Abstract
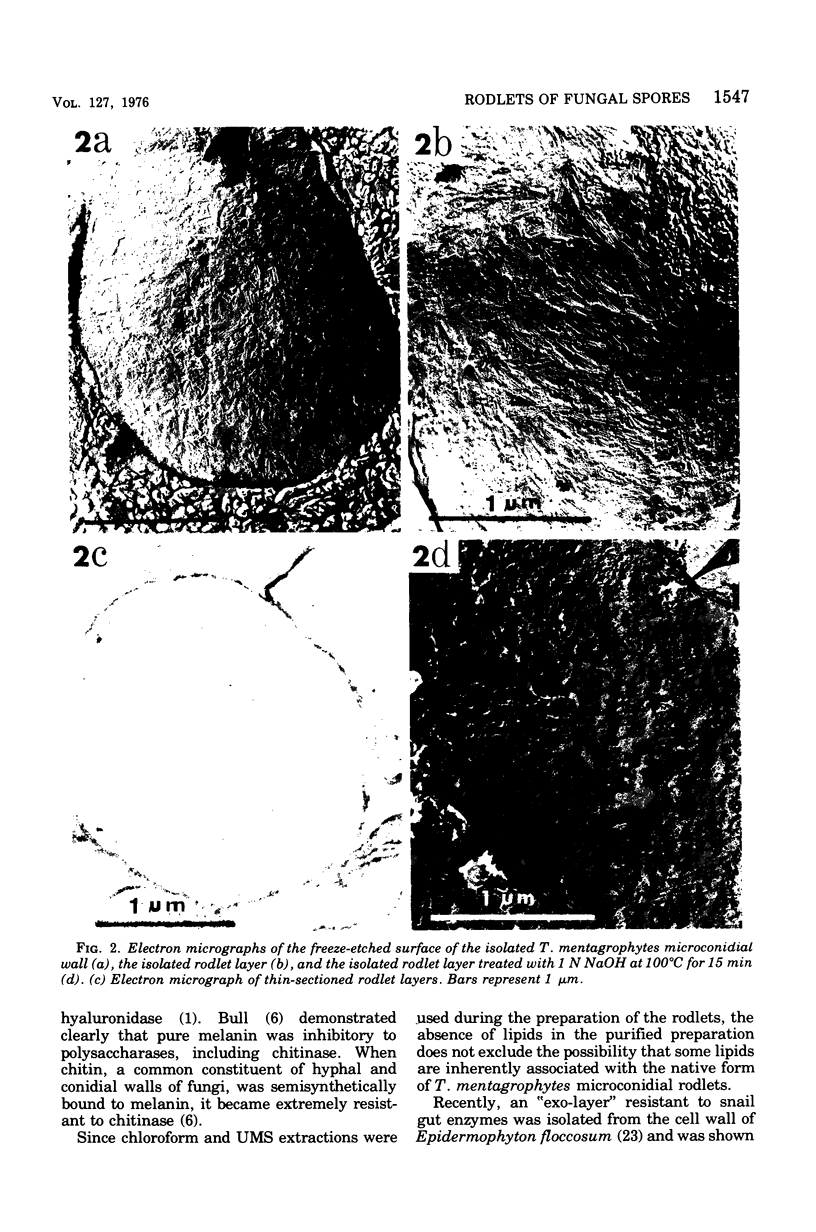
The rodlet layer of the microconidial wall of Trichophyton mentagrophytes was isolated and partially characterized. The purified microconidial walls were first extracted with urea (8M), mercaptoethanol (1%), and sodium dodecyl sulfate (1%) followed by enzymatic digestion with glusulase (snail intestinal enzymes) and purified (1 leads to 3)-beta-D-glucanase and chitinase. The purified rodlet layer was 15 to 30 nm thick and accounted for approximately 10% of the original wall weight. The pattern of rodlet patches, as revealed by electron microscopy of freeze-etched preparations of the isolated layer, was essentially the same as that observed on the intact microconidial wall. The rodlet layer was found to be resistant to most of the common organic solvents, cell wall lytic enzymes, mild acid treatments, and surface-active agents, but was solubilized in boiling 1 N NaOH with concomitant disorientation of the rodlet patterns. A melanin or melanin-like pigment appeared to be intimately associated with this rodlet layer and was solubilized during a hot-alkali treatment. Protein (80 to 85%) and glucomannan (7 to 10%) were the major components of the rodlet layer. The rodlet layer did not contain any appreciable amounts of lipid or phosphorus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., REYES E. CHEMISTRY OF SPORE WALL DIFFERENTIATION IN MUCOR ROUXII. Arch Biochem Biophys. 1964 Oct;108:125–133. doi: 10.1016/0003-9861(64)90363-7. [DOI] [PubMed] [Google Scholar]
- Bradley S. G., Ritzi D. Composition and ultrastructure of Streptomyces venezuelae. J Bacteriol. 1968 Jun;95(6):2358–2364. doi: 10.1128/jb.95.6.2358-2364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull A. T. Chemical composition of wild-type and mutant Aspergillus nidulans cell walls. The nature of polysaccharide and melanin constituents. J Gen Microbiol. 1970 Sep;63(1):75–94. doi: 10.1099/00221287-63-1-75. [DOI] [PubMed] [Google Scholar]
- Bull A. T. Inhibition of polysaccharases by melanin: enzyme inhibition in relation to mycolysis. Arch Biochem Biophys. 1970 Apr;137(2):345–356. doi: 10.1016/0003-9861(70)90448-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fisher D. J., Richmond D. V. The electrophoretic properties and some surface components of penicillium conidia. J Gen Microbiol. 1970 Dec;64(2):205–214. doi: 10.1099/00221287-64-2-205. [DOI] [PubMed] [Google Scholar]
- Florance E. R., Denison W. C., Allen T. C., Jr Ultrastructure of dormant and germinating conidia of Aspergillus nidulans. Mycologia. 1972 Jan-Feb;64(1):115–123. [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R., Pfister R. M., Remsen C. C. Fine structure and composition of the zoogloeal matrix surrounding Zoogloea ramigera. J Bacteriol. 1968 Dec;96(6):2144–2153. doi: 10.1128/jb.96.6.2144-2153.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C., Edwards M. R. Ultrastructure of Aspergillus fumigatus conidia development and maturation. Protoplasma. 1973;76(1):49–59. doi: 10.1007/BF01279672. [DOI] [PubMed] [Google Scholar]
- Griffiths D. A. Development and structure of the aleuriospores of Humicola grisea Traaen. Can J Microbiol. 1974 Jan;20(1):55–58. doi: 10.1139/m74-009. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Wu C. D., Blumenthal H. J. Characterization of L-leucine-induced germination of Trichophyton mentagrophytes microconidia. J Bacteriol. 1972 Nov;112(2):967–976. doi: 10.1128/jb.112.2.967-976.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess W. M., Sassen M. M., Remsen C. C. Surface characteristics of Penicillum conidia. Mycologia. 1968 Mar-Apr;60(2):290–303. [PubMed] [Google Scholar]
- Hess W. M., Stocks D. L. Surface characteristics of Aspergillus conidia. Mycologia. 1969 May-Jun;61(3):560–571. [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima Y., Nozawa Y. Isolation, ultrastructure and chemical composition of the outermost layer ("exo-layer") of the Epidermophyton floccosum cell wall. Biochim Biophys Acta. 1975 Jul 18;394(4):558–568. doi: 10.1016/0005-2736(75)90141-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SUSSMAN A. S., LINGAPPA Y., BERNSTEIN I. A. EFFECT OF LIGHT AND MEDIA UPON GROWTH AND MELANIN FORMATION IN CLADOSPORIUM MANSONI. Mycopathol Mycol Appl. 1963 Oct 30;20:307–314. doi: 10.1007/BF02089218. [DOI] [PubMed] [Google Scholar]
- Sassen M. M., Remsen C. C., Hess W. M. Fine structure of Penicillium megasporum conidiospores. Protoplasma. 1967;64(1):75–88. doi: 10.1007/BF01257383. [DOI] [PubMed] [Google Scholar]
- Wessels J. G., Kreger D. R., Marchant R., Regensburg B. A., De Vries O. M. Chemical and morphological characterization of the hyphal wall surface of the basidiomycete Schizophyllum commune. Biochim Biophys Acta. 1972 Jul 19;273(2):346–358. doi: 10.1016/0304-4165(72)90226-7. [DOI] [PubMed] [Google Scholar]
- Williams S. T., Bradshaw R. M., Costerton J. W., Forge A. Fine structure of the spore sheath of some Streptomyces species. J Gen Microbiol. 1972 Sep;72(2):249–258. doi: 10.1099/00221287-72-2-249. [DOI] [PubMed] [Google Scholar]




