Abstract
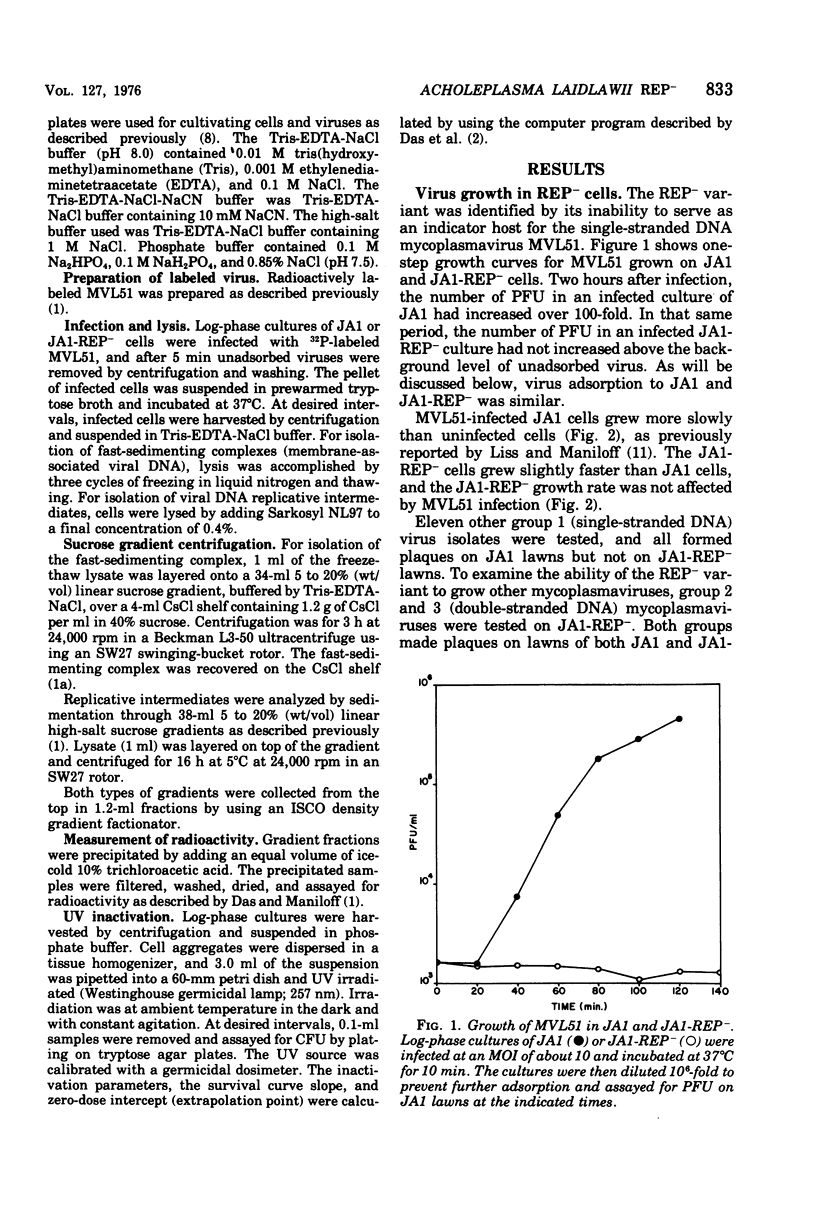
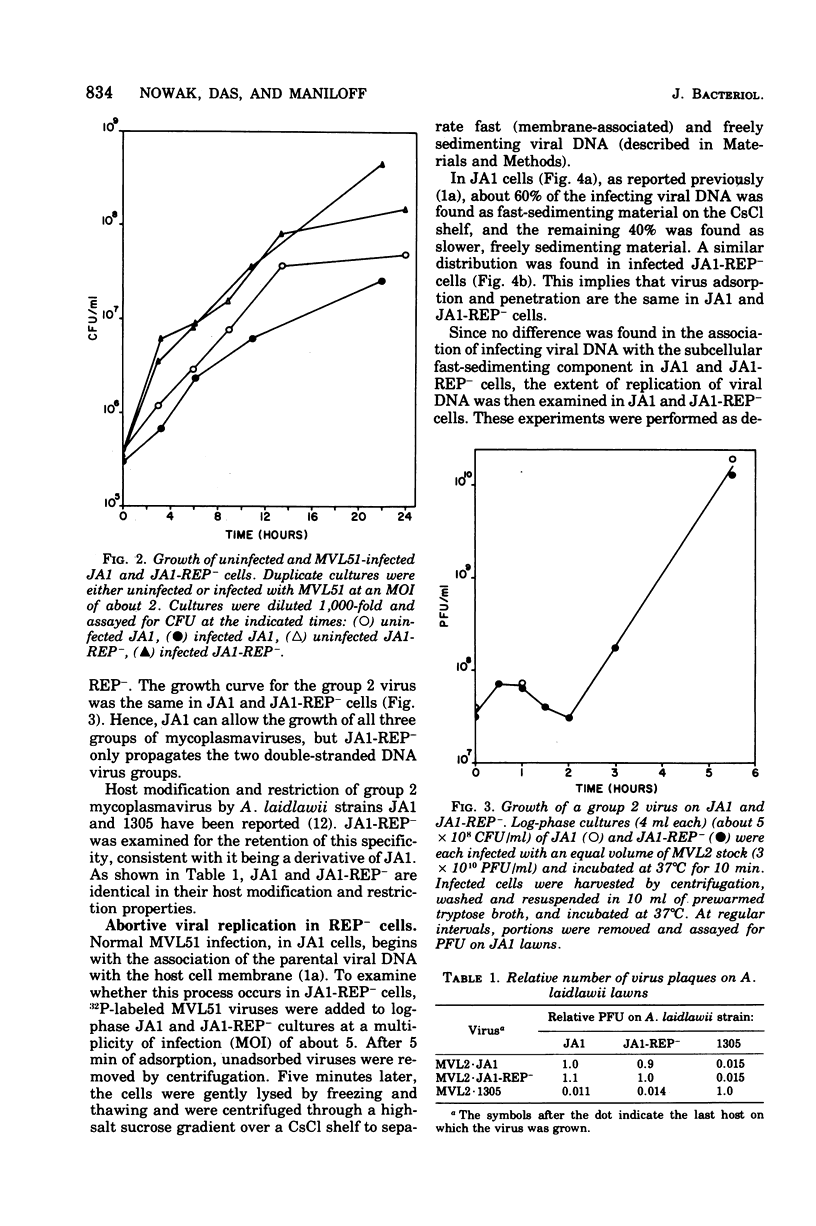
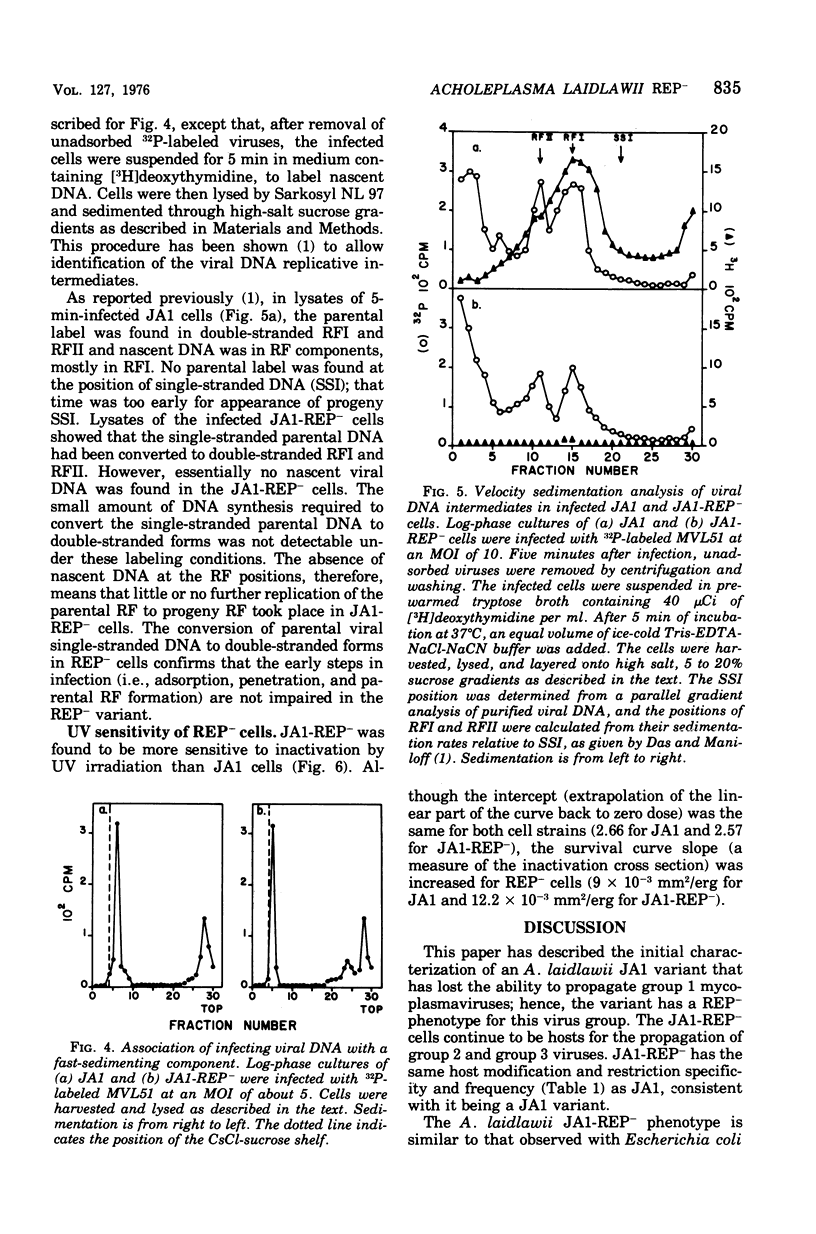
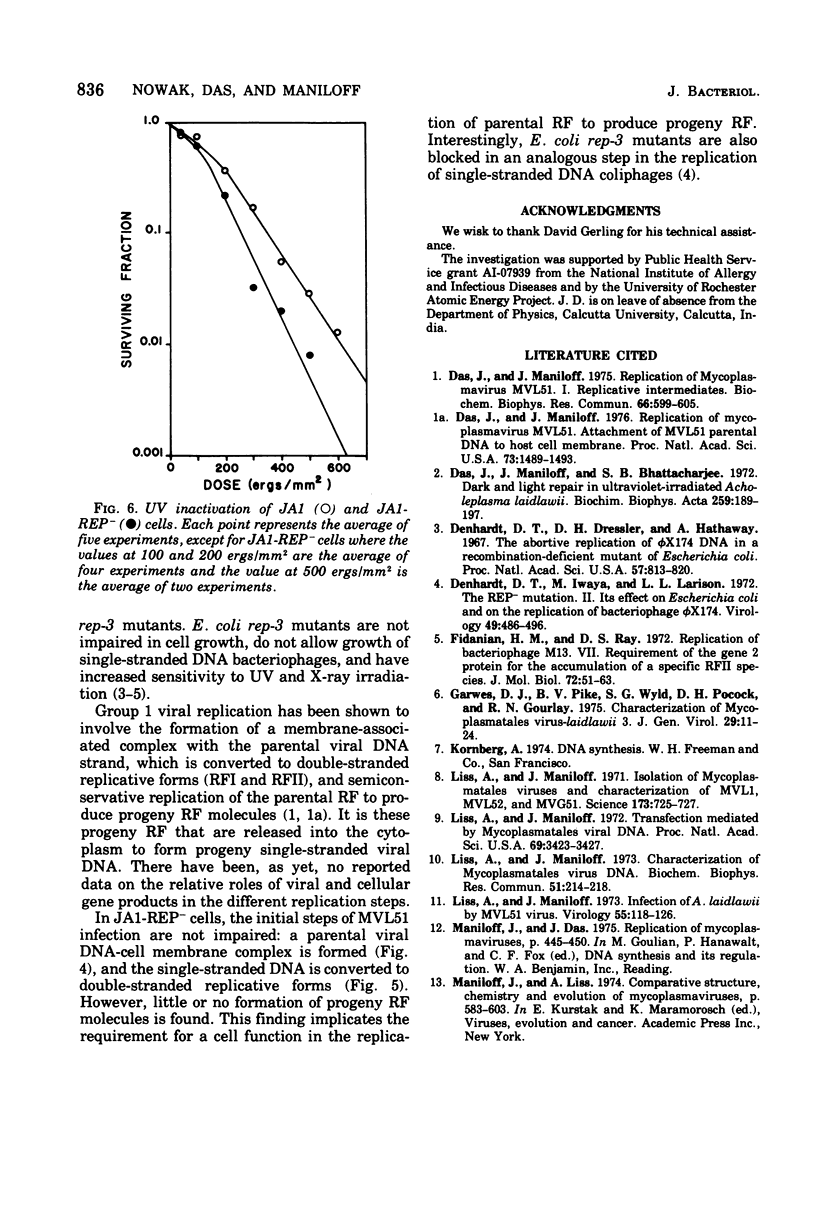
An Acholeplasma laidlawii variant has been isolated that has a REP- phenotype. The properties of this variant, relative to parental cells, are: (i) it exhibits no change in cell growth kinetics; (ii) it does not propagate single-stranded deoxyribonucleic acid (DNA) mycoplasmaviruses but does propagate double-stranded DNA mycoplasmaviruses; (iii) it converts parental circular single-stranded mycoplasmavirus DNA to double-stranded replicative forms that are not replicated further; (iv) it exhibits no change in host modification and restriction; and (v) it has an increased ultraviolet light sensitivity. The REP- isolate is the first stable mycoplasma variant to which a physiological defect has been attributed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Das J., Maniloff J., Bhattacharjee S. B. Dark and light repair in ultraviolet-irradiated Acholeplasma laidlawii. Biochim Biophys Acta. 1972 Jan 31;259(2):189–197. doi: 10.1016/0005-2787(72)90058-5. [DOI] [PubMed] [Google Scholar]
- Das J., Maniloff J. Replication of mycoplasmavirus MVL51: I. Replicative intermediates. Biochem Biophys Res Commun. 1975 Sep 16;66(2):599–605. doi: 10.1016/0006-291x(75)90552-5. [DOI] [PubMed] [Google Scholar]
- Das J., Maniloff J. Replication of mycoplasmavirus MVL51: attachment of MVL51 parental DNA to host cell membrane. Proc Natl Acad Sci U S A. 1976 May;73(5):1489–1493. doi: 10.1073/pnas.73.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. VII. Requirement of the gene 2 protein for the accumulation of a specific RFII species. J Mol Biol. 1972 Dec 14;72(1):51–63. doi: 10.1016/0022-2836(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Pike B. V., Wyld S. G., Pocock D. H., Gourlay R. N. Characterization of Mycoplasmatales virus-laidlawii 3. J Gen Virol. 1975 Oct;29(1):11–24. doi: 10.1099/0022-1317-29-1-11. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Characterization of mycoplasmatales virus DNA. Biochem Biophys Res Commun. 1973 Mar 5;51(1):214–218. doi: 10.1016/0006-291x(73)90530-5. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Infection of Acholeplasma laidlawii by MVL51 virus. Virology. 1973 Sep;55(1):118–126. doi: 10.1016/s0042-6822(73)81013-x. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Transfection mediated by Mycoplasmatales viral DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3423–3427. doi: 10.1073/pnas.69.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]


