]
- Barone L. M., Tassinari M. S., Bortell R., Owen T. A., Zerogian J., Gagne K., Stein G. S., Lian J. B. Inhibition of induced endochondral bone development in caffeine-treated rats. J Cell Biochem. 1993 Jun;52(2):171–182. doi: 10.1002/jcb.240520209. [DOI] [PubMed] [Google Scholar]
- Baserga R., Rubin R. Cell cycle and growth control. Crit Rev Eukaryot Gene Expr. 1993;3(1):47–61. [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Beck L. S., Amento E. P., Xu Y., Deguzman L., Lee W. P., Nguyen T., Gillett N. A. TGF-beta 1 induces bone closure of skull defects: temporal dynamics of bone formation in defects exposed to rhTGF-beta 1. J Bone Miner Res. 1993 Jun;8(6):753–761. doi: 10.1002/jbmr.5650080614. [DOI] [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E., Heersche J. N. Physiological concentrations of glucocorticoids stimulate formation of bone nodules from isolated rat calvaria cells in vitro. Endocrinology. 1987 Dec;121(6):1985–1992. doi: 10.1210/endo-121-6-1985. [DOI] [PubMed] [Google Scholar]
- Bhargava U., Bar-Lev M., Bellows C. G., Aubin J. E. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 1988;9(3):155–163. doi: 10.1016/8756-3282(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Bidwell J. P., Van Wijnen A. J., Fey E. G., Dworetzky S., Penman S., Stein J. L., Lian J. B., Stein G. S. Osteocalcin gene promoter-binding factors are tissue-specific nuclear matrix components. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3162–3166. doi: 10.1073/pnas.90.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortell R., Owen T. A., Shalhoub V., Heinrichs A., Aronow M. A., Rochette-Egly C., Lutz Y., Stein J. L., Lian J. B., Stein G. S. Constitutive transcription of the osteocalcin gene in osteosarcoma cells is reflected by altered protein-DNA interactions at promoter regulatory elements. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2300–2304. doi: 10.1073/pnas.90.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A. L., Wians F. H., Jr, Hauschka P. V. The effect of osteocalcin on in vitro lipid-induced hydroxyapatite formation and seeded hydroxyapatite growth. Calcif Tissue Int. 1985 Jan;37(1):57–62. doi: 10.1007/BF02557680. [DOI] [PubMed] [Google Scholar]
- Breen E. C., van Wijnen A. J., Lian J. B., Stein G. S., Stein J. L. In vivo occupancy of the vitamin D responsive element in the osteocalcin gene supports vitamin D-dependent transcriptional upregulation in intact cells. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12902–12906. doi: 10.1073/pnas.91.26.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder S. P., Caplan A. I. Discrete stages within the osteogenic lineage are revealed by alterations in the cell surface architecture of embryonic bone cells. Connect Tissue Res. 1989;20(1-4):73–79. doi: 10.3109/03008208909023876. [DOI] [PubMed] [Google Scholar]
- Buckley M. J., Banes A. J., Jordan R. D. The effects of mechanical strain on osteoblasts in vitro. J Oral Maxillofac Surg. 1990 Mar;48(3):276–283. doi: 10.1016/0278-2391(90)90393-g. [DOI] [PubMed] [Google Scholar]
- Canalis E., Centrella M., McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest. 1988 May;81(5):1572–1577. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. Effect of glucocorticoids on type I collagen synthesis, alkaline phosphatase activity, and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983 Mar;112(3):931–939. doi: 10.1210/endo-112-3-931. [DOI] [PubMed] [Google Scholar]
- Canalis E., Lian J. B. 1,25-Dihydroxyvitamin D3 effects on collagen and DNA synthesis in periosteum and periosteum-free calvaria. Bone. 1985;6(6):457–460. doi: 10.1016/8756-3282(85)90224-8. [DOI] [PubMed] [Google Scholar]
- Canalis E., Pash J., Varghese S. Skeletal growth factors. Crit Rev Eukaryot Gene Expr. 1993;3(3):155–166. [PubMed] [Google Scholar]
- Centrella M., Horowitz M. C., Wozney J. M., McCarthy T. L. Transforming growth factor-beta gene family members and bone. Endocr Rev. 1994 Feb;15(1):27–39. doi: 10.1210/edrv-15-1-27. [DOI] [PubMed] [Google Scholar]
- Cheng S. L., Yang J. W., Rifas L., Zhang S. F., Avioli L. V. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994 Jan;134(1):277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- Chenu C., Colucci S., Grano M., Zigrino P., Barattolo R., Zambonin G., Baldini N., Vergnaud P., Delmas P. D., Zallone A. Z. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994 Nov;127(4):1149–1158. doi: 10.1083/jcb.127.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart D., Ramsey-Ewing A., Bortell R., Lian J., Stein J., Stein G. Isolation and characterization of a cDNA from a human histone H2B gene which is reciprocally expressed in relation to replication-dependent H2B histone genes during HL60 cell differentiation. Biochemistry. 1991 Feb 12;30(6):1610–1617. doi: 10.1021/bi00220a024. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Dallas S. L., Park-Snyder S., Miyazono K., Twardzik D., Mundy G. R., Bonewald L. F. Characterization and autoregulation of latent transforming growth factor beta (TGF beta) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF beta-binding protein. J Biol Chem. 1994 Mar 4;269(9):6815–6821. [PubMed] [Google Scholar]
- DeFranco D. J., Glowacki J., Cox K. A., Lian J. B. Normal bone particles are preferentially resorbed in the presence of osteocalcin-deficient bone particles in vivo. Calcif Tissue Int. 1991 Jul;49(1):43–50. doi: 10.1007/BF02555901. [DOI] [PubMed] [Google Scholar]
- Demay M. B., Gerardi J. M., DeLuca H. F., Kronenberg H. M. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor and confer responsiveness to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Jan;87(1):369–373. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demay M. B., Kiernan M. S., DeLuca H. F., Kronenberg H. M. Characterization of 1,25-dihydroxyvitamin D3 receptor interactions with target sequences in the rat osteocalcin gene. Mol Endocrinol. 1992 Apr;6(4):557–562. doi: 10.1210/mend.6.4.1316548. [DOI] [PubMed] [Google Scholar]
- Detke S., Lichtler A., Phillips I., Stein J., Stein G. Reassessment of histone gene expression during cell cycle in human cells by using homologous H4 histone cDNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4995–4999. doi: 10.1073/pnas.76.10.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q. P., Markell P. J., Pardee A. B. Thymidine kinase transcription is regulated at G1/S phase by a complex that contains retinoblastoma-like protein and a cdc2 kinase. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3256–3260. doi: 10.1073/pnas.89.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Feteih R., Tassinari M. S., Lian J. B. Effect of sodium warfarin on vitamin K-dependent proteins and skeletal development in the rat fetus. J Bone Miner Res. 1990 Aug;5(8):885–894. doi: 10.1002/jbmr.5650050813. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Franceschi R. T., Romano P. R., Park K. Y. Regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D3 in human osteosarcoma cells. J Biol Chem. 1988 Dec 15;263(35):18938–18945. [PubMed] [Google Scholar]
- Franceschi R. T., Young J. Regulation of alkaline phosphatase by 1,25-dihydroxyvitamin D3 and ascorbic acid in bone-derived cells. J Bone Miner Res. 1990 Nov;5(11):1157–1167. doi: 10.1002/jbmr.5650051111. [DOI] [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D., Price P. A. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 1988 Aug 15;263(23):11033–11036. [PubMed] [Google Scholar]
- Fried A., Benayahu D., Wientroub S. Marrow stroma-derived osteogenic clonal cell lines: putative stages in osteoblastic differentiation. J Cell Physiol. 1993 Jun;155(3):472–482. doi: 10.1002/jcp.1041550306. [DOI] [PubMed] [Google Scholar]
- Friedenstein A. J., Chailakhyan R. K., Gerasimov U. V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987 May;20(3):263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Chipman S. D., Glowacki J., Lian J. B. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987 Jul;122(1):49–60. doi: 10.1016/0012-1606(87)90331-9. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Gotoh Y., McKee M. D., Nanci A., Landis W. J., Glimcher M. J. Expression and ultrastructural immunolocalization of a major 66 kDa phosphoprotein synthesized by chicken osteoblasts during mineralization in vitro. Anat Rec. 1990 Sep;228(1):93–103. doi: 10.1002/ar.1092280113. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N., Harris M. A., Feng J. Q., Mundy G. R., Harris S. E. Expression of the BMP 2 gene during bone cell differentiation. Crit Rev Eukaryot Gene Expr. 1994;4(2-3):345–355. doi: 10.1615/critreveukargeneexpr.v4.i2-3.30. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Silkworth J. B., Tassinari M., Stein G. S., Lian J. B. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits differentiation of normal diploid rat osteoblasts in vitro. J Cell Biochem. 1994 Feb;54(2):231–238. doi: 10.1002/jcb.240540211. [DOI] [PubMed] [Google Scholar]
- Glimcher M. J. Mechanism of calcification: role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec. 1989 Jun;224(2):139–153. doi: 10.1002/ar.1092240205. [DOI] [PubMed] [Google Scholar]
- Globus R. K., Patterson-Buckendahl P., Gospodarowicz D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology. 1988 Jul;123(1):98–105. doi: 10.1210/endo-123-1-98. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Lian J. B. Impaired recruitment and differentiation of osteoclast progenitors by osteocalcin-deplete bone implants. Cell Differ. 1987 Sep;21(4):247–254. doi: 10.1016/0045-6039(87)90479-9. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Rey C., Glimcher M. J., Cox K. A., Lian J. A role for osteocalcin in osteoclast differentiation. J Cell Biochem. 1991 Mar;45(3):292–302. doi: 10.1002/jcb.240450312. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Goldring S. R. Skeletal tissue response to cytokines. Clin Orthop Relat Res. 1990 Sep;(258):245–278. [PubMed] [Google Scholar]
- Gruss P., Walther C. Pax in development. Cell. 1992 May 29;69(5):719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Bonewald L. F., Harris M. A., Sabatini M., Dallas S., Feng J. Q., Ghosh-Choudhury N., Wozney J., Mundy G. R. Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res. 1994 Jun;9(6):855–863. doi: 10.1002/jbmr.5650090611. [DOI] [PubMed] [Google Scholar]
- Heinrichs A. A., Bortell R., Bourke M., Lian J. B., Stein G. S., Stein J. L. Proximal promoter binding protein contributes to developmental, tissue-restricted expression of the rat osteocalcin gene. J Cell Biochem. 1995 Jan;57(1):90–100. doi: 10.1002/jcb.240570110. [DOI] [PubMed] [Google Scholar]
- Heinrichs A. A., Bortell R., Rahman S., Stein J. L., Alnemri E. S., Litwack G., Lian J. B., Stein G. S. Identification of multiple glucocorticoid receptor binding sites in the rat osteocalcin gene promoter. Biochemistry. 1993 Oct 26;32(42):11436–11444. doi: 10.1021/bi00093a022. [DOI] [PubMed] [Google Scholar]
- Hodgkinson J. E., Davidson C. L., Beresford J., Sharpe P. T. Expression of a human homeobox-containing gene is regulated by 1,25(OH)2D3 in bone cells. Biochim Biophys Acta. 1993 Jul 18;1174(1):11–16. doi: 10.1016/0167-4781(93)90086-s. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. M., Catron K. M., van Wijnen A. J., McCabe L. R., Lian J. B., Stein G. S., Stein J. L. Transcriptional control of the tissue-specific, developmentally regulated osteocalcin gene requires a binding motif for the Msx family of homeodomain proteins. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12887–12891. doi: 10.1073/pnas.91.26.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis J., Owen T. A., van Wijnen A. J., Wright K. L., Ramsey-Ewing A., Kennedy M. B., Carter R., Cosenza S. C., Soprano K. J., Lian J. B. Tumor cells exhibit deregulation of the cell cycle histone gene promoter factor HiNF-D. Science. 1990 Mar 23;247(4949 Pt 1):1454–1457. doi: 10.1126/science.247.4949.1454. [DOI] [PubMed] [Google Scholar]
- Howe A. M., Webster W. S. The warfarin embryopathy: a rat model showing maxillonasal hypoplasia and other skeletal disturbances. Teratology. 1992 Oct;46(4):379–390. doi: 10.1002/tera.1420460408. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Hurley M. M., Abreu C., Harrison J. R., Lichtler A. C., Raisz L. G., Kream B. E. Basic fibroblast growth factor inhibits type I collagen gene expression in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 Mar 15;268(8):5588–5593. [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M., Loose-Mitchell D. S. Steroid hormone-induced expression of oncogene encoded nuclear proteins. Crit Rev Eukaryot Gene Expr. 1994;4(1):55–116. doi: 10.1615/critreveukargeneexpr.v4.i1.30. [DOI] [PubMed] [Google Scholar]
- Ibaraki K., Termine J. D., Whitson S. W., Young M. F. Bone matrix mRNA expression in differentiating fetal bovine osteoblasts. J Bone Miner Res. 1992 Jul;7(7):743–754. doi: 10.1002/jbmr.5650070704. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Nakata K., Nakahara H., Nakase T., Kimura T., Kimata K., Caplan A. I., Ono K. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993 Apr;132(4):1603–1608. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Shalhoub V., Lian J. B., Stein G. S., Marks S. C., Jr Aberrant gene expression in cultured mammalian bone cells demonstrates an osteoblast defect in osteopetrosis. J Cell Biochem. 1994 Jul;55(3):366–372. doi: 10.1002/jcb.240550314. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Spiegelman B. M., Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Katagiri T., Yamaguchi A., Komaki M., Abe E., Takahashi N., Ikeda T., Rosen V., Wozney J. M., Fujisawa-Sehara A., Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994 Dec;127(6 Pt 1):1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi N., DeLuca H. F., Noda M. Id gene expression and its suppression by 1,25-dihydroxyvitamin D3 in rat osteoblastic osteosarcoma cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4569–4572. doi: 10.1073/pnas.89.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeting P. E., Scott R. E., Colvard D. S., Anderson M. A., Oursler M. J., Spelsberg T. C., Riggs B. L. Development and characterization of a rapidly proliferating, well-differentiated cell line derived from normal adult human osteoblast-like cells transfected with SV40 large T antigen. J Bone Miner Res. 1992 Feb;7(2):127–136. doi: 10.1002/jbmr.5650070203. [DOI] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Jackson P. K., Kirschner M. W. Mitosis in transition. Cell. 1994 Nov 18;79(4):563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Stein J., Stein G. Dephosphorylation of nonhistone proteins specifically alters the pattern of gene transcription in reconstituted chromatin. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1174–1178. doi: 10.1073/pnas.73.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 1993 Apr;9(4):106–112. doi: 10.1016/0168-9525(93)90203-t. [DOI] [PubMed] [Google Scholar]
- Kuno H., Kurian S. M., Hendy G. N., White J., deLuca H. F., Evans C. O., Nanes M. S. Inhibition of 1,25-dihydroxyvitamin D3 stimulated osteocalcin gene transcription by tumor necrosis factor-alpha: structural determinants within the vitamin D response element. Endocrinology. 1994 Jun;134(6):2524–2531. doi: 10.1210/endo.134.6.8194478. [DOI] [PubMed] [Google Scholar]
- Kyeyune-Nyombi E., Lau K. H., Baylink D. J., Strong D. D. Stimulation of cellular alkaline phosphatase activity and its messenger RNA level in a human osteosarcoma cell line by 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1989 Dec;275(2):363–370. doi: 10.1016/0003-9861(89)90383-4. [DOI] [PubMed] [Google Scholar]
- Leboy P. S., Beresford J. N., Devlin C., Owen M. E. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991 Mar;146(3):370–378. doi: 10.1002/jcp.1041460306. [DOI] [PubMed] [Google Scholar]
- Levine A. J. Tumor suppressor genes. Bioessays. 1990 Feb;12(2):60–66. doi: 10.1002/bies.950120203. [DOI] [PubMed] [Google Scholar]
- Li Y. P., Stashenko P. Characterization of a tumor necrosis factor-responsive element which down-regulates the human osteocalcin gene. Mol Cell Biol. 1993 Jun;13(6):3714–3721. doi: 10.1128/mcb.13.6.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. B., Dunn K., Key L. L., Jr In vitro degradation of bone particles by human monocytes is decreased with the depletion of the vitamin K-dependent bone protein from the matrix. Endocrinology. 1986 Apr;118(4):1636–1642. doi: 10.1210/endo-118-4-1636. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Gundberg C. M. Osteocalcin. Biochemical considerations and clinical applications. Clin Orthop Relat Res. 1988 Jan;(226):267–291. [PubMed] [Google Scholar]
- Lian J. B., Marks S. C., Jr Osteopetrosis in the rat: coexistence of reductions in osteocalcin and bone resorption. Endocrinology. 1990 Feb;126(2):955–962. doi: 10.1210/endo-126-2-955. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Stein G. S., Bortell R., Owen T. A. Phenotype suppression: a postulated molecular mechanism for mediating the relationship of proliferation and differentiation by Fos/Jun interactions at AP-1 sites in steroid responsive promoter elements of tissue-specific genes. J Cell Biochem. 1991 Jan;45(1):9–14. doi: 10.1002/jcb.240450106. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Tassinari M., Glowacki J. Resorption of implanted bone prepared from normal and warfarin-treated rats. J Clin Invest. 1984 Apr;73(4):1223–1226. doi: 10.1172/JCI111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., Collart D., Zambetti G., Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Malaval L., Gupta A. K., Aubin J. E. Simultaneous detection of multiple bone-related mRNAs and protein expression during osteoblast differentiation: polymerase chain reaction and immunocytochemical studies at the single cell level. Dev Biol. 1994 Nov;166(1):220–234. doi: 10.1006/dbio.1994.1309. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Mark M., Hart C. P., Dollé P., LeMeur M., Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992 Oct 29;359(6398):835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Lynch M. P., Stein J. L., Stein G. S., Lian J. B. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995 Jan;216(1):35–45. doi: 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- MacDonald P. N., Dowd D. R., Nakajima S., Galligan M. A., Reeder M. C., Haussler C. A., Ozato K., Haussler M. R. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Mol Cell Biol. 1993 Sep;13(9):5907–5917. doi: 10.1128/mcb.13.9.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeska R. J., Rodan G. A. The effect of 1,25(OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma cells. J Biol Chem. 1982 Apr 10;257(7):3362–3365. [PubMed] [Google Scholar]
- Malaval L., Modrowski D., Gupta A. K., Aubin J. E. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994 Mar;158(3):555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- Marcelli C., Yates A. J., Mundy G. R. In vivo effects of human recombinant transforming growth factor beta on bone turnover in normal mice. J Bone Miner Res. 1990 Oct;5(10):1087–1096. doi: 10.1002/jbmr.5650051013. [DOI] [PubMed] [Google Scholar]
- Marie P. J., Hott M., Garba M. T. Contrasting effects of 1,25-dihydroxyvitamin D3 on bone matrix and mineral appositional rates in the mouse. Metabolism. 1985 Aug;34(8):777–783. doi: 10.1016/0026-0495(85)90030-7. [DOI] [PubMed] [Google Scholar]
- Markose E. R., Stein J. L., Stein G. S., Lian J. B. Vitamin D-mediated modifications in protein-DNA interactions at two promoter elements of the osteocalcin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1701–1705. doi: 10.1073/pnas.87.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi L., Franchi A., Santucci M., Danielli D., Arganini L., Giannone V., Formigli L., Benvenuti S., Tanini A., Beghè F. Adhesion, growth, and matrix production by osteoblasts on collagen substrata. Calcif Tissue Int. 1992 Sep;51(3):202–212. doi: 10.1007/BF00334548. [DOI] [PubMed] [Google Scholar]
- Merry K., Dodds R., Littlewood A., Gowen M. Expression of osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone. J Cell Sci. 1993 Apr;104(Pt 4):1013–1020. doi: 10.1242/jcs.104.4.1013. [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Alvarez J., Greenfield E. M., Teti A., Grano M., Colucci S., Zambonin-Zallone A., Ross F. P., Teitelbaum S. L., Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991 Oct 25;266(30):20369–20374. [PubMed] [Google Scholar]
- Morrison N. A., Shine J., Fragonas J. C., Verkest V., McMenemy M. L., Eisman J. A. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989 Dec 1;246(4934):1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- Murray S. S., Glackin C. A., Winters K. A., Gazit D., Kahn A. J., Murray E. J. Expression of helix-loop-helix regulatory genes during differentiation of mouse osteoblastic cells. J Bone Miner Res. 1992 Oct;7(10):1131–1138. doi: 10.1002/jbmr.5650071004. [DOI] [PubMed] [Google Scholar]
- Nagata T., Bellows C. G., Kasugai S., Butler W. T., Sodek J. Biosynthesis of bone proteins [SPP-1 (secreted phosphoprotein-1, osteopontin), BSP (bone sialoprotein) and SPARC (osteonectin)] in association with mineralized-tissue formation by fetal-rat calvarial cells in culture. Biochem J. 1991 Mar 1;274(Pt 2):513–520. doi: 10.1042/bj2740513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Hanada K., Tamura M., Shibanushi T., Nigi H., Tagawa M., Fukumoto S., Matsumoto T. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology. 1995 Mar;136(3):1276–1284. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- Nanes M. S., Rubin J., Titus L., Hendy G. N., Catherwood B. D. Interferon-gamma inhibits 1,25-dihydroxyvitamin D3-stimulated synthesis of bone GLA protein in rat osteosarcoma cells by a pretranslational mechanism. Endocrinology. 1990 Aug;127(2):588–594. doi: 10.1210/endo-127-2-588. [DOI] [PubMed] [Google Scholar]
- Nicolas V., Nefussi J. R., Collin P., Forest N. Effects of acidic fibroblast growth factor and epidermal growth factor on subconfluent fetal rat calvaria cell cultures: DNA synthesis and alkaline phosphatase activity. Bone Miner. 1990 Feb;8(2):145–156. doi: 10.1016/0169-6009(90)90117-x. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Mulder R. J. Identification of osteocytes in osteoblast-like cell cultures using a monoclonal antibody specifically directed against osteocytes. Histochemistry. 1986;84(4-6):342–347. doi: 10.1007/BF00482961. [DOI] [PubMed] [Google Scholar]
- Noda M., Camilliere J. J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989 Jun;124(6):2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Noff D., Pitaru S., Savion N. Basic fibroblast growth factor enhances the capacity of bone marrow cells to form bone-like nodules in vitro. FEBS Lett. 1989 Jul 3;250(2):619–621. doi: 10.1016/0014-5793(89)80808-7. [DOI] [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994 Nov 18;79(4):547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ogata T., Noda M. Expression of Id, a negative regulator of helix-loop-helix DNA binding proteins, is down-regulated at confluence and enhanced by dexamethasone in a mouse osteoblastic cell line, MC3T3E1. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1194–1199. doi: 10.1016/s0006-291x(05)81322-1. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T. A., Aronow M. S., Barone L. M., Bettencourt B., Stein G. S., Lian J. B. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology. 1991 Mar;128(3):1496–1504. doi: 10.1210/endo-128-3-1496. [DOI] [PubMed] [Google Scholar]
- Owen T. A., Aronow M., Shalhoub V., Barone L. M., Wilming L., Tassinari M. S., Kennedy M. B., Pockwinse S., Lian J. B., Stein G. S. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990 Jun;143(3):420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Owen T. A., Bortell R., Shalhoub V., Heinrichs A., Stein J. L., Stein G. S., Lian J. B. Postproliferative transcription of the rat osteocalcin gene is reflected by vitamin D-responsive developmental modifications in protein-DNA interactions at basal and enhancer promoter elements. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1503–1507. doi: 10.1073/pnas.90.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T. A., Bortell R., Yocum S. A., Smock S. L., Zhang M., Abate C., Shalhoub V., Aronin N., Wright K. L., van Wijnen A. J. Coordinate occupancy of AP-1 sites in the vitamin D-responsive and CCAAT box elements by Fos-Jun in the osteocalcin gene: model for phenotype suppression of transcription. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9990–9994. doi: 10.1073/pnas.87.24.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H., Imamura K., Abe E., Takahashi N., Hiraide T., Shibasaki Y., Fukuhara T., Suda T. Effect of a continuously applied compressive pressure on mouse osteoblast-like cells (MC3T3-E1) in vitro. J Cell Physiol. 1990 Jan;142(1):177–185. doi: 10.1002/jcp.1041420122. [DOI] [PubMed] [Google Scholar]
- Ozono K., Sone T., Pike J. W. The genomic mechanism of action of 1,25-dihydroxyvitamin D3. J Bone Miner Res. 1991 Oct;6(10):1021–1027. doi: 10.1002/jbmr.5650061002. [DOI] [PubMed] [Google Scholar]
- Pauli U., Chrysogelos S., Stein G., Stein J., Nick H. Protein-DNA interactions in vivo upstream of a cell cycle-regulated human H4 histone gene. Science. 1987 Jun 5;236(4806):1308–1311. doi: 10.1126/science.3035717. [DOI] [PubMed] [Google Scholar]
- Pechak D. G., Kujawa M. J., Caplan A. I. Morphological and histochemical events during first bone formation in embryonic chick limbs. Bone. 1986;7(6):441–458. doi: 10.1016/8756-3282(86)90004-9. [DOI] [PubMed] [Google Scholar]
- Pockwinse S. M., Stein J. L., Lian J. B., Stein G. S. Developmental stage-specific cellular responses to vitamin D and glucocorticoids during differentiation of the osteoblast phenotype: interrelationship of morphology and gene expression by in situ hybridization. Exp Cell Res. 1995 Jan;216(1):244–260. doi: 10.1006/excr.1995.1031. [DOI] [PubMed] [Google Scholar]
- Pockwinse S. M., Wilming L. G., Conlon D. M., Stein G. S., Lian J. B. Expression of cell growth and bone specific genes at single cell resolution during development of bone tissue-like organization in primary osteoblast cultures. J Cell Biochem. 1992 Jul;49(3):310–323. doi: 10.1002/jcb.240490315. [DOI] [PubMed] [Google Scholar]
- Quarles L. D., Yohay D. A., Lever L. W., Caton R., Wenstrup R. J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992 Jun;7(6):683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Ramsey-Ewing A., Van Wijnen A. J., Stein G. S., Stein J. L. Delineation of a human histone H4 cell cycle element in vivo: the master switch for H4 gene transcription. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4475–4479. doi: 10.1073/pnas.91.10.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Flanders K. C., Roche N. S., Kondaiah P., Reddi A. H., Termine J. D., Sporn M. B., Roberts A. B. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987 Jul;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Thomas K., Rodan G. A. Growth stimulation of rat calvaria osteoblastic cells by acidic fibroblast growth factor. Endocrinology. 1987 Dec;121(6):1917–1923. doi: 10.1210/endo-121-6-1917. [DOI] [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Riggs B. L., Mann K. G. Inhibition of hydroxyapatite crystal growth by bone-specific and other calcium-binding proteins. Biochemistry. 1986 Mar 11;25(5):1176–1180. doi: 10.1021/bi00353a035. [DOI] [PubMed] [Google Scholar]
- Rüther U., Garber C., Komitowski D., Müller R., Wagner E. F. Deregulated c-fos expression interferes with normal bone development in transgenic mice. 1987 Jan 29-Feb 4Nature. 325(6103):412–416. doi: 10.1038/325412a0. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Autio-Harmainen H., Vuorio E. Localization of the expression of types I, III, and IV collagen, TGF-beta 1 and c-fos genes in developing human calvarial bones. Dev Biol. 1988 Nov;130(1):324–334. doi: 10.1016/0012-1606(88)90438-1. [DOI] [PubMed] [Google Scholar]
- Schräder M., Bendik I., Becker-André M., Carlberg C. Interaction between retinoic acid and vitamin D signaling pathways. J Biol Chem. 1993 Aug 25;268(24):17830–17836. [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Shakoori A. R., Owen T. A., Shalhoub V., Stein J. L., Bustin M., Stein G. S., Lian J. B. Differential expression of the chromosomal high mobility group proteins 14 and 17 during the onset of differentiation in mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1993 Apr;51(4):479–487. doi: 10.1002/jcb.2400510413. [DOI] [PubMed] [Google Scholar]
- Shalhoub V., Bortell R., Jackson M. E., Marks S. C., Jr, Stein J. L., Lian J. B., Stein G. S. Transcriptionally active nuclei isolated from intact bone reflect modified levels of gene expression in skeletal development and pathology. J Cell Biochem. 1994 Jun;55(2):182–189. doi: 10.1002/jcb.240550205. [DOI] [PubMed] [Google Scholar]
- Shalhoub V., Conlon D., Tassinari M., Quinn C., Partridge N., Stein G. S., Lian J. B. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem. 1992 Dec;50(4):425–440. doi: 10.1002/jcb.240500411. [DOI] [PubMed] [Google Scholar]
- Shalhoub V., Jackson M. E., Lian J. B., Stein G. S., Marks S. C., Jr Gene expression during skeletal development in three osteopetrotic rat mutations. Evidence for osteoblast abnormalities. J Biol Chem. 1991 May 25;266(15):9847–9856. [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Pannese M., D'Esposito M., Stornaiuolo A., Gulisano M., Mallamaci A., Kastury K., Druck T., Huebner K. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Spiess Y. H., Price P. A., Deftos J. L., Manolagas S. C. Phenotype-associated changes in the effects of 1,25-dihydroxyvitamin D3 on alkaline phosphatase and bone GLA-protein of rat osteoblastic cells. Endocrinology. 1986 Apr;118(4):1340–1346. doi: 10.1210/endo-118-4-1340. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Lian J. B., Dworetzky S. I., Owen T. A., Bortell R., Bidwell J. P., van Wijnen A. J. Regulation of transcription-factor activity during growth and differentiation: involvement of the nuclear matrix in concentration and localization of promoter binding proteins. J Cell Biochem. 1991 Dec;47(4):300–305. doi: 10.1002/jcb.240470403. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Lian J. B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993 Aug;14(4):424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Lian J. B., Owen T. A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990 Oct;4(13):3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L., van Wijnen A. J., Lian J. B. Histone gene transcription: a model for responsiveness to an integrated series of regulatory signals mediating cell cycle control and proliferation/differentiation interrelationships. J Cell Biochem. 1994 Apr;54(4):393–404. doi: 10.1002/jcb.240540406. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L., van Wijnen A. J., Lian J. B. Regulation of histone gene expression. Curr Opin Cell Biol. 1992 Apr;4(2):166–173. doi: 10.1016/0955-0674(92)90028-b. [DOI] [PubMed] [Google Scholar]
- Strauss P. G., Closs E. I., Schmidt J., Erfle V. Gene expression during osteogenic differentiation in mandibular condyles in vitro. J Cell Biol. 1990 Apr;110(4):1369–1378. doi: 10.1083/jcb.110.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömstedt P. E., Poellinger L., Gustafsson J. A., Carlstedt-Duke J. The glucocorticoid receptor binds to a sequence overlapping the TATA box of the human osteocalcin promoter: a potential mechanism for negative regulation. Mol Cell Biol. 1991 Jun;11(6):3379–3383. doi: 10.1128/mcb.11.6.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M., Colvard D., Keeting P. E., Rasmussen K., Riggs B. L., Spelsberg T. C. Glucocorticoid regulation of alkaline phosphatase, osteocalcin, and proto-oncogenes in normal human osteoblast-like cells. J Cell Biochem. 1992 Dec;50(4):411–424. doi: 10.1002/jcb.240500410. [DOI] [PubMed] [Google Scholar]
- Tamura M., Noda M. Identification of a DNA sequence involved in osteoblast-specific gene expression via interaction with helix-loop-helix (HLH)-type transcription factors. J Cell Biol. 1994 Aug;126(3):773–782. doi: 10.1083/jcb.126.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari M. S., Gerstenfeld L. C., Stein G. S., Lian J. B. Effect of caffeine on parameters of osteoblast growth and differentiation of a mineralized extracellular matrix in vitro. J Bone Miner Res. 1991 Oct;6(10):1029–1036. doi: 10.1002/jbmr.5650061003. [DOI] [PubMed] [Google Scholar]
- Terpening C. M., Haussler C. A., Jurutka P. W., Galligan M. A., Komm B. S., Haussler M. R. The vitamin D-responsive element in the rat bone Gla protein gene is an imperfect direct repeat that cooperates with other cis-elements in 1,25-dihydroxyvitamin D3- mediated transcriptional activation. Mol Endocrinol. 1991 Mar;5(3):373–385. doi: 10.1210/mend-5-3-373. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Bennett C. D., Rodan G. A. Activity of the rat osteocalcin basal promoter in osteoblastic cells is dependent upon homeodomain and CP1 binding motifs. Mol Endocrinol. 1994 May;8(5):614–624. doi: 10.1210/mend.8.5.7914673. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Rutledge S. J., Rodan G. A. Msx-2/Hox 8.1: a transcriptional regulator of the rat osteocalcin promoter. Mol Endocrinol. 1994 Nov;8(11):1484–1493. doi: 10.1210/mend.8.11.7877617. [DOI] [PubMed] [Google Scholar]
- Turksen K., Aubin J. E. Positive and negative immunoselection for enrichment of two classes of osteoprogenitor cells. J Cell Biol. 1991 Jul;114(2):373–384. doi: 10.1083/jcb.114.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Kleinman H. K., Reddi A. H. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990 Oct 19;63(2):437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- Webber D., Osdoby P., Hauschka P., Krukowski M. Correlation of an osteoclast antigen and ruffled border on giant cells formed in response to resorbable substrates. J Bone Miner Res. 1990 Apr;5(4):401–410. doi: 10.1002/jbmr.5650050414. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weinreb M., Shinar D., Rodan G. A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J Bone Miner Res. 1990 Aug;5(8):831–842. doi: 10.1002/jbmr.5650050806. [DOI] [PubMed] [Google Scholar]
- White E. Tumour biology. p53, guardian of Rb. Nature. 1994 Sep 1;371(6492):21–22. doi: 10.1038/371021a0. [DOI] [PubMed] [Google Scholar]
- Wronski T. J., Halloran B. P., Bikle D. D., Globus R. K., Morey-Holton E. R. Chronic administration of 1,25-dihydroxyvitamin D3: increased bone but impaired mineralization. Endocrinology. 1986 Dec;119(6):2580–2585. doi: 10.1210/endo-119-6-2580. [DOI] [PubMed] [Google Scholar]
- Yao K. L., Todescan R., Jr, Sodek J. Temporal changes in matrix protein synthesis and mRNA expression during mineralized tissue formation by adult rat bone marrow cells in culture. J Bone Miner Res. 1994 Feb;9(2):231–240. doi: 10.1002/jbmr.5650090212. [DOI] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- van Wijnen A. J., Aziz F., Graña X., De Luca A., Desai R. K., Jaarsveld K., Last T. J., Soprano K., Giordano A., Lian J. B. Transcription of histone H4, H3, and H1 cell cycle genes: promoter factor HiNF-D contains CDC2, cyclin A, and an RB-related protein. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12882–12886. doi: 10.1073/pnas.91.26.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., Wright K. L., Lian J. B., Stein J. L., Stein G. S. Human H4 histone gene transcription requires the proliferation-specific nuclear factor HiNF-D. Auxiliary roles for HiNF-C (Sp1-like) and HiNF-A (high mobility group-like). J Biol Chem. 1989 Sep 5;264(25):15034–15042. [PubMed] [Google Scholar]
- van Wijnen A. J., van den Ent F. M., Lian J. B., Stein J. L., Stein G. S. Overlapping and CpG methylation-sensitive protein-DNA interactions at the histone H4 transcriptional cell cycle domain: distinctions between two human H4 gene promoters. Mol Cell Biol. 1992 Jul;12(7):3273–3287. doi: 10.1128/mcb.12.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F. M., van Wijnen A. J., Lian J. B., Stein J. L., Stein G. S. Cell cycle controlled histone H1, H3, and H4 genes share unusual arrangements of recognition motifs for HiNF-D supporting a coordinate promoter binding mechanism. J Cell Physiol. 1994 Jun;159(3):515–530. doi: 10.1002/jcp.1041590316. [DOI] [PubMed] [Google Scholar]
- van der Houven van Oordt C. W., van Wijnen A. J., Carter R., Soprano K., Lian J. B., Stein G. S., Stein J. L. Protein-DNA interactions at the H4-site III upstream transcriptional element of a cell cycle regulated histone H4 gene: differences in normal versus tumor cells. J Cell Biochem. 1992 May;49(1):93–110. doi: 10.1002/jcb.240490115. [DOI] [PubMed] [Google Scholar]
- van der Plas A., Aarden E. M., Feijen J. H., de Boer A. H., Wiltink A., Alblas M. J., de Leij L., Nijweide P. J. Characteristics and properties of osteocytes in culture. J Bone Miner Res. 1994 Nov;9(11):1697–1704. doi: 10.1002/jbmr.5650091105. [DOI] [PubMed] [Google Scholar]




