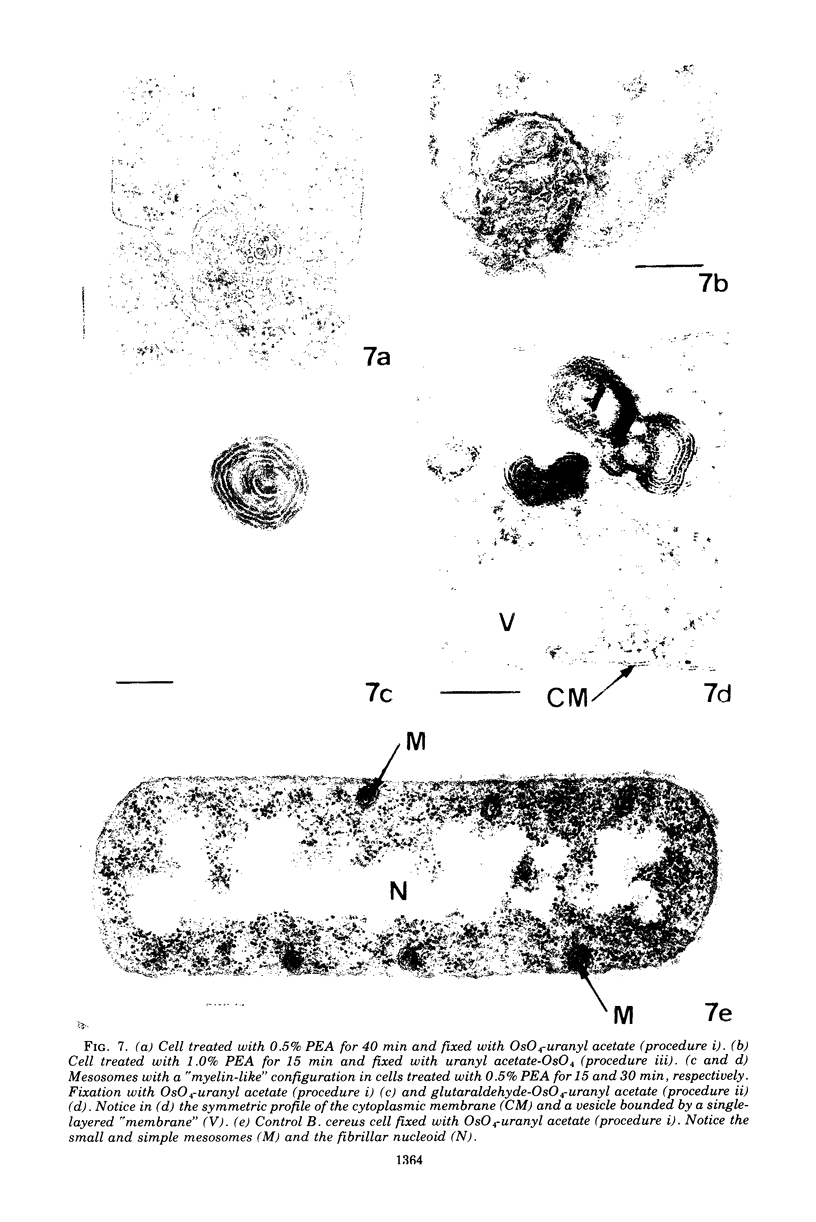
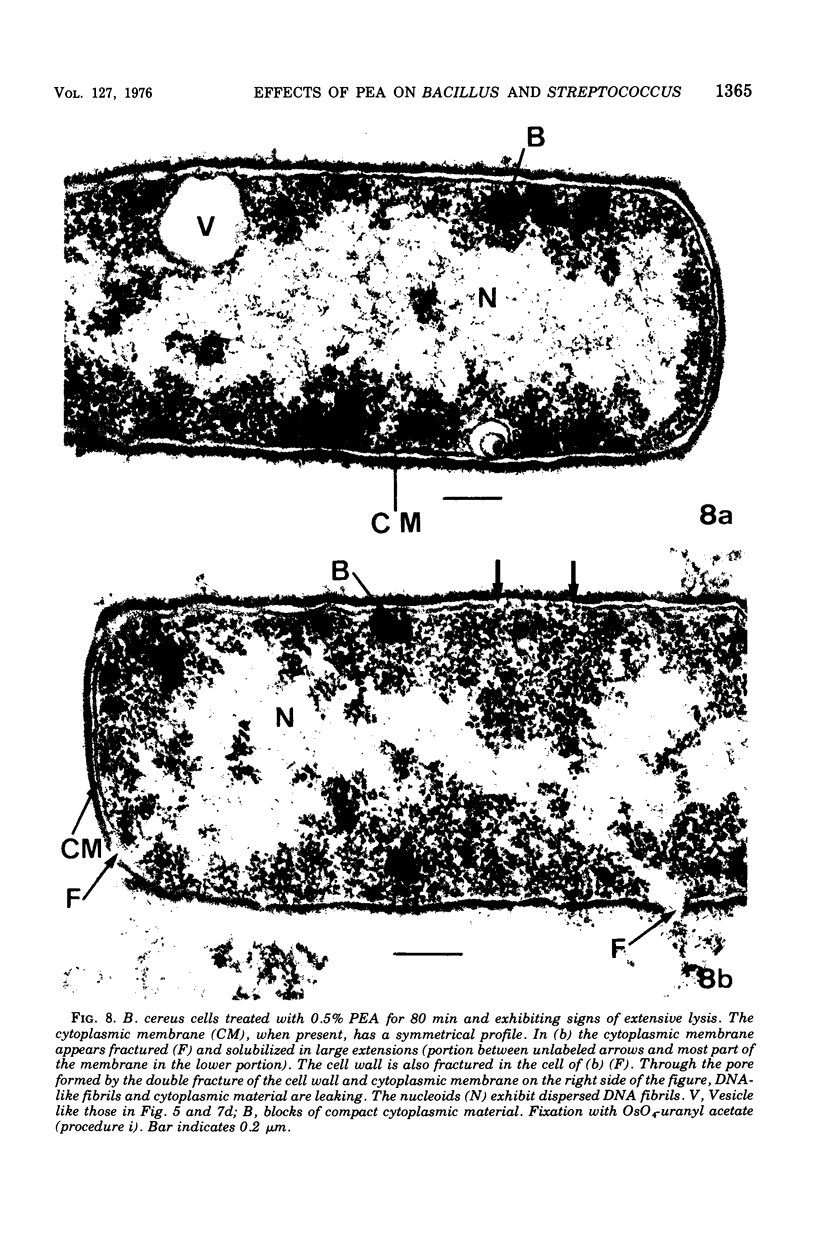
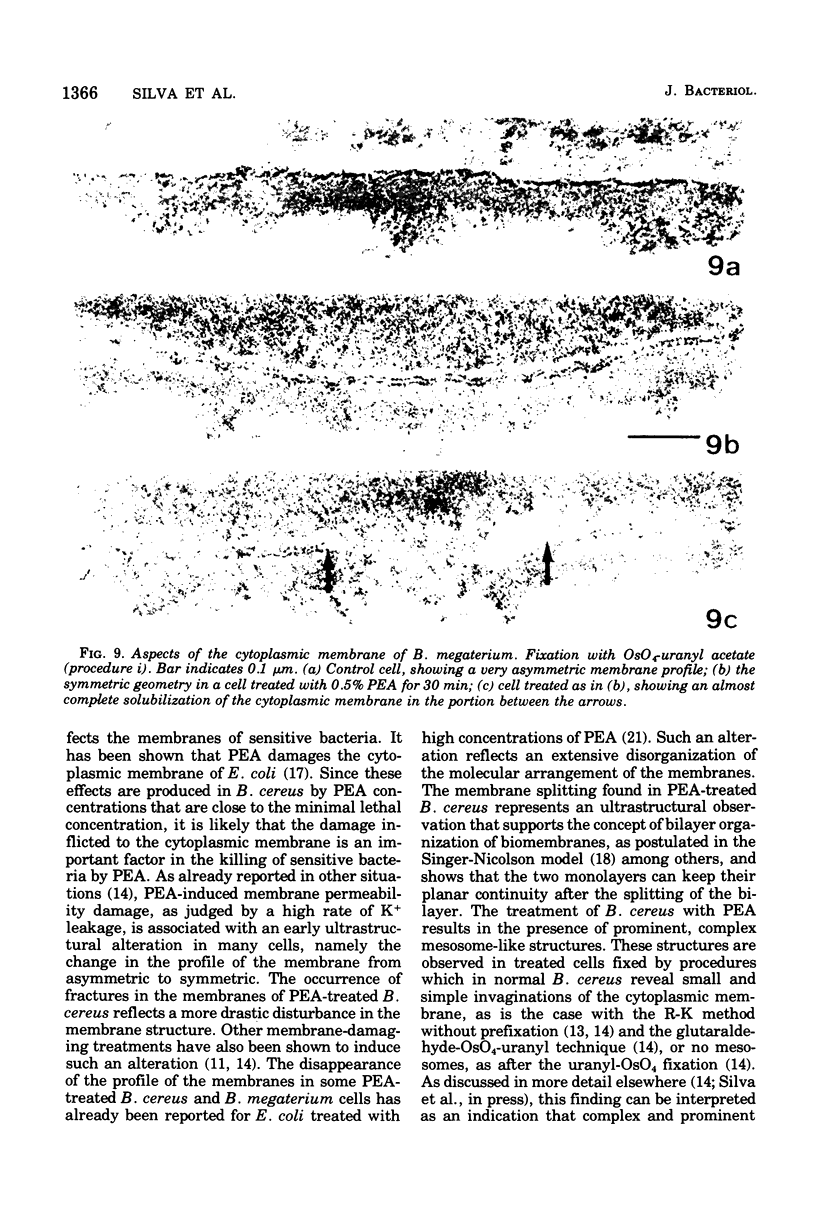
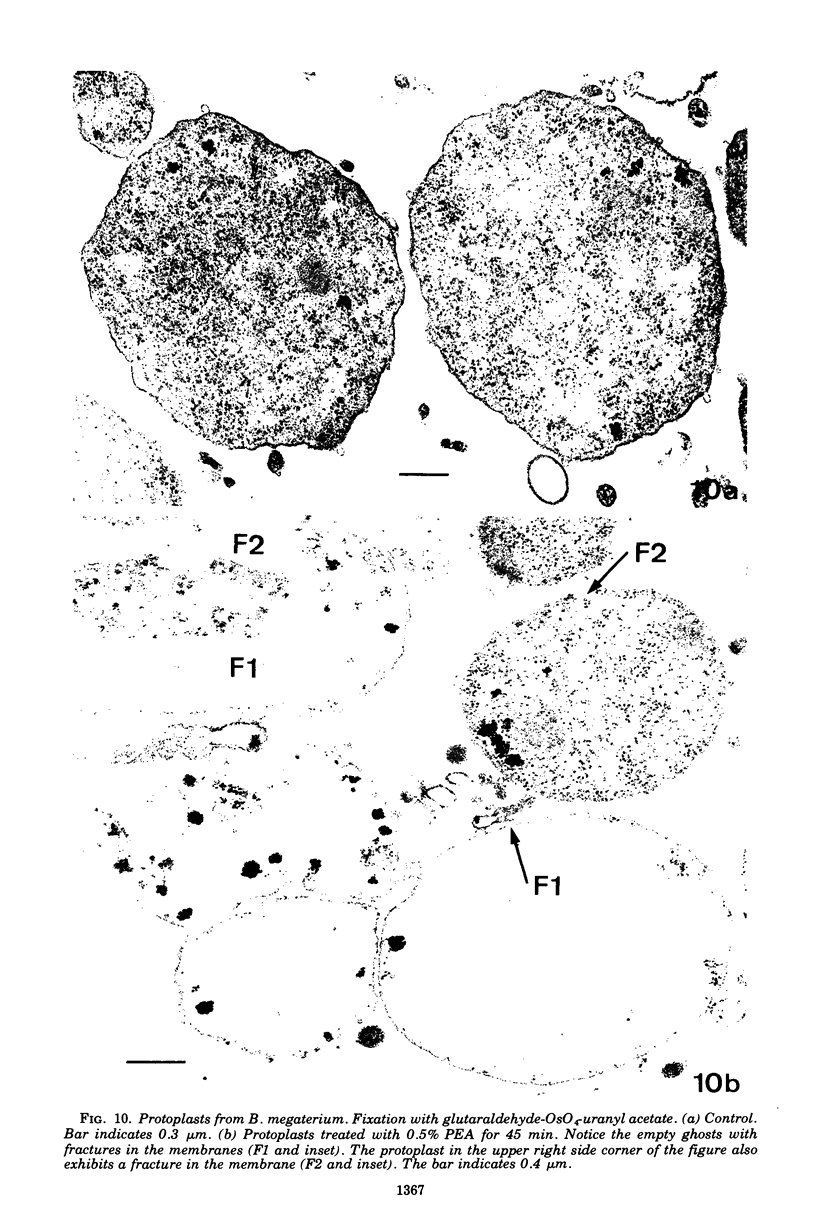
Abstract
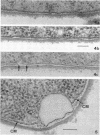
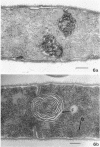
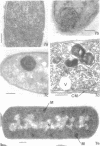
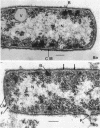
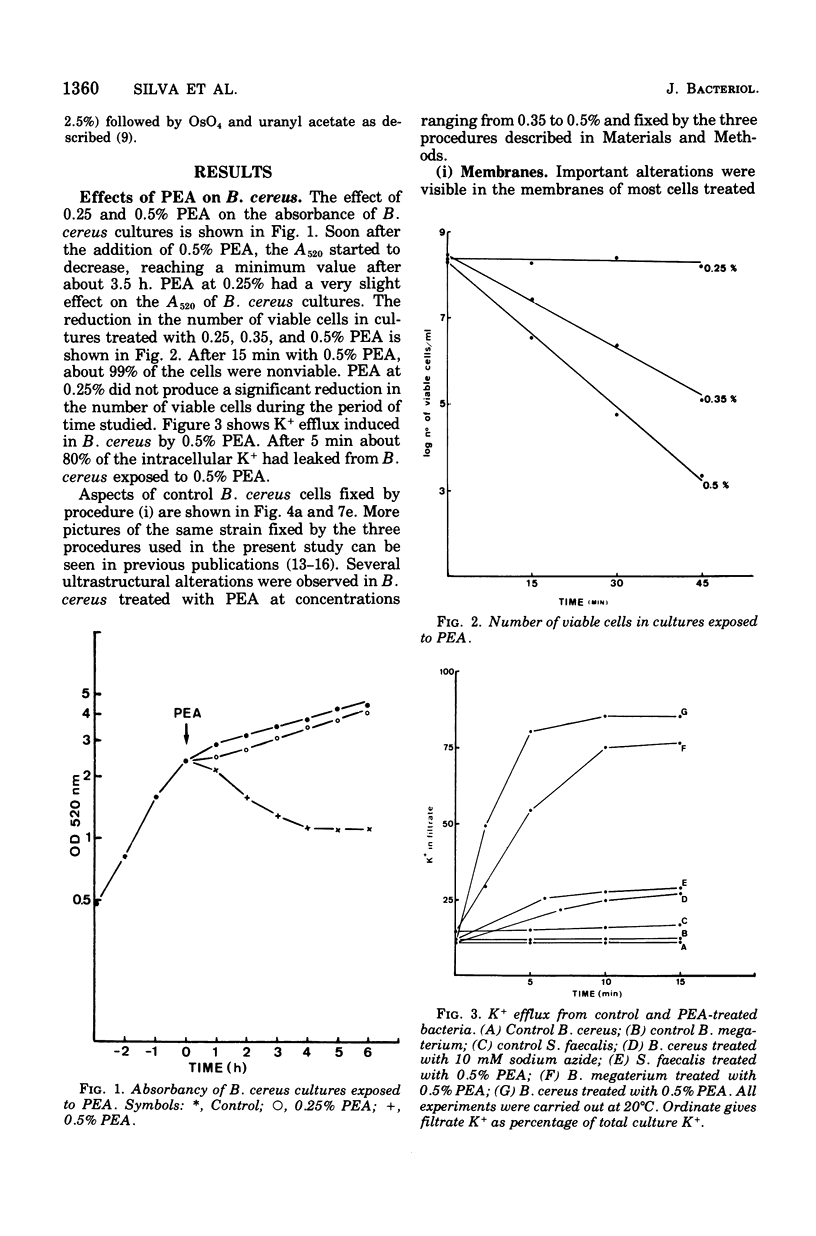
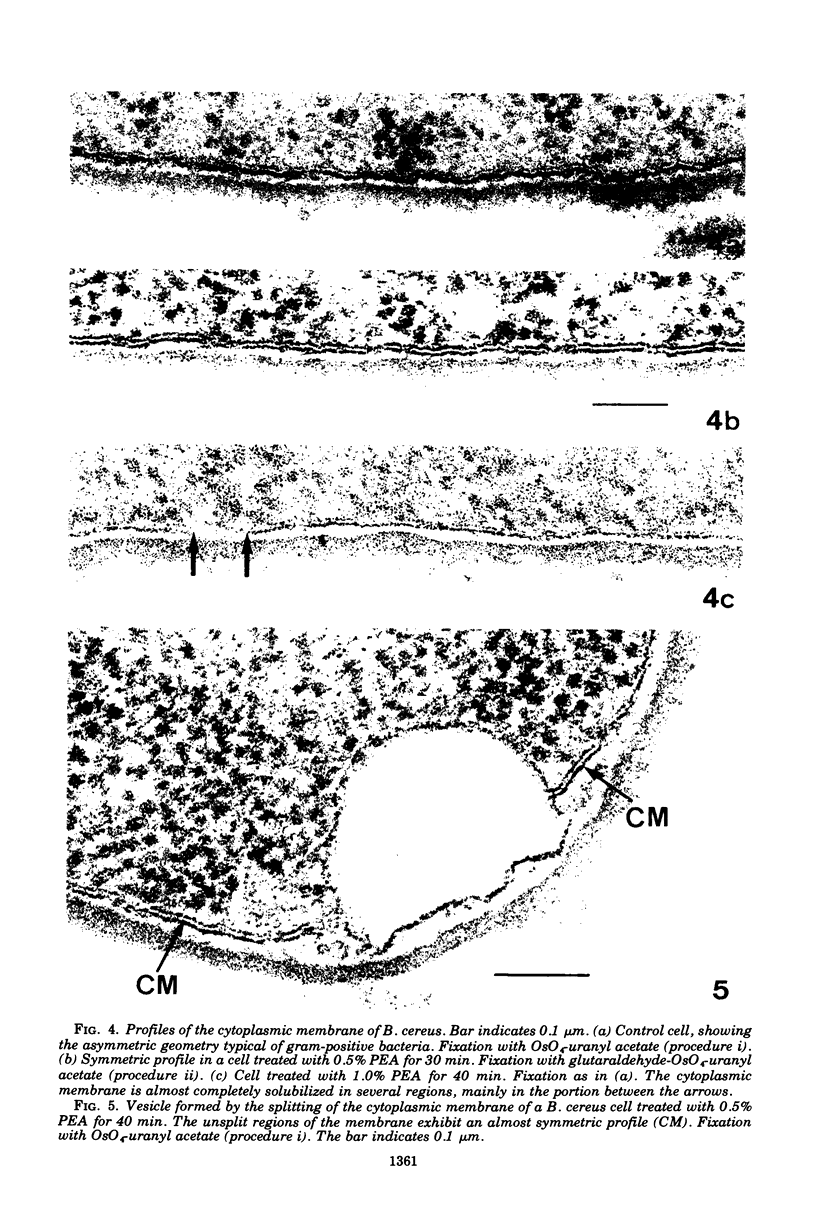
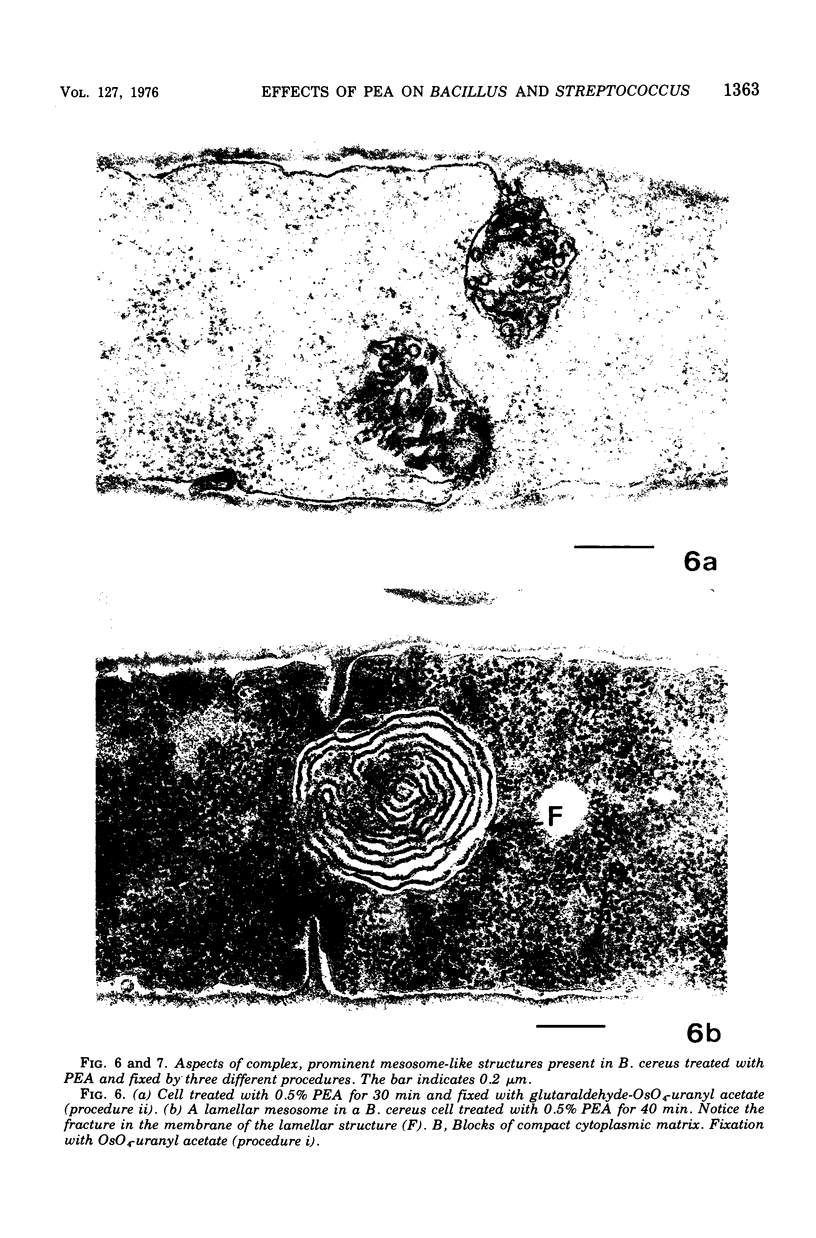
The activity of phenethyl alcohol (PEA) on Bacillus cereus, B. megaterium, and Streptococcus faecalis was studied by electron microscopy of thin sections and by the assay of intracellular K+ leakage. S. faecalis was unaffected by PEA at concentrations up to 0.5%, B. cereus was severely damaged by 0.5% PEA, and B. megaterium behaved intermediately. Important membrane ultrastructural alterations were observed in B. cereus cells treated with 0.5% PEA, namely the change in the geometry of the membrane profile from asymmetric to symmetric, the occurrence of prominent, complex mesosome-like structures, and membrane fracturing and solubilization. Protoplasts from B. megaterium were found to be quickly lysed by 0.5% PEA due to the disruption of the cytoplasmic membrane. The electron microscopic observations, together with the results of the study of the K+ efflux from B. cereus and B. megaterium, indicate that PEA primarily and directly damages the cytoplasmic membrane of sensitive bacteria. The breakdown of the permeability barrier probably is responsible for the observed bactericidal action of 0.5% PEA on B. cereus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRAH G., KONETZKA W. A. Selective and reversible inhibition of the synthesis of bacterial deoxyribonucleic acid by phenethyl alcohol. J Bacteriol. 1962 Apr;83:738–744. doi: 10.1128/jb.83.4.738-744.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B., Fernandez S. M., Sha'afi R. I. Fluidity of natural membranes and phosphatidylserine and ganglioside dispersions. Effect of local anesthetics, cholesterol and protein. Biochim Biophys Acta. 1975 Dec 16;413(3):354–370. doi: 10.1016/0005-2736(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Fitz-James P. Electron microscopy of Bacillus megaterium undergoing isolation of its nuclear bodies. J Bacteriol. 1964 May;87(5):1202–1210. doi: 10.1128/jb.87.5.1202-1210.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shaw T. J., Tillman M. C., Leach F. R. Effect of phenethyl alcohol on cell culture growth. II. Isolated cell components and lysosomal enzymes. Exp Cell Res. 1969 Jul;56(1):24–28. doi: 10.1016/0014-4827(69)90387-5. [DOI] [PubMed] [Google Scholar]
- LEACH F. R., BEST N. H., DAVIS E. M., SANDERS D. C., GIMLIN D. M. EFFECT OF PHENETHYL ALCOHOL ON CELL CULTURE GROWTH. I. CHARACTERIZATION OF THE EFFECT. Exp Cell Res. 1964 Dec;36:524–532. doi: 10.1016/0014-4827(64)90309-x. [DOI] [PubMed] [Google Scholar]
- LILLEY B. D., BREWER J. H. The selective antibacterial action of phenylethyl alcohol. J Am Pharm Assoc Am Pharm Assoc. 1953 Jan;42(1):6–8. doi: 10.1002/jps.3030420103. [DOI] [PubMed] [Google Scholar]
- Mota J. S., Silva M. T., Guerra F. C. Variations in the membranes of Streptococcus faecalis related to different cultural conditions. Arch Mikrobiol. 1972;83(4):293–302. doi: 10.1007/BF00425241. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Silva M. T. Changes induced in the ultrastructure of the cytoplasmic and intracytoplasmic membranes of several Gram-positive bacteria by variations in OsO 4 fixation. J Microsc. 1971 Jun;93(3):227–232. doi: 10.1111/j.1365-2818.1971.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Electron microscopic aspects of membrane alterations during bacterial cell lysis. Exp Cell Res. 1967 May;46(2):245–251. doi: 10.1016/0014-4827(67)90062-6. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Electron microscopic study on the effect of the oxidation of ultrathin sections of Bacillus cereus and Bacillus megaterium. J Ultrastruct Res. 1967 May;18(3):345–353. doi: 10.1016/s0022-5320(67)80123-0. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Santos Mota J. M., Melo J. V., Guerra F. C. Uranyl salts as fixatives for electron microscopy. Study of the membrane ultrastructure and phospholipid loss in bacilli. Biochim Biophys Acta. 1971 Jun 1;233(3):513–520. doi: 10.1016/0005-2736(71)90151-9. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C. Ultrastructural alterations induced by moist heat in Bacillus cereus. Appl Microbiol. 1972 Sep;24(3):463–476. doi: 10.1128/am.24.3.463-476.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J Bacteriol. 1967 Feb;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L. Effects of toluene and phenethyl alcohol on the ultrastructure of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1359–1361. doi: 10.1128/jb.114.3.1359-1361.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]