Abstract
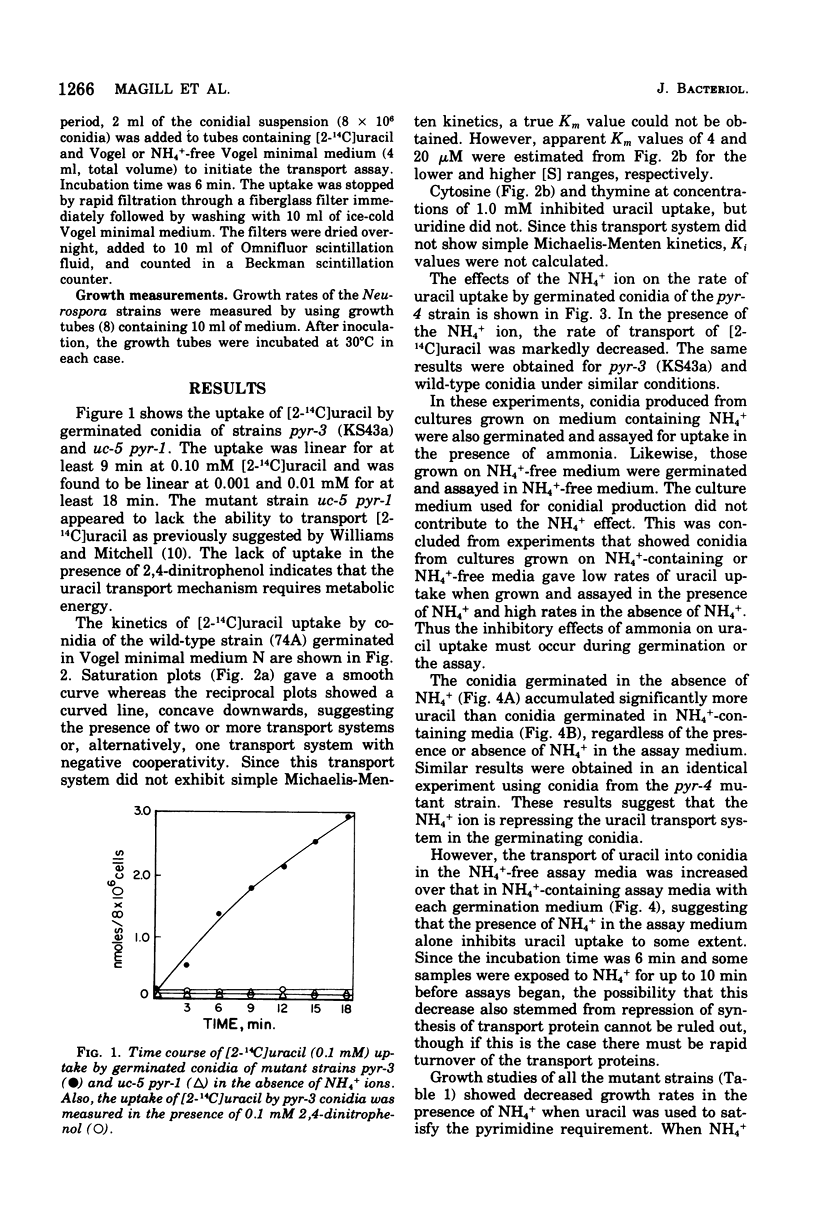
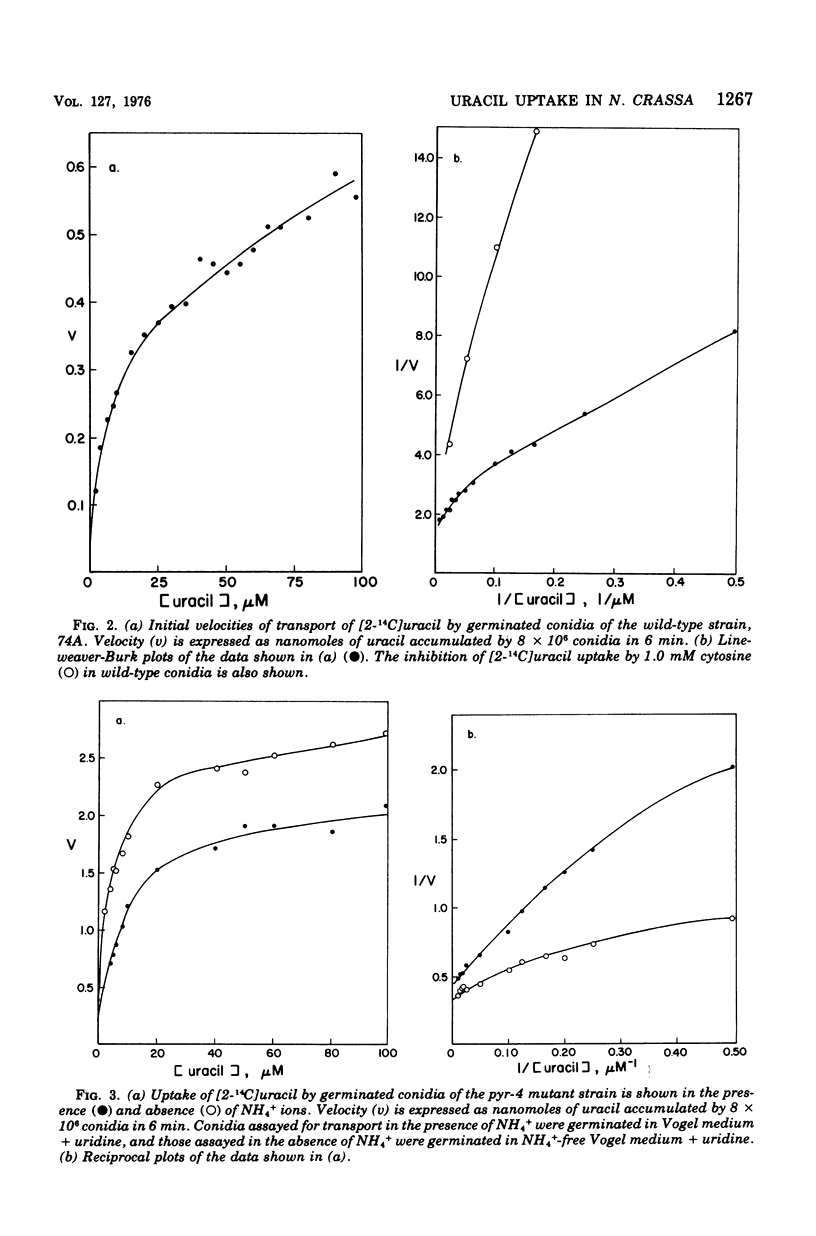
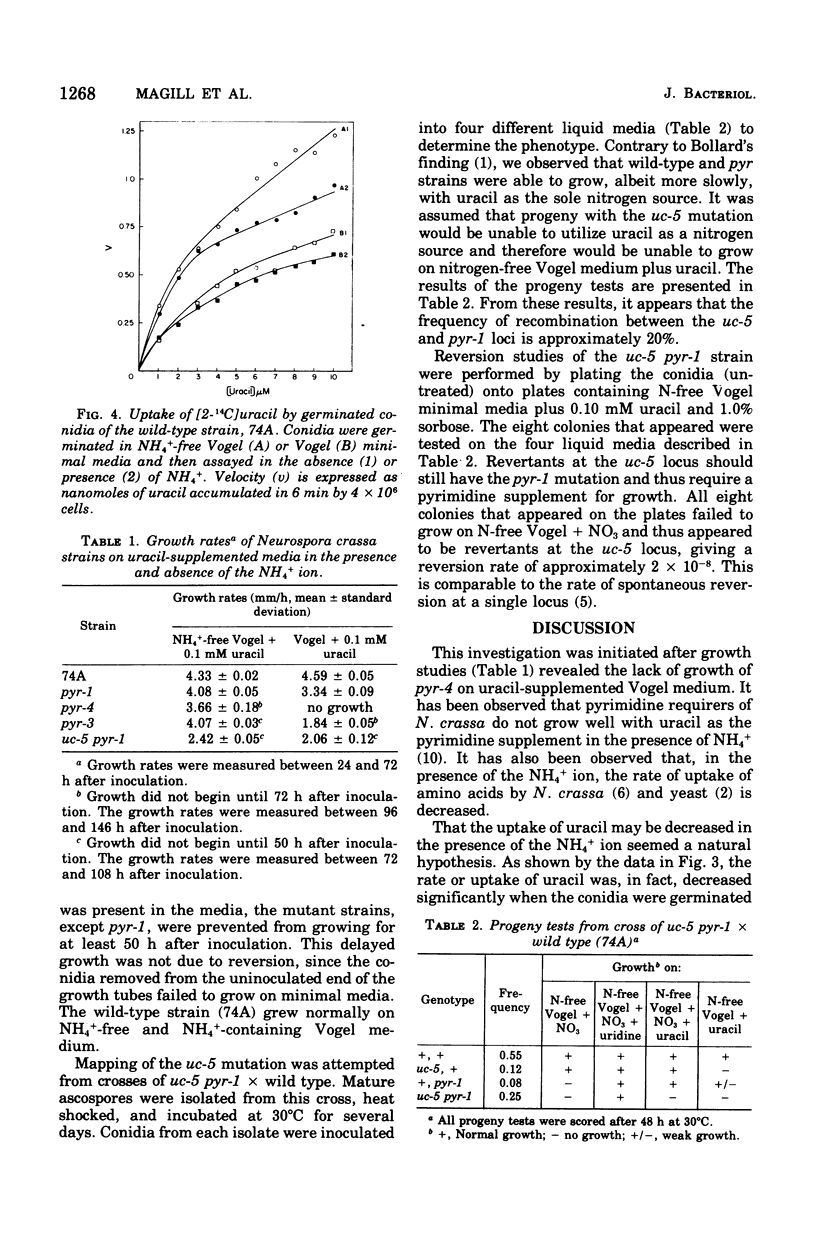
The mechanism of uracil uptake and one aspect of its regulation were studied in germinated conidia of Neurospora crassa. Uracil was found to be taken up by a transport mechanism that did not exhibit Michaelis-Menten kinetics. Rather, the kinetic patterns indicated two separate systems or a single transport mechanism with negative cooperativity. Cytosine and thymine inhibited uracil uptake, but uridine did not. The mutant strain uc-5-pyr-1, which failed to transport uracil, was used in reversion studies and to map the uc-5 locus. Spontaneous reversion rates at the uc-5 locus were found to be approximately 2 x 10(-8), indicating that the uc-5 lesion results from a single mutation. Loss of the uracil transport function through a single mutation favors the model of a single transport mechanism with negative cooperativity. Uracil uptake was significantly decreased in the presence of NH 4+, and evidence is presented for repression by NH4+ of a uracil transport system. Growth rates of pyrimidine-requiring and wild-type strains measured in the presence and absence of NH4+, with uracil as the pyrimidine supplement, showed that NH4+ decreased the growth rates of the pyrimidine-requiring strains significantly, while having no effect on wild-type growth rates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grenson M., Hou C. Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenaseless mutants of saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Aug 21;48(4):749–756. doi: 10.1016/0006-291x(72)90670-5. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malling H. V., de Serres F. J. Correlation between base-pair transition and complementation pattern in nitrous acid-induced ad-3B mutants of Neurospora crassa. Mutat Res. 1968 May-Jun;5(3):359–371. doi: 10.1016/0027-5107(68)90006-7. [DOI] [PubMed] [Google Scholar]
- Rao E. Y., Rao T. K., Debusk A. G. Isolation and characterization of a mutant of Neurospora crassa deficient in general amino acid permease activity. Biochim Biophys Acta. 1975 Nov 17;413(1):45–51. doi: 10.1016/0005-2736(75)90057-7. [DOI] [PubMed] [Google Scholar]
- Reinert W. R., Marzluf G. A. Regulation of the purine catabolic enzymes in Neurospora crassa. Arch Biochem Biophys. 1975 Feb;166(2):565–574. doi: 10.1016/0003-9861(75)90421-x. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Mitchell H. K. Mutants affecting thymidine metabolism in Neurospora crassa. J Bacteriol. 1969 Oct;100(1):383–389. doi: 10.1128/jb.100.1.383-389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


