Abstract
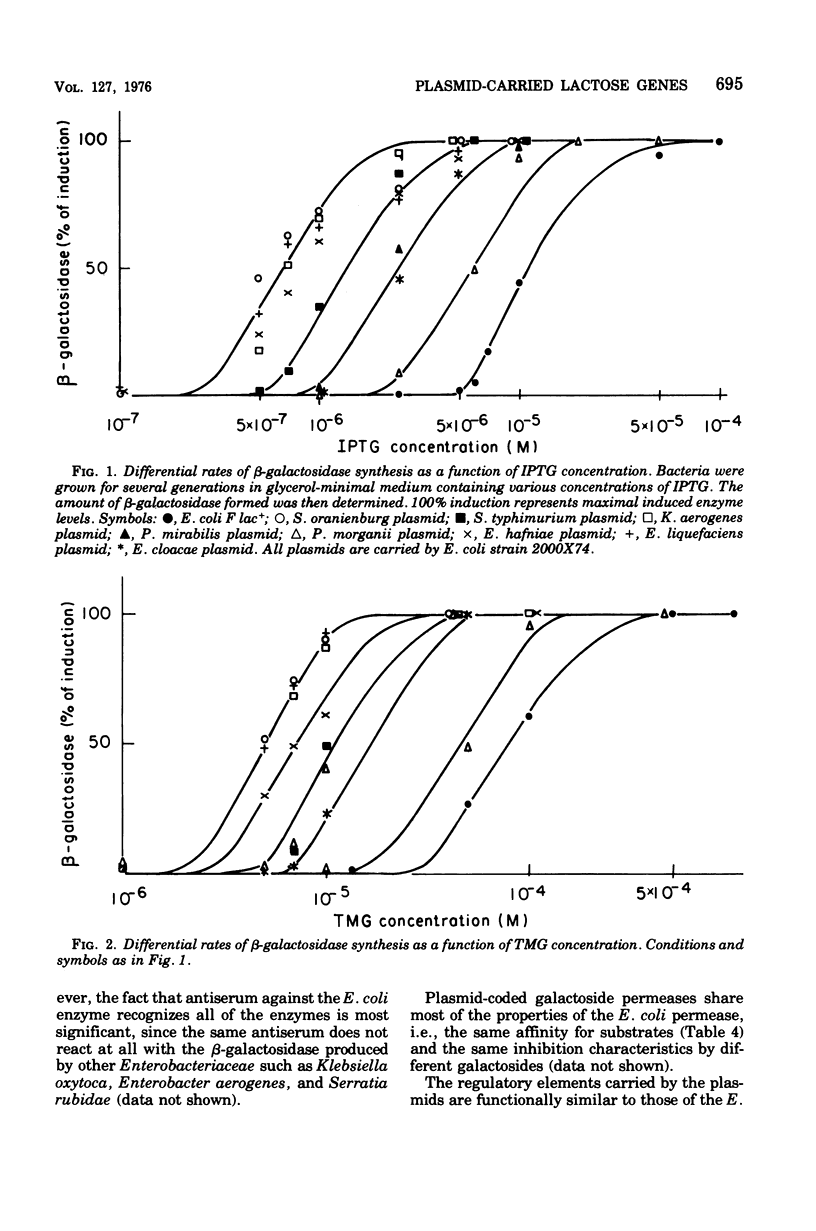
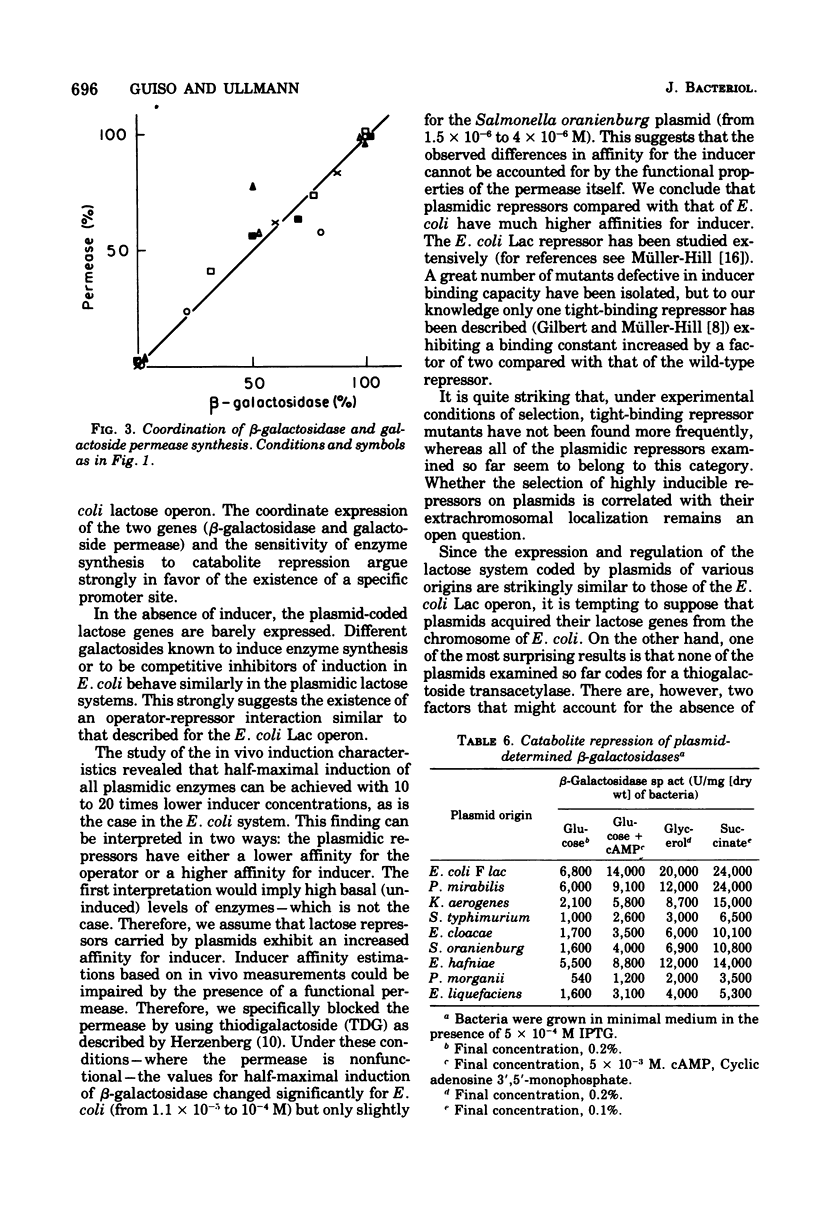
A number of plasmids carrying the lactose character have been studied. All of the plasmids examined so far code for proteins essential for lactose utilization, i.e., beta-galactosidase and galactoside permease. None of them carries enzymatically or immunologically detectable thiogalactoside transacetylase. The expression of the two enzymes is both negatively and positively controlled: they are inducible by different galactosides and are sensitive to catabolite repression. Since the plasmid-coded lactose systems have many features in common with the Escherichia coli lactose operon, it is suggested that the plasmids could have acquired the lactose genes from an E. coli chromosome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Magasanik B. Klebsiella aerogenes strain carrying drug-resistance determinants and a lac plasmid. J Bacteriol. 1972 Oct;112(1):200–205. doi: 10.1128/jb.112.1.200-205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Easterling S. B., Johnson E. M., Wohlhieter J. A., Baron L. S. Nature of lactose-fermenting Salmonella strains obtained from clinical sources. J Bacteriol. 1969 Oct;100(1):35–41. doi: 10.1128/jb.100.1.35-41.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. II. NATURE OF LAC+ PROTEUS STRAINS ISOLATED FROM CLINICAL SPECIMENS. J Bacteriol. 1964 Dec;88:1598–1601. doi: 10.1128/jb.88.6.1598-1601.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcão D. P., Trabulsi L. R., Hickman F. W., Farmer J. J., 3rd Unusual Enterobacteriaceae: lactose-positive Salmonella typhimurium which is endemic in São Paulo, Brazil. J Clin Microbiol. 1975 Oct;2(4):349–353. doi: 10.1128/jcm.2.4.349-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A. B. Lactose-fermenting Salmonella. J Bacteriol. 1966 Apr;91(4):1661–1662. doi: 10.1128/jb.91.4.1661-1662.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KUNZ L. J., EWING W. H. LABORATORY INFECTION WITH A LACTOSE-FERMENTING STRAIN OF SALMONELLA TYPHI. J Bacteriol. 1965 Jun;89:1629–1629. doi: 10.1128/jb.89.6.1629-1629.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor L., Coynault C., Pessoa G. Déterminisme plasmidique du caractère atypique "lactose positif" de souches de S. typhi-murium et de S. oranienburg isolées au Brésil lors d'épidémies de 1971 à 1973. Ann Microbiol (Paris) 1974 Apr;125A(3):261–285. [PubMed] [Google Scholar]
- Le Minor L., Coynault C., Schwartz M. Déterminisme plasmidique du caractère "lactose positif" de Serratia liquefaciens. Ann Microbiol (Paris) 1974 Oct-Nov;125B(3):357–366. [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B. Lac repressor and lac operator. Prog Biophys Mol Biol. 1975;30(2-3):227–252. doi: 10.1016/0079-6107(76)90011-0. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Braithwaite J. A. Fk-lac, an episome with unusual properties found in a wild strain of a Klebsiella species. Nature. 1970 Oct 10;228(5267):162–164. doi: 10.1038/228162a0. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Braithwaite J. A. Lac-plus plasmids are responsible for the strong lactose-positive phenotype found in many strains of Klebsiella species. Genet Res. 1973 Dec;22(3):329–333. doi: 10.1017/s0016672300013124. [DOI] [PubMed] [Google Scholar]
- SUTTER V. L., FOECKING F. J. Biochemical characteristics of lactose-fermenting Proteus rettgeri from clinical specimens. J Bacteriol. 1962 Apr;83:933–935. doi: 10.1128/jb.83.4.933-935.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierno P. M., Jr, Steinberg P. Isolation and characterization of a lactose-positive strain of Proteus morganii. J Clin Microbiol. 1975 Jan;1(1):108–109. doi: 10.1128/jcm.1.1.108-109.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOHLHIETER J. A., FALKOW S., CITARELLA R. V., BARON L. S. CHARACTERIZATION OF DNA FROM A PROTEUS STRAIN HARBORING AN EPISOME. J Mol Biol. 1964 Aug;9:576–588. doi: 10.1016/s0022-2836(64)80228-x. [DOI] [PubMed] [Google Scholar]


