Abstract
Treatment with thymidine dinucleotide (pTT) has well documented DNA-protective effects and reduces development of squamous cell carcinoma in UV-irradiated mice. The preventive effect of pTT on basal cell carcinoma (BCC) was evaluated in UV-irradiated Ptch-1+/− mice, a model of the human disease Gorlin syndrome. Topical pTT treatment significantly reduced the number and size (P < 0.001) of BCCs in murine skin after 7 months of chronic irradiation. Skin biopsies collected 24 hours after the final UV exposure showed that pTT reduced the number of nuclei positive for cyclobutane pyrimidine dimers by 40% (P < 0.0002) and for 8-hydroxy-2′-deoxyguanosine by 61% (P < 0.01 compared with vehicle control). Immunostaining with an antibody specific for mutated p53 revealed 63% fewer positive patches in BCCs of pTT-treated mice compared with controls (P < 0.01), and the number of Ki-67-positive cells was decreased by 56% (P < 0.01) in pTT-treated tumor-free epidermis and by 76% (P < 0.001) in BCC tumor nests (P < 0.001). Terminal dUTP nick-end labeling staining revealed a 213% increase (P < 0.04) in the number of apoptotic cells in BCCs of pTT-treated mice. Cox-2 immunostaining was decreased by 80% in tumor-free epidermis of pTT-treated mice compared with controls (P < 0.01). We conclude that topical pTT treatment during a prolonged period of intermittent UV exposure decreases the number and size of UV-induced BCCs through several anti-cancer mechanisms.
Basal cell carcinoma (BCC) is the most common human cancer. More than 1 million new cases are reported each year in the United States.1 Like squamous cell carcinomas (SCCs), BCCs are associated with fair skin and chronic sunlight exposure,2,3 but the relationship is more complex and studies to date have failed to show that sun protection reduces development of BCCs, in contrast to SCCs.1 Moreover, BCCs and SCCs differ in their responsiveness to certain chemopreventive agents. For example, BCC development is inhibited strongly by topical retinoids but not by oral nonsteroidal anti-inflammatory drugs (NSAIDs) or green tea.4,5 By contrast, murine SCC photocarcinogenesis is inhibited poorly by topical retinoids and quite well by oral NSAIDs or green tea.4,6 These differing effects of UV radiation and chemopreventive agents on BCC versus SCC, in combination with the large socioeconomic burden of BCCs,7 stimulated us to test thymidine dinucleotide (pTT), an agent already demonstrated to prevent SCCs in UV-irradiated mice8 for its ability to reduce BCC development.
Studies during the past decade have tied BCC tumorigenesis quite firmly to activated hedgehog signaling, a pathway most likely not aberrant in SCCs.9,10 This uncovering of aberrant HH signaling as the pivotal molecular defect in BCCs has allowed the construction of Ptch-1+/− mice.9,10,11 Like humans with a germline mutation in the Ptch gene (Gorlin syndrome, OMIM no. 109400), these mice develop cutaneous BCCs after mutagenic environmental insults such as UV or ionizing radiation and are the first practical model of BCC carcinogenesis.
Gorlin syndrome, also termed basal cell nevus syndrome, is a dominantly inherited human disorder characterized by various developmental abnormalities and large numbers of BCCs.12 Mutation in the Ptch gene also characterizes sporadic BCCs.13 Ptch-1 heterozygote knockout (Ptch-1+/−) mice develop multiple BCCs after ionizing radiation or multiple UV exposures because of mutation of the remaining intact Ptch-1 allele in irradiated keratinocytes.9,14 Numerous microscopic BCCs develop within 6 months of chronic UV irradiation, progressing to visible tumors after 9 to 12 months.5,9 Cyclobutane pyrimidine dimers (CPDs) are the predominant photoproducts generated after UV exposure and are removed by the nucleotide excision repair pathway within several days.15 UV exposure also induces oxidative DNA damage, manifest principally as 8-hydroxy-2′-deoxyguanosine (8-oxo-dG) formation that is also linked to UV-induced gene mutations and carcinogenesis.16 p53 activation after UV irradiation protects genomic integrity through multiple effects including increased DNA repair capacity, transient cell cycle arrest to allow more time for DNA repair, and with severe damage programmed cell death or apoptosis.17 Photoproducts that are not removed efficiently may lead to mutations in key tumor suppressor genes including p53 and Ptch.18,19
Protection against photocarcinogenesis is multifaceted and appears in part to result from DNA damage recognition at telomeres.20 Telomeres consist of several thousand bp of TTAGGG and its complement at the end of each chromosome that form a large loop structure21 anchored by a single-stranded 100- to 400-base 3′ overhang and secured by a specific binding protein TRF-2.22 de Lange’s group23 has shown that disruption of the telomere loop leads to exposure of the 3′ overhang and DNA damage responses in part through activation of ATM and its effector protein p53. Our group has shown that 2- to 20-base oligonucleotides homologous to the 3′ telomere overhang, termed T-oligos, induce very similar DNA damage responses through the same signaling pathway but without telomeric disruption and overhang loss.24,25,26 Moreover, both in vitro and in vivo, T-oligos lead to apoptosis and/or senescence of malignant cells, while inducing only protective responses in normal cells.25,26
Complete telomere homologs have not been used for topical application because of anticipated poor percutaneous penetration. However, topical application of thymine dinucleotide (pTT), one third of the mammalian telomere repeat sequence TTAGGG,20 reduces UV-induced mutations and photocarcinogenesis in hairless mice via p53 activation, transient cell cycle arrest, and increased DNA repair capacity.8 In human or rodent skin with epidermal melanocytes, pTT also stimulates protective melanogenesis.28,29 Additionally, inhibition of cyclooxygenese-2 (Cox-2), the proinflammatory enzyme whose overexpression has been linked to photocarcinogenesis, reduces skin cancer development in UV-irradiated mice.6 We have shown that pTT treatment of cells in vitro, of mouse skin in vivo, or of human skin ex vivo decreases the constitutive and UV-induced levels of Cox-2 through p53 activation, known to inhibit Cox-2 expression,30 by reducing NF-κB-dependent Cox-2 transcription.31 These and other effects of T-oligos may be broadly construed as cancer preventative.
In this study we evaluate the effect of topical pTT treatment on BCC development in chronically irradiated Ptch-1+/− mice. We find that these telomere homolog oligonucleotides are as effective in prevention of BCC as in prevention of SCCs.
Materials and Methods
Ptch-1+/− Mice
Ptch-1+/− mice generation, maintenance on a mixed C57BL/6 DBA/2 background, and housing were as described.9 Mice were housed at 70°F with 50% humidity and shaved with electrical clippers when needed for topical applications or UV exposure.
UV Exposure
A UV irradiation unit (Daavlin Corp., Bryan, OH) that emits 75 to 80% UVB (290 to 320 nm) and 28% UVA (320 to 380 nm)32 was used with a filter through Kodacel plastic sheeting to remove the 280 nm (UVC) light. Mice received a 3 minimal erythemal dose, previously predetermined for these mice, three times per week from age 2 to 9 months in a protocol previously shown to result in numerous BCCs.9
T-Oligo Treatment
pTT was purchased from the Midland Certified Reagent Company (Midland, TX) and stored at −20°C as a 2 mmol/L stock solution for applications. It was diluted to 100 μmol/L in propylene glycol 75%:dimethyl sulfoxide 25%, previously shown to deliver a biologically active dose of pTT to mouse and guinea pig skin.8,29
Study Design
Beginning at age 60 days, mice were treated topically with pTT or vehicle alone 5 days per week and UV irradiated three times per week for 7 months. Twenty-four hours after the last UV exposure, a piece of skin 1 × 1.5 cm was excised from the UV-irradiated back area and sliced into five strips each 1 cm in length and 3 to 4 mm thick. All five strips from the same animal were embedded in one cassette (Supplemental Figure S1, see http://ajp.amjpathol.org). Sections 5 to 6 μm thick were cut from each strip and placed on a single slide. To evaluate the effect of pTT treatment on DNA repair after a single UV exposure, another set of Ptch-1+/− mice never treated or irradiated before were treated topically with pTT or vehicle alone daily excluding the weekend (seven applications; week 1, Monday through Friday once a day; then week 2, Monday and Tuesday once a day), then irradiated with a single dose of UV 24 hours after the last application. Punch biopsies were taken from UV-irradiated and sham-irradiated areas 0, 48, and 72 hours after UV exposure.
Histology and Immunohistology
Skin samples were paraffin-embedded after fixation in 10% formalin and processed for β-galactosidase staining as described to highlight blue cells lacking both Ptch alleles (BCCs).9 Cross-sections of all five strips from each mice were placed on the same slide and processed for hematoxylin and eosin (H&E) staining. The number and size of BCCs were evaluated by a blinded investigator using computer-assisted image analysis (Image J NIH software; National Institutes of Health, Bethesda, MD).28,31 Adjacent sections were processed for immunohistology. After dewaxing and dehydration, antigen unmasking was done by boiling the slides in citrate buffer, pH = 6, for 20 minutes. Slides were then blocked with 10% goat serum for 20 minutes, and incubated with primary antibodies against CPDs (1/100; MBL, Nagoya, Japan), 8-oxo-dG antibody (1/100; Trevigen, Inc., Gaithersburg, MD), p53 Ab-240 mouse monoclonal antibody (1/50; Visionbiosystems Inc., Norwell, MA), Ki-67 rabbit polyclonal antibody (1/100; Vector Laboratories, Burlingame, CA), and Cox-2 mouse monoclonal antibody (1/50; BD Biosciences, San Jose, CA) overnight. Next day the slides were incubated with secondary goat anti-mouse antibody and followed by the use of an AEC detection kit (Labvision Corp., Fremont CA). Terminal dUTP nick-end labeling (TUNEL) staining was performed using the Apoptag Kit (Chemicon International, Temecula, CA) and tetramethyl-rhodamine isothiocyanate-labeled goat anti-rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used as secondary antibody for Ki-67 immunostaining.
Statistical Analysis
To evaluate the differences in the number and cross-sectional area of tumors, and number of positive cells after immunostaining, and percent Cox-2(+) epidermal area, we used analysis of variance Fisher protected least significant difference and unpaired t-test (StatView program; SAS Institute, Cary, NC). Differences were considered significant when P < 0.05.
Results
pTT Treatment Decreases the Number and Area Occupied by BCC Nests
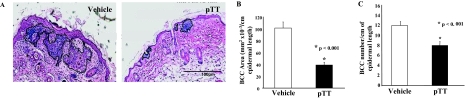
Computer-assisted (Image J, NIH software) measurements revealed decreases of 57% and 35% (P < 0.001 for both) in total BCC cross-sectional area and number of microscopic BCCs per cm of epidermal length, respectively, in pTT- versus vehicle-treated chronically irradiated mice after 7 months (Figure 1).
Figure 1.
pTT treatment decreases the number of BCCs and cross-sectional area occupied by BCCs after chronic UV irradiation. A: Representative images of H&E-stained biopsies obtained 24 hours after the last UV irradiation. BCCs are outlined by solid lines and are darker blue than normal epidermis because of β-galactosidase expression in cells lacking a normal Ptch-1 allele (see Material and Methods). B: Graphic representation of computer-assisted measurement of tumor area per cm of epidermal length in five sections each of 9 vehicle-treated and 11 pTT-treated mice. C: Graphic representation of the number of tumors per cm of epidermal length.
pTT Treatment Accelerates Removal of Photoproducts in Epidermis
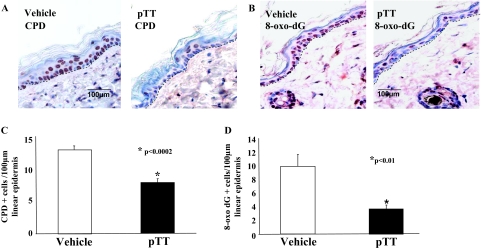
To determine the effect of pTT on DNA repair, skin samples were processed for CPD immunostaining (Figure 2A). Compared to vehicle, pTT treatment reduced CPD(+) cells per 100 μm epidermal length by 40% (vehicle, 13.8 ± 1.3 versus pTT 8.4 ± 1.3; P < 0.002) (Figure 2C), confirming earlier results in acutely UV-irradiated murine8 and human skin28 and indicating that pTT treatment persistently reduces the number of cells with a detectable level of CPDs, even after 7 months of chronic UV exposure. In addition, 8-oxo-dG immunostaining was used to detect UV-induced oxidative DNA damage repair (Figure 2B). The number of 8-oxo-dG(+) cells per 100 μm was reduced 61% in pTT- versus vehicle-treated mice (vehicle, 9.95 ± 2.9 versus 3.8 ± 0.9; P < 0.01) (Figure 2D).
Figure 2.
pTT treatment enhances removal of photoproducts after chronic UV irradiation. A: CPD(+) nuclei, stained red-brown, were reduced in pTT- versus vehicle-treated skin 24 hours after the last UV irradiation in mice intermittently treated with pTT and UV irradiated for 7 months. B: Adjacent sections were processed for 8-oxo-dG immunostaining (red-brownish nuclear staining). CPD(+) cells (C) and 8-oxo-dG(+) (D) cells were quantified in 5 to 10 randomly selected areas 100 μm in epidermal length in eight mice per group treated with pTT or vehicle alone.
To determine the effect of pTT treatment on DNA repair after a single UV exposure in Ptch-1+/− mice, we treated previously unirradiated, untreated mice topically with pTT or vehicle alone daily for 7 of 9 days, omitting the weekend, and then evaluated CPD immunostaining 0, 48, and 72 hours after a single 3 minimal erythemal dose UV exposure (Supplemental Figure S2A, see http://ajp.amjpathol.org). The effect of pTT on the removal of DNA photoproducts was quantified by adjusting the number of CPD(+) cells immediately after UV to 100% and each subsequent time point was calculated as a percentage of initial (time 0) value (Supplemental Figure S2B, see http://ajp.amjpathol.org). The number of the CPD(+) cells per 100 μm epidermal length in pTT-treated mice compared to vehicle-treated mice was reduced 63.5% and 60% after 48 hours and 72 hours, respectively: 48 hours: pTT 30 ± 14 (P < 0.005) versus vehicle 82 ± 16; and 72 hours: pTT 15 ± 12 versus vehicle 36.7 ± 18 (P < 0.01) (Supplemental Figure S2B, see http://ajp.amjpathol.org). These data establish that pTT accelerates DNA repair in mice of a second genetic background, not just in wild-type and Xpc+/− hairless mice,8 and that the degree of acceleration is similar after either acute or chronic UV exposure.
pTT Treatment Reduces p53 Mutations
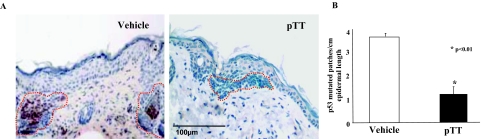
To evaluate the status of p53 in these chronically irradiated mice, we stained cross-sections of skin samples with an antibody (Ab-240) that specifically recognizes mutated p53 (m-p53). The m-p53(+) cells were detected predominantly within BCCs (Figure 3A) as patches of red-brown cells, presumably representing clones derived from a single mutated cell with an acquired survival advantage over neighboring cells.33 The number of m-p53(+) clones per cm of epidermal length was reduced by 63% in pTT versus vehicle-treated mice: 3.8 ± 0.2 versus 1.4 ± 0.6, P < 0.01 (Figure 3B). This finding is consistent with the previously reported ability of pTT to decrease mutation rate in a LacZ mutation-indicator gene in both acutely and chronically irradiated hairless mice.8
Figure 3.
pTT treatment decreases p53 mutation. A: Mutated m-p53(+) cells, stained red-brown, were detected predominantly in BCC nests (outlined by dotted red lines). More m-p53(+) cells were detected in BCC arising in vehicle- versus pTT-treated skin. B: Mutated p53(+) patches were quantified per cm epidermal length of five sections each for three mice per treatment group.
pTT Treatment Inhibits Epidermal and Tumor Proliferation
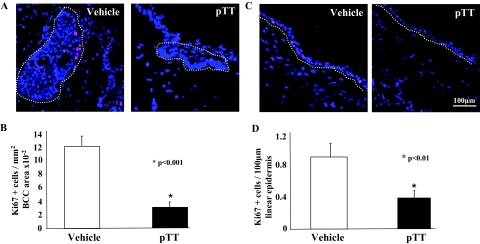
To examine the effect of chronic pTT treatment on cellular proliferation, we used Ki-67 immunostaining (Figure 4, A and B). pTT treatment reduced Ki-67(+) presumptively proliferating cells by 76% per unit cross-sectional area in BCCs (vehicle 11.9 ± 1.9 versus pTT 2.9 ± 1.2 per mm2 × 10−2; P < 0.001) and by 56% in the nontumor-bearing epidermis (vehicle 0.9 ± 0.4 versus pTT 0.4 ± 0.2, per 100 μm epidermal length; P < 0.01) (Figure 4, C and D).
Figure 4.
pTT treatment inhibits epidermal and tumor proliferation. Ki-67 antibody was used to detect proliferative cells (red nuclear staining). Nuclei were stained blue with DAPI. More Ki-67(+) cells were detected in skin of vehicle-treated versus pTT-treated mice in tumor nests (A, outlined by a dotted line) and per unit epidermal length (C, dermo-epidermal junction is indicated by dotted line), as calculated for five randomly selected sections of eight mice per treatment group. B and D: Ki-67(+) cells were counted in BCC nests (B) and uninvolved epidermis (D) in 8 to 10 randomly selected areas of 100 μm epidermal length in five sections each of eight mice per treatment group.
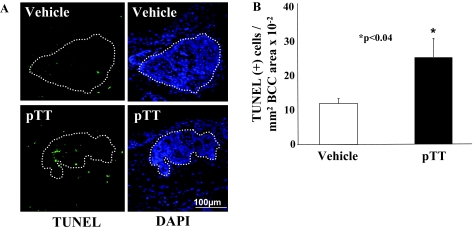
pTT Treatment Induces Apoptosis in BCC Nests
TUNEL(+) cells were infrequent in tumor-free epidermis (Figure 5) with a slight but insignificant increase in pTT- versus vehicle-treated skin (1.0 ± 0.8 versus 0.7 ± 0.4 TUNEL(+) cells/100 μm epidermal length, data not shown). Interestingly there was a 213% increase in TUNEL(+) cells within BCCs per mm2 tumor area in pTT- versus vehicle-treated mice at the single time point examined, 24 hours after the last UV exposure: vehicle 12.7 ± 2.6 versus pTT 27.1 ± 5.6 per mm2 × 10−2 (P < 0.04) (Figure 5, A and B). These data suggest that pTT preferentially promotes apoptosis of malignant cells in skin.
Figure 5.
pTT treatment induces apoptosis in BCCs in chronically irradiated Ptch-1+/− mice. A: TUNEL(+) cells (green) were detected in BCCs (outlined by dotted lines). Nuclei are stained blue (DAPI). B: The number of TUNEL(+) cells were quantified in tumor areas of five sections in eight mice treated either with pTT or vehicle.
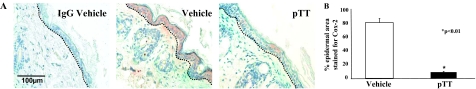
pTT Treatment Inhibits Cox-2 Expression in the Epidermis
pTT treatment markedly decreased Cox-2 protein level, as determined by immunostaining in chronically irradiated skin (Figure 6A). Computer-assisted image analysis revealed an 80% decrease (P < 0.01) in epidermal Cox-2 expression in pTT- versus vehicle-treated mice (Figure 6B). This extends the earlier finding that pTT decreases Cox-2 transcription and protein expression acutely, after a single UV exposure of murine or human skin.31
Figure 6.
pTT treatment inhibits Cox-2 expression in chronically irradiated epidermis. A: Adjacent sections were processed for Cox-2 immunostaining that appears as brown-red cytoplasmic staining in the epidermis. Cox-2 expression was strikingly decreased in the epidermis of pTT-treated versus control mice. There was no staining in control IgG-stained sections lacking the primary antibody. B: Computer-assisted image analysis (Image J NIH software) was used to quantify Cox-2 expression in 8 to 10 randomly selected areas in the epidermis of three mice per treatment group.
Discussion
UV-induced mutation of tumor suppressor genes is a well known contributing factor in photocarcinogenesis generally, and in BCC development specifically, with the majority of these mutations resulting from unrepaired photoproducts.34 Activation of the sonic hedgehog pathway because of Ptch mutation, often with activation of other oncogenes, leads to keratinocyte proliferation and BCC development.9,13
The incidence of BCCs, like that of most malignancies, increases exponentially with age,35 very likely attributable in part to an age-associated decrease in DNA repair capacity.36 Reduction in DNA repair capacity appears to increase the risk of BCC development specifically because young individuals with a history of BCC have lower DNA repair capacity than age-matched controls.36 Our group has previously shown that pTT treatment corrects the age-associated decline in DNA repair capacity37 at least in part through p53 activation, known to play a key role in DNA damage repair, with up-regulation of multiple DNA repair proteins, some but not all of which are induced by p53.38,39 Within 24 to 48 hours pTT treatment enhances DNA repair and induces other photoprotective responses in cultured cells, hairless mice, and human skin explants.8,28,40
In this study we showed that pTT treatment decreases the number of epidermal cells with a detectable level of DNA damage, CPDs by 40% and of 8-oxo-dG by 61% as assessed 24 hours after the last UVB irradiation in a chronic UV irradiation protocol. Because photoproduct removal kinetics after chronic UV irradiation may be influenced by several interrelated biological processes such as enhanced DNA repair capacity, increased apoptosis, epidermal growth arrest versus hyperproliferation, as well as by mutations in one or several genes, we also examined DNA repair after a single UV exposure in Ptch-1+/− mice. Topical pTT application led to an ∼60% decrease in the number of cells with a detectable level of photoproducts 48 and 72 hours after a single UV irradiation, as measured by positive nuclear immunostaining for photoproducts, an indirect measure of DNA repair rate confirming our previous findings of enhanced repair rate in irradiated Xpc+/− mice.8
Our group has reported previously that T-oligos increase anti-oxidative defense through up-regulation of mRNA and protein levels for Cu/Zn- and Mn-dependent superoxide dismutases in cultured epidermal cells.41 We have also noted in preliminary studies (unpublished data) that cultured cells show accelerated base excision repair, the presumptive major mechanism for removal of oxidative DNA damage.42 In this article we demonstrate that 8-hydroxy-2′-diguanosine (8-oxo-dG), known to be generated by UV-induced oxidative DNA damage,43 was reduced significantly in pTT- versus vehicle-treated UV-irradiated murine skin. This suggests that in vivo pTT not only up-regulates nucleotide excision repair,8,28,37 but may also up-regulate base excision repair and/or reduce reactive oxygen species by increasing superoxide dismutase levels.
p53 mutation is an early event in skin carcinogenesis and occurs in 40 to 60% of BCCs.18,44 Mutated p53 correlates positively with tumor hyperproliferation and negatively with apoptosis.45 Moreover, the interactions between p53 and activation of sonic hedgehog pathway have been implicated directly in initiation and development of BCC.46 Because of the central role of p53 in DNA damage responses, p53 mutations may lead to genomic instability, chromosomal deletions or aberrations,47 and tumor progression. Indeed, both BCC and SCC are reported to have losses of chromosomal fragments, that are very specific for BCC (restricted to chromosome arm 9q, site of the ptch gene) and diverse for SCC (chromosomes 3, 9, 13, 17).48
Tumor progression, including BCC development, usually depends on inappropriate cell proliferation, impaired apoptosis and cell senescence, and some degree of dedifferentiation.7,49 Our data show a significant decrease in Ki-67(+) presumptively proliferative cells in pTT- versus vehicle-treated BCC nests, as well as an increase in the number of TUNEL(+) cells, consistent with anti-proliferative and proapoptotic effects of T-oligos that we have previously reported for several malignant cell lines.20,26,27,50
UV exposure induces Cox-2 expression in murine skin49 and Cox-2 is known to be anti-apoptotic and proangiogenetic in BCCs and other tumors.51 Oral administration of cyclooxygenase inhibitors (coxibs) reduces development of SCCs in hairless mice.4,6 Possibly the profound decrease in epidermal Cox-2 protein level by topical pTT application has a greater effect on Cox-2 activity in the skin than does oral administration of a coxib at tolerable doses.
Several agents have been examined for their ability to prevent BCC. They include topical retinoids such as tazarotene, ornithine decarboxylase, cyclopamine, and a synthetic Smo antagonist, CUR61414.5,7,32 It has also been suggested that cholesterol reduction using statins may decrease signaling through Shh pathway.7 The ability of T-oligos to act via multiple relevant signaling pathways, as well as to decrease causative UV mutations, makes pTT an attractive addition to this list.
Acknowledgments
We thank Yefim Khaimsky for providing excellent technical assistance.
Footnotes
Address reprint requests to David A. Goukassian, M.D., Ph.D., or Barbara A Gilchrest, M.D., Department of Dermatology, Boston University School of Medicine, 609 Albany St., Boston, MA 02118. E-mail: dgoukass@bu.edu and bgilchre@bu.edu.
Supported by the National Institutes of Health (grants CA 10515 to B.A.G., and AR 050440 and CA 109584 to E.H.E.).
S.A. and E.Z. contributed equally to this study.
Research involving animal experiments, including intermittent topical T-oligo applications, UV-irradiations, as well as tissue harvesting and embedding, was performed at Children’s Hospital of Oakland Research Institute, Oakland, CA.
Research involving processing tissue for evaluation of microscopic BCCs, routine histology, immunofluorostaining image, and statistical analyses, and manuscript preparation were performed at the Department of Dermatology, Boston University School of Medicine, Boston, MA.
Conflict of Interest: Aspects of this work are patent-protected and licensed to SemaCo, Inc., a for-profit company in which B.A.G. is a shareholder and D.A.G. has served as a consultant.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current Address of E.Z.: Clinica Dermatologica di Padova, Padova, Italy.
References
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- MacKie RM. Long-term health risk to the skin of ultraviolet radiation. Prog Biophys Mol Biol. 2006;92:92–96. doi: 10.1016/j.pbiomolbio.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ramos J, Villa J, Ruiz A, Armstrong R, Matta J. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:2006–2011. [PubMed] [Google Scholar]
- An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- So PL, Lee K, Hebert J, Walker P, Lu Y, Hwang J, Kopelovich L, Athar M, Bickers D, Aszterbaum M, Epstein EH., Jr Topical tazarotene chemoprevention reduces basal cell carcinoma number and size in Ptch1+/− mice exposed to ultraviolet or ionizing radiation. Cancer Res. 2004;64:4385–4389. doi: 10.1158/0008-5472.CAN-03-1927. [DOI] [PubMed] [Google Scholar]
- Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- Tang JY, So PL, Epstein EH., Jr Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol Appl Pharmacol. 2007;224:257–264. doi: 10.1016/j.taap.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goukassian DA, Helms E, van Steeg H, van Oostrom C, Bhawan J, Gilchrest BA. Topical DNA oligonucleotide therapy reduces UV-induced mutations and photocarcinogenesis in hairless mice. Proc Natl Acad Sci USA. 2004;101:3933–3938. doi: 10.1073/pnas.0306389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ. Nevoid basal-cell carcinoma syndrome. Medicine (Baltimore) 1987;66:98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- Reifenberger J, Wolter M, Knobbe CB, Kohler B, Schonicke A, Scharwachter C, Kumar K, Blaschke B, Ruzicka T, Reifenberger G. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pazzaglia S, Tanori M, Hahn H, Merola P, Rebessi S, Atkinson MJ, Di Majo V, Covelli V, Saran A. Basal cell carcinoma and its development: insights from radiation-induced tumors in Ptch1-deficient mice. Cancer Res. 2004;64:934–941. doi: 10.1158/0008-5472.can-03-2460. [DOI] [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994;266:1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Kunisada M, Sakumi K, Tominaga Y, Budiyanto A, Ueda M, Ichihashi M, Nakabeppu Y, Nishigori C. 8-Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res. 2005;65:6006–6010. doi: 10.1158/0008-5472.CAN-05-0724. [DOI] [PubMed] [Google Scholar]
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Berg RJ, van Kranen HJ, Rebel HG, de Vries A, van Vloten WA, Van Kreijl CF, van der Leun JC, de Gruijl FR. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc Natl Acad Sci USA. 1996;93:274–278. doi: 10.1073/pnas.93.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D, Peacocke M, Zhang H, Ping XL, Tsou HC. UV-specific p53 and PTCH mutations in sporadic basal cell carcinoma of sun-exposed skin. J Am Acad Dermatol. 2001;44:293–297. doi: 10.1067/mjd.2001.112361. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS. The tale of the telomere: implications for prevention and treatment of skin cancers. J Invest Dermatol Symp Proc. 2005;10:124–130. doi: 10.1111/j.1087-0024.2005.200406.x. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- Li GZ, Eller MS, Firoozabadi R, Gilchrest BA. Evidence that exposure of the telomere 3′ overhang sequence induces senescence. Proc Natl Acad Sci USA. 2003;100:527–531. doi: 10.1073/pnas.0235444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N, Yaar M, Eller MS, Truzzi F, Gilchrest BA. Features that determine telomere homolog oligonucleotide-induced therapeutic DNA damage-like responses in cancer cells. J Cell Physiol. 2007;210:582–595. doi: 10.1002/jcp.20848. [DOI] [PubMed] [Google Scholar]
- Yaar M, Eller MS, Panova I, Kubera J, Wee LH, Cowan KH, Gilchrest BA. Telomeric DNA induces apoptosis and senescence of human breast carcinoma cells. Breast Cancer Res. 2007;9:R13. doi: 10.1186/bcr1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N, Eller MS, Byers HR, Dykstra S, Kubera J, Gilchrest BA. Telomere-based DNA damage responses: a new approach to melanoma. FASEB J. 2004;18:1373–1381. doi: 10.1096/fj.04-1774com. [DOI] [PubMed] [Google Scholar]
- Arad S, Konnikov N, Goukassian DA, Gilchrest BA. T-oligos augment UV-induced protective responses in human skin. FASEB J. 2006;20:1895–1897. doi: 10.1096/fj.06-5964fje. [DOI] [PubMed] [Google Scholar]
- Eller MS, Yaar M, Gilchrest BA. DNA damage and melanogenesis. Nature. 1994;372:413–414. doi: 10.1038/372413a0. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ. Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem. 1999;274:10911–10915. doi: 10.1074/jbc.274.16.10911. [DOI] [PubMed] [Google Scholar]
- Marwaha V, Chen YH, Helms E, Arad S, Inoue H, Bord E, Kishore R, Sarkissian RD, Gilchrest BA, Goukassian DA. T-oligo treatment decreases constitutive and UVB-induced COX-2 levels through p53- and NFkappaB-dependent repression of the COX-2 promoter. J Biol Chem. 2005;280:32379–32388. doi: 10.1074/jbc.M503245200. [DOI] [PubMed] [Google Scholar]
- Athar M, Li C, Tang X, Chi S, Zhang X, Kim AL, Tyring SK, Kopelovich L, Hebert J, Epstein EH, Jr, Bickers DR, Xie J. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker T, Siller G, Saunders N. Molecular and cellular biology of basal cell carcinoma. Australas J Dermatol. 2002;43:241–246. doi: 10.1046/j.1440-0960.2002.00609.x. [DOI] [PubMed] [Google Scholar]
- Scotto J, Fears T, Fraumeni J. Bethesda: National Institutes of Health,; Incidence of non-melanoma skin cancer in the United States. 1983:pp 43–45. Publication 83-2433. [Google Scholar]
- Wei Z, Mantanoski GM, Farmer ER, Hedayati MA, Grossman L. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA. 1993;90:1614–1618. doi: 10.1073/pnas.90.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goukassian DA, Bagheri S, el-Keeb L, Eller MS, Gilchrest BA. DNA oligonucleotide treatment corrects the age-associated decline in DNA repair capacity. FASEB J. 2002;16:754–756. doi: 10.1096/fj.01-0829fje. [DOI] [PubMed] [Google Scholar]
- Eller MS, Maeda T, Magnoni C, Atwal D, Gilchrest BA. Enhancement of DNA repair in human skin cells by thymidine dinucleotides: evidence for a p53-mediated mammalian SOS response. Proc Natl Acad Sci USA. 1997;94:12627–12632. doi: 10.1073/pnas.94.23.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goukassian DA, Eller MS, Yaar M, Gilchrest BA. Thymidine dinucleotide mimics the effect of solar simulated irradiation on p53 and p53-regulated proteins. J Invest Dermatol. 1999;112:25–31. doi: 10.1046/j.1523-1747.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- Eller MS, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci USA. 1996;93:1087–1092. doi: 10.1073/pnas.93.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Yaar M, Eller MS, Gilchrest BA. Oligonucleotides homologous to the telomeric 3′ overhang stimulate p21-dependent adaptive responses to oxidative damage. J Invest Dermatol. 2003;121(1) [Google Scholar]
- Wilson DM, III, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nishigori C, Tanaka T, Uchida K, Nikaido O, Osawa T, Hiai H, Imamura S, Toyokuni S. 8-Hydroxy-2′-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol. 1996;107:733–737. doi: 10.1111/1523-1747.ep12365625. [DOI] [PubMed] [Google Scholar]
- Gailani MR, Leffell DJ, Ziegler A, Gross EG, Brash DE, Bale AE. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996;88:349–354. doi: 10.1093/jnci/88.6.349. [DOI] [PubMed] [Google Scholar]
- Stratigos AJ, Kapranos N, Petrakou E, Anastasiadou A, Pagouni A, Christofidou E, Petridis A, Papadopoulos O, Kokka E, Antoniou C, Georgala S, Katsambas AD. Immunophenotypic analysis of the p53 gene in non-melanoma skin cancer and correlation with apoptosis and cell proliferation. J Eur Acad Dermatol Venereol. 2005;19:180–186. doi: 10.1111/j.1468-3083.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- Athar MTX, Kim KH, Kim AL, Kopelovich L, Bickers DR. Interaction of p53 and sonic hedgehog signaling pathways in the development of ultraviolet-B (UVB)-induced basal cell carcinomas (BCCs) in murine skin. J Invest Dermatol. 2007;127:S25. [Google Scholar]
- Emri G, Wenczl E, Van Erp P, Jans J, Roza L, Horkay I, Schothorst AA. Low doses of UVB or UVA induce chromosomal aberrations in cultured human skin cells. J Invest Dermatol. 2000;115:435–440. doi: 10.1046/j.1523-1747.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- Quinn AG, Sikkink S, Rees JL. Basal cell carcinomas and squamous cell carcinomas of human skin show distinct patterns of chromosome loss. Cancer Res. 1994;54:4756–4759. [PubMed] [Google Scholar]
- Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, Epstein EH, Jr, Bickers DR. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280:1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- Aoki H, Iwado E, Eller MS, Kondo Y, Fujiwara K, Li GZ, Hess KR, Siwak DR, Sawaya R, Mills GB, Gilchrest BA, Kondo S. Telomere 3′ overhang-specific DNA oligonucleotides induce autophagy in malignant glioma cells. FASEB J. 2007;21:2918–2930. doi: 10.1096/fj.06-6941com. [DOI] [PubMed] [Google Scholar]
- Tjiu JW, Liao YH, Lin SJ, Huang YL, Tsai WL, Chu CY, Kuo ML, Jee SH. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–1151. doi: 10.1038/sj.jid.5700191. [DOI] [PubMed] [Google Scholar]