Abstract
Photosynthesis is often limited by the rate of CO2 diffusion from the atmosphere to the chloroplast. The primary resistances for CO2 diffusion are thought to be at the stomata and at photosynthesizing cells via a combination resulting from resistances of aqueous solution as well as the plasma membrane and both outer and inner chloroplast membranes. In contrast with stomatal resistance, the resistance of biological membranes to gas transport is not widely recognized as a limiting factor for metabolic function. We show that the tobacco (Nicotiana tabacum) plasma membrane and inner chloroplast membranes contain the aquaporin Nt AQP1. RNA interference–mediated decreases in Nt AQP1 expression lowered the CO2 permeability of the inner chloroplast membrane. In vivo data show that the reduced amount of Nt AQP1 caused a 20% change in CO2 conductance within leaves. Our discovery of CO2 aquaporin function in the chloroplast membrane opens new opportunities for mechanistic examination of leaf internal CO2 conductance regulation.
INTRODUCTION
The existence of a water-facilitating component in membranes was hypothesized after the discovery that the transepithelial water flow across amphibian skin was ∼5 times higher than expected if it was only controlled by simple diffusion across a lipid bilayer (Hevesy et al., 1935). Aquaporins were identified as this component in the early nineties (Preston et al., 1992; Agre, 2004). Since then, these proteins were identified in almost all organisms. Aquaporins display variable conductivity for water, in some cases along with permeability to solutes such as glycerol, urea, amino acids, and even ions (Biela et al., 1999; Eckert et al., 1999; Yasui et al., 1999; Ikeda et al., 2002). Furthermore, some aquaporins were found to facilitate gas transport through biomembranes in heterologous expression systems (Nakhoul et al., 1998; Jahn et al., 2004).
Within plant membranes, Uehlein et al. (2003) provided evidence for a protein-mediated pathway for CO2 transport in vivo by altering the expression of the aquaporin 1 from Nicotiana tabacum (Nt AQP1). The Nt AQP1 protein belongs to the PIP1 subfamily (plasma membrane intrinsic proteins) and shares functionally important amino acid residues in the pore region with human AQP1 (de Groot et al., 2001). High expression of Nt AQP1 resulted in increased photosynthesis and growth. However, the precise mechanism of action and mechanistic relation to photosynthesis was uncertain (Uehlein et al., 2003).
In many cases, photosynthesis is limited by the availability of CO2, which is dependent on the process of diffusion from the bulk atmosphere to the site of photosynthetic CO2 fixation in the chloroplast stroma. There are three commonly defined resistances in this diffusion pathway: the leaf boundary layer, the stomata, and the internal leaf structure. Over the past 30 years, speculation about the magnitude and variability of resistance to CO2 diffusion inside leaves (ri) has ranged from assumptions that it is insignificant to that it is large and variable. The importance of characterizing ri for improving models of photosynthesis has recently been revisited (Ethier et al., 2006; Warren and Adams, 2006), though the mechanism of its variability remains poorly understood. Internal leaf resistance was subdivided into resistance within the intercellular air space, liquid phase resistance in cell walls and cytosol, and resistance of biological membranes (Evans et al., 1994; Gillon and Yakir, 2000).
Because they have been difficult to measure, membrane resistances cause the greatest uncertainties. Using measurements of the CO2 permeability of a lecithin-cholesterol bilayer, and assuming an equal resistance for each of the three membranes in the diffusive pathway from the air spaces to the chloroplast stroma, Evans et al. (1994) calculated that the plasma membrane and the chloroplast outer and inner membranes together account for 49% of ri. However, not all three membranes may have equal permeability.
Developmentally controlled morphological changes in leaves, such as the amount of surface area exposed to intercellular airspaces, are commonly invoked to explain differences in ri (Evans et al., 1994; Evans and Loreto, 2000). The hypothesis that protein expression may reduce ri was initially proposed as a function of carbonic anhydrase, as it could maintain equilibrium CO2 concentrations adjacent to membranes (Evans et al., 1994; Price et al., 1995; Gillon and Yakir, 2000; Bernacchi et al., 2002). This helps to control ri because CO2 diffuses through membranes more readily than HCO3−. This mechanism moves CO2 between inorganic carbon pools separated by a membrane and is thus also modulated by membrane CO2 permeability.
More recently, Terashima and Ono (2002) showed an effect of HgCl2 treatment on CO2 dependence of leaf photosynthesis, indicating an involvement of aquaporins in CO2 diffusion across the plasma membrane. The first studies to provide evidence for involvement of aquaporins in CO2 transport were encouraging but complicated by morphological variation and expression of non-native proteins (Hanba et al., 2004). Flexas et al. (2006) generated the most rigorous data showing that expression levels of the endogenous aquaporin Nt AQP1 in tobacco leaves is correlated with ri. As CO2 diffuses through the mesophyll to the site of the CO2 fixing enzyme ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco), it must cross the plasma membrane and chloroplast membranes. An obvious assumption is that Nt AQP1 fulfils its function as a CO2 transport facilitator in the plasma membrane since it belongs to the PIP1 family of plant plasma membrane intrinsic proteins (Biela et al., 1999). However, plasma membrane CO2 permeability only varies slightly with the level of Nt AQP1 expression (see results below). Consequently, the chloroplast membranes are another possible location for aquaporin-mediated modification of CO2 flux. To date, we know of no reports describing aquaporins in organelles apart from mitochondria in animals (Amiry-Moghaddam et al., 2005; Calamita et al., 2006). However, no conclusive proof of organelle aquaporin function was provided (Yang et al., 2006).
Here, we show that Nt AQP1 is localized in chloroplast membranes in addition to plasma membranes, and we give evidence for its function in CO2 transport in isolated chloroplasts in situ, in membrane vesicles, and in vivo using mature leaves. By demonstrating the CO2 (rather than H2O) transporting function for this organelle located aquaporin, we call into question the appropriateness of referring to these proteins as aquaporins and challenge the concept of free diffusion of gases like CO2 through biomembranes.
RESULTS
Localization of Nt AQP1
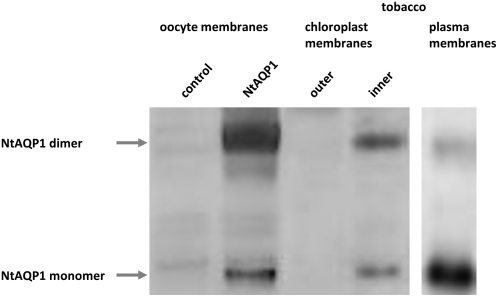
The subcellular localization of Nt AQP1 in chloroplast membranes was determined by protein gel blot analysis, electron microscopy, and fluorescence microscopy using an Nt AQP1-mGFP (for modified green fluorescent protein) fusion protein. For protein gel blotting, chloroplast membranes were isolated and separated into outer, inner, and thylakoid membranes. The identity of envelope membranes was verified with antibodies directed to the phosphate translocator in the inner chloroplast membrane and the 24-kD outer chloroplast membrane protein, respectively (see Supplemental Figure 1 online). Using an antibody directed to the N terminus of Nt AQP1, we confirmed that this aquaporin is a constituent of plasma membranes. In addition, a clear signal corresponding to the inner chloroplast membrane was detected (Figure 1). The antibody revealed a signal corresponding to that detected on plasma membranes of Xenopus oocytes expressing Nt AQP1.
Figure 1.
Identification of Nt AQP1 Dimers and Monomers in Various Membranes.
Protein gel blot analysis was performed using purified chloroplast envelopes and plasma membranes of tobacco, plasma membranes of Xenopus oocytes expressing Nt AQP1, and water-injected control oocytes not expressing Nt AQP1. Oocyte membranes were prepared as described by Yang and Verkman (1997). An antibody against Nt AQP1 was used to reveal signals corresponding to Nt AQP1 monomers and dimers in the membranes indicated at the top of the figure.
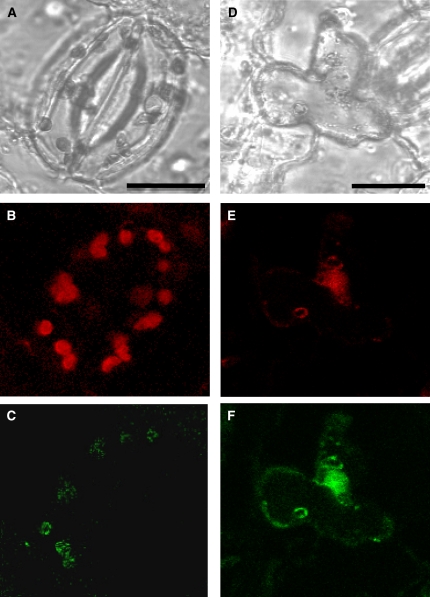
We confirmed these findings by electron microscopy using an immunogold-labeled secondary antibody. As depicted in Figure 2A, gold particles were observed in the chloroplast membrane region and in regions of the plasma membrane (i.e., at the borders between cell wall and cytoplasm) (Figure 2B). Transient expression of a translational fusion of Nt AQP1 and mGFP in plants provided a tool to analyze cellular protein distribution independently of immunological approaches. As demonstrated in Figure 3, AQP1-GFP fluorescence colocalized with chlorophyll autofluorescence only in guard cells when leaf disks with an intact epidermal layer were transformed by particle bombardment (Figures 3A to 3C). Cells from the epidermal layer, which usually do not harbor chloroplasts, displayed GFP fluorescence solely in regions of the plasma membrane. We also observed mesophyll cells after removal of the epidermal layer. As depicted in Figures 3D to 3F, these cells showed a fluorescence signal in regions of the plasma membrane and in regions of chlorophyll fluorescence. Taken together, this supports the notion that in mesophyll cells and in guard cells, Nt AQP1 was targeted to the chloroplast in addition to the initially discovered localization in plasma membranes.
Figure 2.
Localization of Nt AQP1 in Tobacco Cells.
(A) Immunogold localization of Nt AQP1 in a section of a tobacco chloroplast.
(B) Immunogold localization of Nt AQP1 in two tobacco plant cell sections divided by a cell wall.
For both (A) and (B), a specific antibody against Nt AQP1 and a secondary antibody coupled to gold particles was employed. Arrows indicate the localization of selected gold particles showing the localization of Nt AQP1. Chloroplast envelope (ev), thylakoids (th), the cytoplasm (cy), and cell wall (cw) are indicated. Bars = 0.5 μm.
Figure 3.
Detection of a Translational Fusion of Nt AQP1 to GFP in Tobacco Chloroplasts after Transient Transformation Using the Biolistic Method.
Panels (A) to (C) show intact guard cells (bar = 20 μm), and (D) to (F) show mesophyll cells (bar = 25 μm). Bright-field images ([A] and [D]), chlorophyll fluorescence ([B] and [E]; red), and mGFP fluorescence ([C] and [F]; green) are shown.
CO2 Permeability of Isolated Membranes
Nt AQP1 function in green leaf cell membranes was analyzed with regard to water and CO2 permeability. Plasma membrane or chloroplast membrane vesicles were loaded with carboxyfluorescein and carbonic anhydrase, and, in a stopped flow spectrophotometer, the suspension was subjected to a CO2-saturated buffer solution. As a consequence of CO2 uptake and conversion into carbonic acid by carbonic anhydrase, the cell acidified and the kinetics of the resulting fluorescence decrease were recorded. Using these data, CO2 permeability could be calculated and revealed just slight differences in plasma membranes isolated from leaf cells of control plants (8.54*10−3 ± 1.71*10−4 cm/s [n = 15]) or those of plants lacking Nt AQP1 from disruption via RNA interference (RNAi) (7.73*10−3 ± 2.95*10−4 cm/s [n = 15]; t test, P = 0.1). The plasma membrane water permeability changed, and it was 6.34*10−3 ± 1.47*10−4 cm/s for controls (n = 20) and 3.39*10−3 ± 9.72*10−5 cm/s for RNAi plants (n = 20; t test, P = 3.4*10−18). However, the water permeability of chloroplast envelopes was not significantly different (i.e., 1.91*10−2 ± 6.27*10−4 cm/s [n = 25] for controls and 1.70*10−2 ± 6.34*10−4 cm/s [n = 40] for RNAi plants; t test, P = 0.22). By contrast, the CO2 permeability of chloroplast envelopes with or without AQP1 differed (Figure 4A). It was 18.5*10−4 ± 4.06*10−4 cm/s (n = 22) for control and 2.06*10−4 ± 4.90*10−5 cm/s (n = 28) for RNAi plants (t test, P = 1.89*10−5). This corresponds to an 89% reduced CO2 uptake rate compared with chloroplast vesicles with a natural expression of Nt AQP1. Comparison of PCO2 data from chloroplast envelope and plasma membranes indicate that the plasma membrane is ∼5 times more permeable to CO2 than the chloroplast membrane (Figure 4B).
Figure 4.
CO2 Permeability of Chloroplast Envelope and Plasma Membranes in Control and RNAi Tobacco Plants.
(A) Time course of acidification in chloroplast envelope vesicles in response to CO2 transport. Vesicles were loaded with carbonic anhydrase and fluorescein and rapidly mixed with a CO2-saturated buffer solution in a stopped flow device. Uptake of CO2 resulted in an intravesicular acidification and consequently a decrease in fluorescein fluorescence. Kinetics from chloroplast vesicles of control plants (WT, black) and Nt AQP1–deficient plants (RNAi, gray) are shown over a period of 100 ms.
(B) CO2 permeability of chloroplast envelope and plasma membranes (WT, black; RNAi, gray).
Photosynthetic Capacity
We also examined the influence of chloroplast envelope CO2 permeability on the photosynthetic capacity of leaves from controls and Nt AQP1–deficient RNAi plants. During illumination, the leaf internal CO2 partial pressure (ci) was similar in RNAi plants and control plants. However, photosynthetic rates were reduced by 15% in the former (Table 1), confirming our findings on Nt AQP1 antisense plants (Uehlein et al., 2003). The inhibition of AQP1 expression also resulted in a slight reduction of stomata conductance that could potentially limit photosynthesis by limiting uptake of CO2 into leaves. However, the ratio of net assimilation rate (A) to ci was significantly higher in controls (Table 1). As Flexas et al. (2006) showed that tobacco plants with impaired expression of AQP1 exhibit the same amount and activity of Rubisco, the change in A/ci points to a change of mesophyll conductance.
Table 1.
Gas Exchange Analysis
| A (μmol m−2 s−1) | gs (mol m−2 s−1) | ci (Pa) | A/ci (μmol m−2 s−1 Pa−1) | |
|---|---|---|---|---|
| Control | 19.1 ± 1.0a | 0.49 ± 0.01a | 22.4 ± 0.4a | 0.85 ± 0.06a |
| RNAi | 16.2 ± 0.8b | 0.38 ± 0.03b | 22.2 ± 0.4a | 0.73 ± 0.03b |
Net photosynthesis (A), stomatal conductance to water vapour (gs), and intercellular CO2 partial pressure (ci) in AQP1 RNAi and control tobacco plants. Measurements reported at an atmospheric pressure of ∼100 kPa and a light intensity of 1500 μmol m−2 s−1. Different letters indicate significant differences (t test, P < 0.05), and se is shown as ± (n = 12).
Leaf Internal Conductance
In a separate experiment, we combined leaf gas exchange with tunable diode laser spectroscopy (TDL) to perform online measurements of carbon isotope discrimination (Δ) by leaves (Flexas et al., 2006; Barbour et al., 2007). Following the procedure given by Evans et al. (1986), and Farquhar (Farquhar et al., 1982; Farquhar and Richards, 1984), we calculated internal leaf conductance to CO2 (gi = 1/ri) and the CO2 partial pressure at the site of carboxylation (cc). Plants for this experiment were grown in New Mexico at higher light intensities (due to greenhouse characteristics) and lower ambient partial pressures of CO2 (due to higher elevation above sea level) than the plants for the previously described gas exchange experiment performed in Darmstadt, Germany.
At the assay light intensity of 2000 μmol photons m−2 s−1, A was lower in the RNAi line, though only significant as a trend (P = 0.10), while gs and ci did not differ significantly between control and RNAi lines (Table 2). The ratio of A to ci in New Mexico–grown wild-type plants was ∼25% higher than that of the wild-type plants grown in Germany, suggesting that the New Mexico plants either had a higher photosynthetic capacity (greater amounts or more active Rubisco) or a higher gi. Applying these data to the Evans et al. (1986) model, we calculated that the mesophyll conductance for CO2 (gi) in the RNAi line is 21% lower (or ri is 27% higher) than for the control line. Since cc = ci- (A/gi), this equates with a 1.7 Pa (13%, P = 0.16) lower cc in the RNAi line (Table 2). Lower rates of photosynthesis in the RNAi line have the effect of increasing the calculated cc, so it is not surprising that the cc decrease is only a trend.
Table 2.
Gas Exchange Analysis and Online Photosynthetic Discrimination
| A (μmol m−2 s−1) | gs (mol H2O m−2 s−1) | ci (Pa) | Δ (‰) | gi (μmol CO2 m−2 s−1 Pa−1) | cc (Pa) | |
|---|---|---|---|---|---|---|
| Control | 20.7 ± 0.4a | 0.66 ± 0.07a | 19.6 ± 0.5a | 17.0 ± 0.4a | 3.0 ± 0.3a | 12.5 ± 0.9a |
| RNAi | 19.6 ± 0.7a | 0.56 ± 0.05a | 19.4 ± 0.7a | 16.3 ± 0.7a | 2.4 ± 0.2b | 10.8 ± 1.3a |
Net photosynthesis (A), stomatal conductance to water vapor (gs), intercellular CO2 partial pressure (ci), online photosynthetic 13CO2 discrimination (Δ), internal leaf conductance (gi), and chloroplast CO2 partial pressures (cc) are reported for AQP1 RNAi and control tobacco plants. All measurements were made at a PPFD of 2000 μmol m−2 s−1, except for gi, which was calculated from measurements at multiple light intensities. Measurements are reported at an atmospheric pressure of ∼79 kPa, and leaves were provided with a ca of ∼25.1 Pa at a light intensity of 2000 μmol m−2 s−1, except gi data, which were generated from multiple light intensities. Values reported for gi can be converted to the more familiar, non-SI units of mol CO2 m−2 s−1 bar−1 by dividing by 10. Different letters indicate significant differences (t test, P < 0.05), and se is shown as ± (n = 6).
Membrane Resistances as a Percentage of Total Resistance
In combination, our data show that the purified inner chloroplast membranes from RNAi plants have a 56% higher resistance to CO2 transport, but in vivo the entire ri only increased 27%. Therefore, if we assume that the changes in the Nt AQP1 content of the inner chloroplast membrane are the sole factor generating the change in ri, we would estimate that the resistance of the inner chloroplast membrane constitutes 48% of ri in tobacco rather than the 16% estimated by Evans et al. (1994) for tobacco.
DISCUSSION
Tobacco AQP1 belongs to the so-called Plasma Membrane Intrinsic Protein family 1 (PIP1). Our data show that the classification with regard to plant aquaporin localization is insufficient because the cellular distribution of this aquaporin is not restricted to the plasma membrane. In addition to its location in the plasma membrane, Nt AQP1 is also a component of the inner chloroplast membrane even though it lacks a classical chloroplast transit peptide. We could demonstrate that Nt AQP1 in chloroplast envelopes fulfills an important physiological function in vivo by showing that CO2 transport through the plant leaf to the site of CO2 fixation in the chloroplast stroma is strongly impacted by the function of Nt AQP1 as a CO2 membrane transport facilitator.
Plants with reduced Nt AQP1 expression have an increased chloroplast membrane resistance to CO2 transport of 56%, which is manifested as a 27% increase of in vivo internal leaf resistance. This causes a reduced rate of photosynthesis in the RNAi line, and, despite this lower sink strength, the chloroplast CO2 partial pressure in this RNAi line is lower than that in controls. Reduced expression of Nt AQP1 has effects on the characteristics of membranes in chloroplast-containing mesophyll cells. The plasma membrane shows decreased water permeability, while the CO2 permeability was not considerably affected. In chloroplast envelopes, the water permeability was just slightly reduced, while the CO2 permeability decreased significantly.
Thus, Nt AQP1 seems to switch function from a water transport facilitating channel to a CO2 transport facilitating channel depending on its cellular location or on the intrinsic CO2 permeability of the membrane where it resides. Comparative analysis of the CO2 permeability properties indicates that the plasma membrane is ∼5 times more permeable to CO2 than the chloroplast envelope. Thus, the chloroplast envelope could potentially meet the requirements for CO2 permeation through aquaporins in a membrane with low CO2 permeability as demanded by Hub and de Groot (2006). The concept that certain membranes could be more mosaic than fluid would support this view (Engelman, 2005) and is consistent with the concept of Nt AQP1 being a CO2 pore.
It is known that aquaporin function can be regulated by posttranslational modification (e.g., via phosphorylation) or by interaction with other aquaporin isoforms (Johansson et al., 1998; Moshelion et al., 2004). Although phosphorylation or regulation by phosphorylation was not demonstrated for PIP1-type aquaporins like Nt AQP1, it is possible that one of the above-mentioned regulatory mechanisms causes the change in transport specificity. Besides aquaporin regulation, simple differences in intrinsic membrane properties between the plasma membrane and chloroplast membranes could explain the assumed switch in aquaporin function. If the intrinsic CO2 permeability of plant plasma membranes is large enough to ensure high enough rates of CO2 transport, the presence or absence of CO2 pores may not have a measurable effect. Theoretical studies have demonstrated that significant aquaporin-mediated CO2 permeation is only relevant in membranes with a low intrinsic CO2 permeability (Hub and de Groot, 2006). As confirmed by our studies in membrane vesicles and in CO2 conductivity measurements in vivo, the chloroplast envelope lacking Nt AQP1 appears to be rate limiting for CO2 transport.
It was shown that an unstirred layer could affect fast membrane transport processes in vesicles of artificial bilayers, and the question could arise if these could also be rate limiting in experiments with cell membranes (Gutknecht et al., 1977; Hill et al., 2004). Because we detect clear differences in membrane CO2 permeability with or without Nt AQP1 and since all CO2 transport experiments were conducted under iso-osmotic conditions, it is unlikely that a membrane water layer, which could be assumed to have the same thickness in membrane vesicles from RNAi or controls, is the rate limiting step. Determination of PCO2 values revealed that CO2 permeates the chloroplast membrane ∼100 to 1000 times slower than estimated for an artificial bilayer. The absolute values for CO2 transport rates are in perfect agreement with those obtained on human aquaporin1 by a comparable experimental setup (Prasad et al., 1998). The determined CO2 permeability is not in the range where unstirred layers significantly affect CO2 transport; it is rather in the range of that for water. Consequently, it is suggested that the contribution of Nt AQP1 to CO2 permeability is as significant as that of water-conducting aquaporins to membrane water permeability. Taken together, we conclude that Nt AQP1 as a CO2 facilitator increases CO2 transport at the inner chloroplast membrane, not at the plasma membrane.
The localization of Nt AQP1 in chloroplast membranes (in addition to the plasma membrane) suggests that its classification and possibly that of other PIP aquaporins as plasma membrane intrinsic protein is misleading. Also, the demonstrated CO2 transporting function in addition to water transport makes the term “CO2 aquaporin” or “COOporin,” as it was already suggested by Terashima et al. (2006) more appropriate than aquaporin. The physiological relevance of this gas transporting function also suggests a mechanism that plants may use to modify photosynthetic function. Furthermore, the possibility that a CO2 transport function or gas transport function in general may be important for mitochondrial membranes in many different species, including animals, has not escaped our notice.
METHODS
Plant Material
Tobacco plants (Nicotiana tabacum cv Petit Havana SR1) were cultivated under standard greenhouse conditions in Germany (25°C, 80% RH, PAR ∼800 μmol m−2 s−1, ∼36.9 Pa CO2, 14 h light) and in New Mexico (26°C, 20% RH, max PAR ∼1200 μmol m−2 s−1, ∼28.8 Pa CO2, 14 h light). The RNAi tobacco plants with reduced expression of Nt AQP1 were a gift from M. Bots and C. Mariani. The production of the transgenic plants was described elsewhere (Bots, 2004). Existence of the RNAi effect was verified on the protein level using a PIP1-specific antiserum kindly provided by C. Mariani and an Nt AQP1–specific antiserum (Otto and Kaldenhoff, 2000; see Supplemental Figure 2 online).
Purification of Plasma Membranes
Prior to purification of plasma membranes, crude membrane fractions (microsomes) were isolated following the procedure of Kjellbom and Larsson (1984). Briefly, 100 g fresh weight leaf material were homogenized three times for 30 s in 300 mL 0.33 M sucrose using a kitchen blender, 50 mM HEPES/KOH, pH 7.5, 5 mM EDTA, 5 mM DTT, 5 mM ascorbic acid, 0.5 mM phenylmethanesulphonylfluoride, 0.2% BSA, 0.2% caseine (boiled for 10 min), and 0.6% polyvinylpolypyrrolidone. The homogenate was filtered through three layers of miracloth, and the bulk of chloroplasts and mitochondria were sedimented by centrifugation at 5000g for 10 min. The membranes in the supernatant were precipitated at 100,000g for 1 h, and the pellet was resuspended to a total volume of 10 mL in 0.33 M sucrose, 5 mM HEPES/KOH, pH 7.5, 5 mM KCl, 1 mM DTT, and 0.1 mM EDTA. Plasma membranes were purified from the crude membrane fraction using the two-phase partitioning system (Widell and Larsson, 1981; Lundborg et al., 1981). Briefly, 9 g of the microsomal suspension were added to 27 g of the phase mixture to give a total phase system of 36 g with a final composition of 6.2% (w/w) Dextran T 500, 6.2% (w/w) polyethylene glycol 3350, 0.33 M sucrose, 5 mM potassium phosphate buffer, pH 7.8, 5 mM KCl, 1 mM DTT, and 0.1 mM EDTA. The phase system was mixed by several inversions and centrifuged in a swing-out rotor for 10 min at 1500g. The upper phase was reextracted three times. The plasma membrane–enriched final upper phase was diluted fivefold with buffer and centrifuged at 100,000g for 1 h. The pellet was resuspended in 3 mM HEPES/KOH, pH 7.5, 0.25 M sucrose, 50 mM KCl, and 1 mM DTT.
Protein Gel Blotting
Ten micrograms of protein per lane were separated on a 12% polyacrylamid gel (SDS-PAGE) (Laemmli, 1970). Equal lane loading was monitored by Coomassie Brilliant Blue stain in a parallel experiment. Protein transfer was performed in a tank transfer system (Amersham) with 10 mM CAPS to a nitrocellulose membrane for 2 h at 80 V. Transfer efficiency was monitored by a reversible, colloidal silver stain. For this, the membrane was incubated in a solution containing 2% sodium citrate, 0.8% FeSO4 heptahydrate, and 0.2% AgNO3 for 10 min and washed with water. The membrane was destained with 15 mM potassium hexacyanoferrat (III) and 50 mM Na-thiosulfate. Subsequently, it was blocked with fat-free milk powder in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4)/0.5% Tween 20, and incubated with the respective antibodies. Detection was achieved via a chemiluminescent substrate according to the protocol provided by the manufacturer (CDP-Star; Applied Biosystems).
Electron Microscopy and Immunocytochemistry
Elelctron microscopy was done essentially following the procedure given in Kaldenhoff et al. (1995). Briefly, tissues were fixed in 2% glutaraldehyde, buffered phosphate, pH 7.2, for 60 min at 4°C. Specimens were washed with ice-cold phosphate buffer and dehydrated in a graded acetone series and embedded in EM resin (TAAB). Sections were cut with a diamond knife on an ultramicrotome. Immunostaining was performed by treating the sections with 1% BSA in 20 mM phosphate buffer, pH 7.2, for 30 min and a 1:200 dilution of the Nt AQP1 antiserum or preimmune serum for 60 min. After washing with PBS, they were incubated with goat anti-rabbit-gold diluted 1:30 for 45 min and rinsed with PBS. All sections were counterstained with uranyl acetate and examined by electron microscopy.
Nt AQP1-mGFP4 Reporter Gene Expression
Nt AQP1 cDNA was inserted into pPILY vector (Ferrando et al., 2000) with SmaI and Eco47III, and its integrity was confirmed by sequencing. By integrating the Nt AQP1 gene, it was fused to an mGFP reporter gene, mGFP4 (Haseloff and Amos, 1995). The fusion protein gene was expressed under the control of a tandem repeat of cauliflower mosaic virus 35S promoters. Plasmid DNA was transformed into leaf disks of 4- to 7-month-old tobacco plants. For this, 0.6-μm gold microcarriers were coated with plasmid DNA according to the manufacturer's guidelines (Sanford et al., 1993) and delivered into plant tissues by biolistic transformation using a pds1000/He device (Bio-Rad). Following bombardment, the leaf tissues were kept on Murashige and Skoog solid medium (Duchefa) and incubated in the dark for 2 to 5 d. Visualization of mGFP4 reporter gene expression in plant cell compartments was achieved using a Zeis LSM5 Pascal confocal laser scanning microscope. For visualization of mGFP4 protein expression in mesophyll cells, the epidermis of the leaf abaxial surface was removed.
Gas Exchange Measurements
Gas exchange measurements in Germany (see above) were performed using the CIRAS-2 portable photosynthesis system (PP Systems). Water and CO2 concentrations at the inlet and outlet of the cuvette were measured using a differential infrared gas analyzer (IRGA). The cuvette flow was adjusted to 200 mL min−1. The cuvette area was 4.5 cm2, and white light of 500 to 1500 μmol m−2 s−1 PPFD was used. Leaf temperature was kept at 25°C. Measurements were made on the youngest fully expanded leaves. Data were recorded at 10-s intervals. Data analysis and calculations were performed using Microsoft Excel. Statistically significant differences between plants with reduced Nt AQP1 expression and control plants were analyzed using a Student's t test (P < 0.05). Data are given as means ± se.
Purification of Chloroplast Envelope Membrane Vesicles
Prior to the isolation of chloroplasts, plants were incubated in darkness for 24 h to reduce the starch content of the chloroplasts. Intact chloroplasts were isolated from tobacco using a Percoll gradient based method as described by Cline (1985) with modifications by Bock (1998) in buffer solutions containing sorbitol as osmoticum. All steps were performed at 4°C. Tobacco leaves were washed with distilled water and homogenized using a modified blender (Kannangara et al., 1977) in ice-cold grinding buffer (330 mM sorbitol, 1 mM sodium pyrophosphate, 2 mM EDTA, pH 8.0, 5 mM MgCl2, 5 mM 2-mercaptoethanol, 5 mM ascorbate, and 50 mM HEPES, pH 6.8). The homogenate was filtered through four layers of miracloth and centrifuged at 4.000 rpm in a Sorvall GSA rotor. The supernatant was discarded and the pellet resuspended in 5 mL of ice-cold grinding buffer and layered on top of a two-step Percoll gradient (80 and 40% percoll in grinding buffer). Centrifugation at 5.750 rpm in an Sorvall HB-4 rotor for 10 min resulted in separation of intact chloroplasts from residual starch granules, mitochondria, broken chloroplasts, and other cellular membranes. Chloroplasts were washed three times by careful resuspension in ice-cold grinding buffer and centrifugation for 1 min at 3.000 rpm in a Sorvall HB-4 rotor. Purified chloroplasts were carefully resuspended in 0.6 M sorbitol in Tricine-EDTA buffer (10 mM Tricine and 1 mM EDTA, pH 7.0) to cause shrinkage for 30 min. Chloroplasts were subjected to a freeze-thaw cycle. After homogenization, thylakoid membranes were removed by centrifugation at 2000g. The sorbitol concentration in the supernatant was diluted to 0.3 M with Tricine-EDTA buffer. Envelope membrane vesicles were collected from the supernatant by centrifugation on a three-step sucrose gradient (0.8, 0.64, and 0.4 M in Tricine buffer) at 113,000g for 3 h. Verification of membrane fraction identity was done using antibodies directed to phosphate translocator in inner or 24-kD outer chloroplast membrane protein from spinach (Spinacia oleracea; kindly provided by U.I. Flügge, University of Cologne, Germany).
Analysis of CO2 Permeability of Membrane Vesicles
Vesicles were suspended in a buffer solution containing 50 mM KCl, 50 mM NaCl, and 20 mM HEPES, pH 7.0, supplemented with 50 μM carboxyfluorescein and 1 mg/mL carbonic anhydrase and incubated at 4°C overnight. Carboxyfluorescein- and carbonic anhydrase–loaded vesicles were pelleted by centrifugation (100,000g, 30 min) and washed once with buffer aerated with N2. Vesicles loaded with carbonic anhydrase and fluorescein were rapidly mixed with the above-mentioned buffer solution aerated with CO2 in a stopped flow spectrometry device (SFM-300; Bio-Logic Scientific Instruments). Uptake of CO2 resulted in an intravesicular acidification and consequently a decrease of fluorescein fluorescence. To calculate membrane CO2 permeability, the intravesicular pH was assessed prior to CO2 uptake and after the reaction was completed following the procedure given by Slavik (1982). For this, the ratio of pH-independent to pH-dependent fluorescein fluorescence (435 nm versus 490 nm) at different pH values was determined. The resulting calibration curve (pH/ratio of fluorescence intensity) was used to calculate the intracellular pH from the ratio of fluorescence intensity as measured prior to and after the reaction. Consequently, the kinetics in fluorescence change as detected by the stopped flow spectrophotometer could be converted into a pH-change kinetics. The exponential time constant of the acidification was determined over the initial 100 ms. CO2 permeability was calculated using the method given by Yang et al. (2006). The average vesicle diameter was 276 and 365 nm for plasma membrane and chloroplast envelope vesicles, respectively, giving surface-to-volume ratios of 2.17*105 cm−1 and 1.64*105 cm−1. Vesicle size was determined using a Zetasizer system (Malvern Instruments).
Analysis of H2O Permeability of Membrane Vesicles
Water permeability of membrane vesicles was analyzed by measuring the intensity of light scattering following an osmotic challenge as described by Maurel et al. (1997). Purified plasma membrane vesicles were diluted to a concentration of 100 μg protein·mL−1 in a hypotonic solution (50 mM NaCl, 50 mM mannitol, and 10 mM Tris-MES, pH 8.3), which induced a transient opening of vesicles and equilibration of the intravesicular material with the buffer solution. The vesicles were mixed with an equal volume of the same solution as used for equilibration supplemented with 500 mM mannitol in an SFM 300 stopped flow spectrometer (Bio-Logic Scientific Instruments). Vesicle shrinkage was monitored via measurement of 90° scattered light intensity increase.
Estimation of gi and cc
Online measurements of photosynthetic 13CO2 discrimination from a combined IRGA-tunable diode laser system were used to calculate gi and cc using the equations of Evans et al. (1986). The TGA-100 (Campbell Scientific) measures absolute concentrations of 13CO2 and 12CO2 at a frequency of 10 Hz from dry air before and after exposure to a photosynthesising leaf. The 10 Hz data were averaged over 15 s every 3 min (between calibrations) to calculate the isotopic composition (δ13C) of sampled air with a precision of 0.05 to 0.09‰. Measurements of photosynthesis were made using IRGAs in an LI-6400 portable gas exchange system with a 6 cm2 leaf chamber and a red/blue LED light source (LI-COR). All data except gi are reported for a light intensity of 2000 μmol m−2 s−1 PPFD. Estimates of gi were generated from online measurements of Δ made at light intensities ranging from 500 to 2000 μmol m−2 s−1 PPFD to vary the ratio of A to ca (ambient partial pressure of CO2). We report the units of gi per Pascal (μmol m−2 s−1 Pa−1) rather than the traditional method that uses non-SI units of per bar (mol m−2 s−1 bar−1). Conversion to the per bar values is achieved by dividing per Pa data by 10. Leaf temperature was kept at 25°C, and leaves were provided with 30 Pa CO2 with a δ13C of approximately −4‰. Measurements were made on the youngest fully expanded leaves.
We calculated cc using the models of photosynthetic discrimination (Δ) presented by Farquhar et al. (Farquhar et al., 1982; Farquhar and Richards, 1984). The simple model describing predicted discrimination (Δi) is  where ca, cs, and ci are the ambient (well mixed air surrounding the leaf), leaf surface, and intercellular partial pressures of CO2, respectively, ab is the fractionation occurring from diffusion through the leaf boundary layer (2.9‰), a is the fractionation occurring from diffusion in air (4.4‰), and b is the net fractionation caused by Rubisco and PEPC (29‰ for tobacco) (Evans et al., 1994). In the simple model, cc is related to Δ by making the additional assumption that gi is infinite (ci = cc). The slope of the plot of Δi − Δ versus A/ca (see Figure 5 for an example) is inversely proportional to gi (Evans et al., 1986). We used the relationship cc = ci − (A/gi) to calculate CO2 partial pressure at the chloroplast. Statistically significant differences were analyzed using a Student's t test (P < 0.05). Data are given as means ± se.
where ca, cs, and ci are the ambient (well mixed air surrounding the leaf), leaf surface, and intercellular partial pressures of CO2, respectively, ab is the fractionation occurring from diffusion through the leaf boundary layer (2.9‰), a is the fractionation occurring from diffusion in air (4.4‰), and b is the net fractionation caused by Rubisco and PEPC (29‰ for tobacco) (Evans et al., 1994). In the simple model, cc is related to Δ by making the additional assumption that gi is infinite (ci = cc). The slope of the plot of Δi − Δ versus A/ca (see Figure 5 for an example) is inversely proportional to gi (Evans et al., 1986). We used the relationship cc = ci − (A/gi) to calculate CO2 partial pressure at the chloroplast. Statistically significant differences were analyzed using a Student's t test (P < 0.05). Data are given as means ± se.
Figure 5.
Raw Data for One Control and One RNAi Leaf Used to Calculate gi from Online Measurements of Photosynthetic Discrimination.
Linear regressions (solid and dashed lines) were fit to a plot of predicted discrimination (Δi) minus observed discrimination (Δobs) versus the ratio of photosytnthesis to the ambient partial pressure of CO2 (A/ca). The inverse of the slope was used to calculate gi for each leaf. Closed symbols, control; open symbols, RNAi leaf.
Accession Numbers
Sequence data of genes appearing in this article can be found in the GenBank/EMBL data libraries under accession numbers AJ001416 (Nt AQP1), NM_198098 (human AQP1), and SCU87624 (mGFP4).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Identification of Chloroplast Envelope Proteins by Immunoblots.
Supplemental Figure 2. Nt AQP1 in RNAi and Control Plants.
Acknowledgments
This work was supported through funding from the Deutsch Forschungsgemeinschaft (Project KA 1032/15-2 to R.K.), the Institute of Geophysics and Planetary Physics at Los Alamos National Laboratory (Project 095566-001-05 to N.M. and D.T.H.), a Laboratory Directed Research and Development-Exploratory Research grant (N.M.), and the University of New Mexico Research Allocation Committee (Grant 05-L-07 to D.T.H.). We thank U.I. Flügge (University of Cologne, Germany) and J. Soll (University of Munich, Germany) for providing antibodies against chloroplast envelope proteins.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Norbert Uehlein (uehlein@bio.tu-darmstadt.de).
Online version contains Web-only data.
References
- Agre, P. (2004). Aquaporin water channels (Nobel lecture). Angew. Chem. Int. Ed. 43 4278–4290. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam, M., Lindland, H., Zelenin, S., Roberg, B.A., Gundersen, B.B., Petersen, P., Rinvik, E., Torgner, I.A., and Ottersen, O.P. (2005). Brain mitochondria contain aquaporin water channels: Evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 19 1459–1467. [DOI] [PubMed] [Google Scholar]
- Barbour, M.M., McDowell, N.G., Tcherkez, G., Bickford, C.P., and Hanson, D.T. (2007). A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ. 30 469–482. [DOI] [PubMed] [Google Scholar]
- Bernacchi,C.J., Portis,A.R., Nakano,H., von Caemmerer, S., and Long, S.P. (2002). Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 130 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biela, A., Grote, K., Otto, B., Hoth, S., Hedrich, R., and Kaldenhoff, R. (1999). The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J. 18 565–570. [DOI] [PubMed] [Google Scholar]
- Bock, R. (1998). Analysis of RNA editing in plastids. Methods 15 75–83. [DOI] [PubMed] [Google Scholar]
- Bots, M. (2004). Control of Water Movement in Reproductive Organs of Tobacco. (Enschede, The Netherlands: PrintPartners Ipskamp).
- Calamita, G., Gena, P., Meleleo, D., Ferri, D., and Svelto, M. (2006). Water permeability of rat liver mitochondria: A biophysical study. Biochim. Biophys. Acta 1758 1018–1024. [DOI] [PubMed] [Google Scholar]
- Cline, K. (1985). Cell components. In Modern Methods of Plant Analysis, H.F.Linskens and J.F.Jackson, eds (Berlin: Springer), pp. 182–198.
- de Groot, B.L., Engel, A., and Grubmuller, H. (2001). A refined structure of human aquaporin-1. FEBS Lett. 504 206–211. [DOI] [PubMed] [Google Scholar]
- Eckert, M., Biela, A., Siefritz, F., and Kaldenhoff, R. (1999). New aspects of plant aquaporin regulation and specificity. J. Exp. Bot. 50 1541–1545. [Google Scholar]
- Engelman, D.M. (2005). Membranes are more mosaic than fluid. Nature 438 578–580. [DOI] [PubMed] [Google Scholar]
- Ethier, G.J., Livingston, N.J., Harrison, D.L., Black, T.A., and Moran, J.A. (2006). Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant Cell Environ. 29 2168–2184. [DOI] [PubMed] [Google Scholar]
- Evans, J.R., and Loreto, F. (2000). Acquisition and diffusion of CO2 in higher plant leaves. In Photosynthesis: Physiology and Metabolism, R.C. Leegood, T.D. Sharkey, and S. von Caemmerer, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 321–351.
- Evans, J.R., Sharkey, T.D., Berry, J.A., and Farquhar, G.D. (1986). Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher plants. Aust. J. Plant Physiol. 13 281–292. [Google Scholar]
- Evans, J.R., von Caemmerer, S., Setchell, B.A., and Hudson, M. (1994). The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust. J. Plant Physiol. 21 475–495. [Google Scholar]
- Farquhar, G.D., O'Leary, M.H., and Berry, J.A. (1982). On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust. J. Plant Physiol. 9 121–137. [Google Scholar]
- Farquhar, G.D., and Richards, L.A. (1984). Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 11 539–552. [Google Scholar]
- Ferrando, A., Farras, R., Jasik, J., Schell, J., and Koncz, C. (2000). Intron-tagged epitope: A tool for facile detection and purification of proteins expressed in Agrobacterium-transformed plant cells. Plant J. 22 553–560. [DOI] [PubMed] [Google Scholar]
- Flexas, J., Ribas-Carbo, M., Hanson, D.T., Bota, J., Otto, B., Cifre, J., McDowell, N., Medrano, H., and Kaldenhoff, R. (2006). Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 48 427–439. [DOI] [PubMed] [Google Scholar]
- Gillon, J.S., and Yakir, D. (2000). Internal conductance to CO(2) diffusion and C(18)OO discrimination in C(3) leaves. Plant Physiol. 123 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht, J., Bisson, M.A., and Tosteson, F.C. (1977). Diffusion of carbon-dioxide through lipid bilayer membranes - Effects of carbonic-anhydrase, bicarbonate, and enstirred layers. J. Gen. Physiol. 69 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba, Y.T., Shibasaka, M., Hayashi, Y., Hayakawa, T., Kasamo, K., Terashima, I., and Katsuhara, M. (2004). Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 45 521–529. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., and Amos, B. (1995). GFP in plants. Trends Genet. 11 328–329. [DOI] [PubMed] [Google Scholar]
- Hevesy, G., Hofer, E., and Krogh, A. (1935). The permeability of the skin of frogs to water as determined by D2O and H2O. Skand. Arch. Physiol. 72 199–214. [Google Scholar]
- Hill, A.E., Shachar-Hill, B., and Shachar-Hill, Y. (2004). What are aquaporins for? J. Membr. Biol. 197 1–32. [DOI] [PubMed] [Google Scholar]
- Hub, J.S., and de Groot, B.L. (2006). Does CO2 permeate through Aquaporin-1? Biophys. J. 93 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M., Beitz, E., Kozono, D., Guggino, W.B., Agre, P., and Yasui, M. (2002). Characterization of Aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J. Biol. Chem. 277 39873–39879. [DOI] [PubMed] [Google Scholar]
- Jahn, T.P., Moller, A.L.B., Zeuthen, T., Holm, L.M., Klaerke, D.A., Mohsin, B., Kuhlbrandt, W., and Schjoerring, J.K. (2004). Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 574 31–36. [DOI] [PubMed] [Google Scholar]
- Johansson, I., Karlsson, M., Shukla, V.K., Chrispeels, M.J., Larsson, C., and Kjellbom, P. (1998). Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff, R., Kolling, A., Meyers, J., Karmann, U., Ruppel, G., and Richter, G. (1995). The blue light-responsive AthH2 gene of Arabidopsis thaliana is primarily expressed in expanding as well as in differentiating cells and encodes a putative channel protein of the plasmalemma. Plant J. 7 87–95. [DOI] [PubMed] [Google Scholar]
- Kannangara, C.G., Gough, S.P., Hansen, B., Rasmussen, J.N., and Simpson, D.J. (1977). A homogenizer with replaceable razor blades for bulk isolation of active barley plastids. Carlsberg Res. Commun. 42 431–439. [Google Scholar]
- Kjellbom, P., and Larsson, C. (1984). Preparation and polypeptide composition of chlorophyll-free plasma membranes from leaves of light-grown spinach and barley. Plant Physiol. 62 501–509. [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Lundborg, T., Widell, S., and Larsson, C. (1981). Distribution of ATPases in wheat root membranes separated by phase partition. Physiol. Plant 52 89–95. [Google Scholar]
- Maurel, C., Tacnet, F., Guclu, J., Guern, J., and Ripoche, P. (1997). Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. Proc. Natl. Acad. Sci. USA 94 7103–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion, M., Moran, N., and Chaumont, F. (2004). Dynamic changes in the osmotic water permeability of protoplast plasma membrane. Plant Physiol. 135 2301–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul, N.L., Davis, B.A., Romero, M.F., and Boron, W.F. (1998). Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. Cell Physiol. 43 C543–C548. [DOI] [PubMed] [Google Scholar]
- Otto, B., and Kaldenhoff, R. (2000). Cell-specific expression of the mercury-insensitive plasma membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 211 167–172. [DOI] [PubMed] [Google Scholar]
- Prasad, G.V.R., Coury, L.A., Finn, F., and Zeidel, M.L. (1998). Reconstituted aquaporin 1 water channels transport CO2 across membranes. J. Biol. Chem. 273 33123–33126. [DOI] [PubMed] [Google Scholar]
- Preston, G.M., Carroll, T.P., Guggino, W.B., and Agre, P. (1992). Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256 385–387. [DOI] [PubMed] [Google Scholar]
- Price,G.D., Evans,J.R., von Caemmerer, S., Yu, J.W., and Badger, M.R. (1995). Specific reduction of chloroplast glyceraldehyde-3-phosphate dehydrogenase activity by antisense RNA reduces CO2 assimilation via a reduction in ribulose bisphosphate regeneration in transgenic tobacco plants. Planta 195 369–378. [DOI] [PubMed] [Google Scholar]
- Sanford, J.C., Smith, F.D., and Russell, J.A. (1993). Optimizing the biolistic process for different biological applications. Methods Enzymol. 217 483–509. [DOI] [PubMed] [Google Scholar]
- Slavik, J. (1982). Intracellular pH of yeast cells measured with fluorescent probes. FEBS Lett. 140 22–26. [DOI] [PubMed] [Google Scholar]
- Terashima, I., Hanba, Y.T., Tazoe, Y., Vyas, P., and Yano, S. (2006). Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 57 343–354. [DOI] [PubMed] [Google Scholar]
- Terashima, I., and Ono, K. (2002). Effects of HgCl2 on CO2 dependence of leaf photosynthesis: Evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol. 43 70–78. [DOI] [PubMed] [Google Scholar]
- Uehlein, N., Lovisolo, C., Siefritz, F., and Kaldenhoff, R. (2003). The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425 734–737. [DOI] [PubMed] [Google Scholar]
- Warren, C.R., and Adams, M.A. (2006). Internal conductance does not scale with photosynthetic capacity: Implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ. 29 192–201. [DOI] [PubMed] [Google Scholar]
- Widell, S., and Larsson, C. (1981). Separation of presumptive plasma membranes from mitochondria by partition in an aqueous polymer two-phase system. Physiol. Plant 51 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B., and Verkman, A.S. (1997). Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 272 16140–16146. [DOI] [PubMed] [Google Scholar]
- Yang, B., Zhao, D., and Verkman, A.S. (2006). Evidence against functionally significant aquaporin expression in mitochondria. J. Biol. Chem. 281 16202–16206. [DOI] [PubMed] [Google Scholar]
- Yasui, M., Hazama, A., Kwon, T.H., Nielsen, S., Guggino, W.B., and Agre, P. (1999). Rapid gating and anion permeability of an intracellular aquaporin. Nature 402 184–187. [DOI] [PubMed] [Google Scholar]