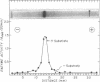
Abstract
Dihydroorotase +4,5-L-dihydro-orotate amidohydrolase [EC 3.5.2.3]), which catalyzes the reversible cyclization of N-carbamyl-L-aspartate to L-dihydroorotate, has been purified from orotate-grown Clostridium oroticum. The enzyme is homogeneous when subjected to polyacrylamide gel electrophoresis and is stable at pH 7.6 in 0.3 M NaCl containing 10 muM ZnSO4. The enzyme has a molecular weight of approximately 110,000. Sodium dodecyl sulfate gel electrophoresis, using three different buffer systems, indicated the enzyme is composed of two subunits, each having a molecular weight of 55,000. Dihydroorotase is shown by atomic absorption spectroscopy to be a zinc-containing metalloenzyme with 4 g-atoms of zinc per 110,000 g of protein. The pH optima for the conversion of N-carbamyl-L-aspartate to L-dihydroorotate and for L-dihydroorotate to N-carbamyl-L-aspartate are pH 6.0 and 8.2, respectively. The Km values for N-carbamyl-L-aspartate and for L-dihydroorotate are 0.13 and 0.07 mM, respectively. Inhibitor studies indicate that zinc may be involved in the catalytic activity of the enzyme.
Full text
PDF



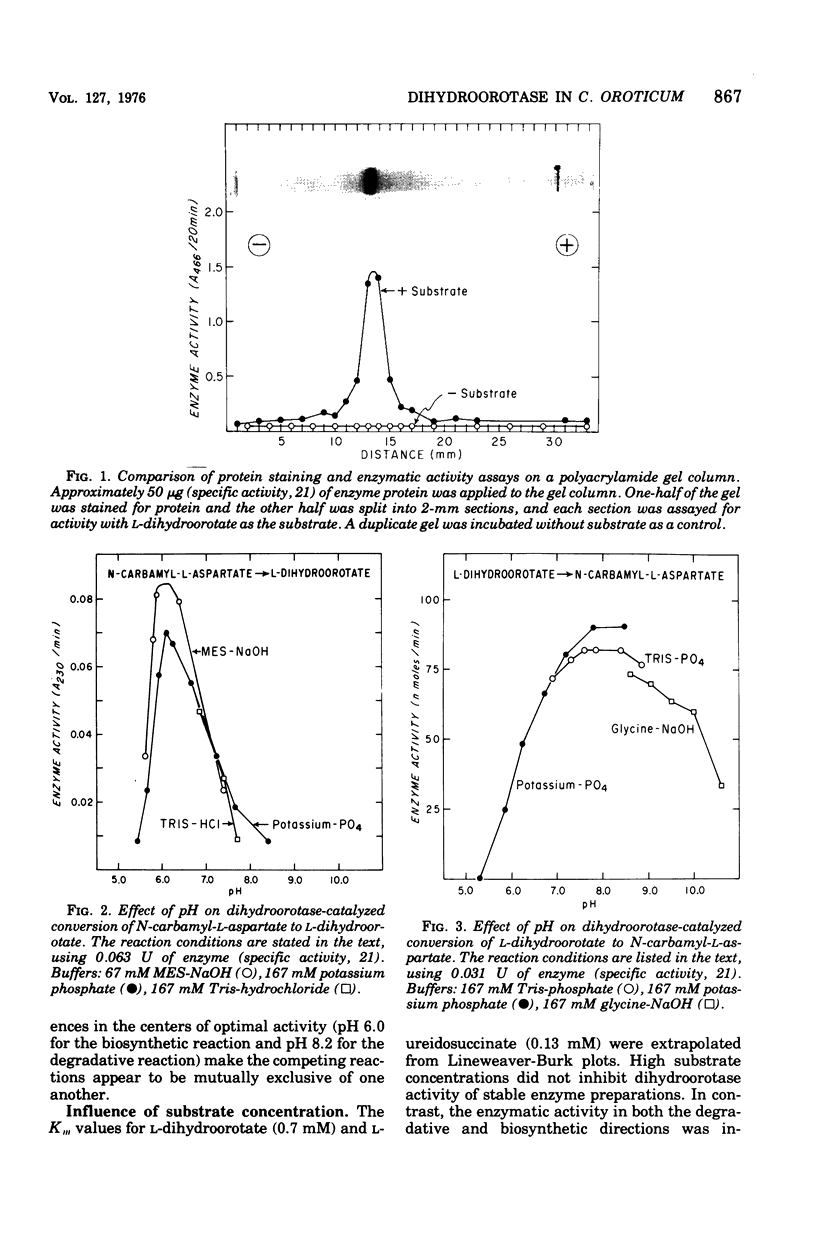
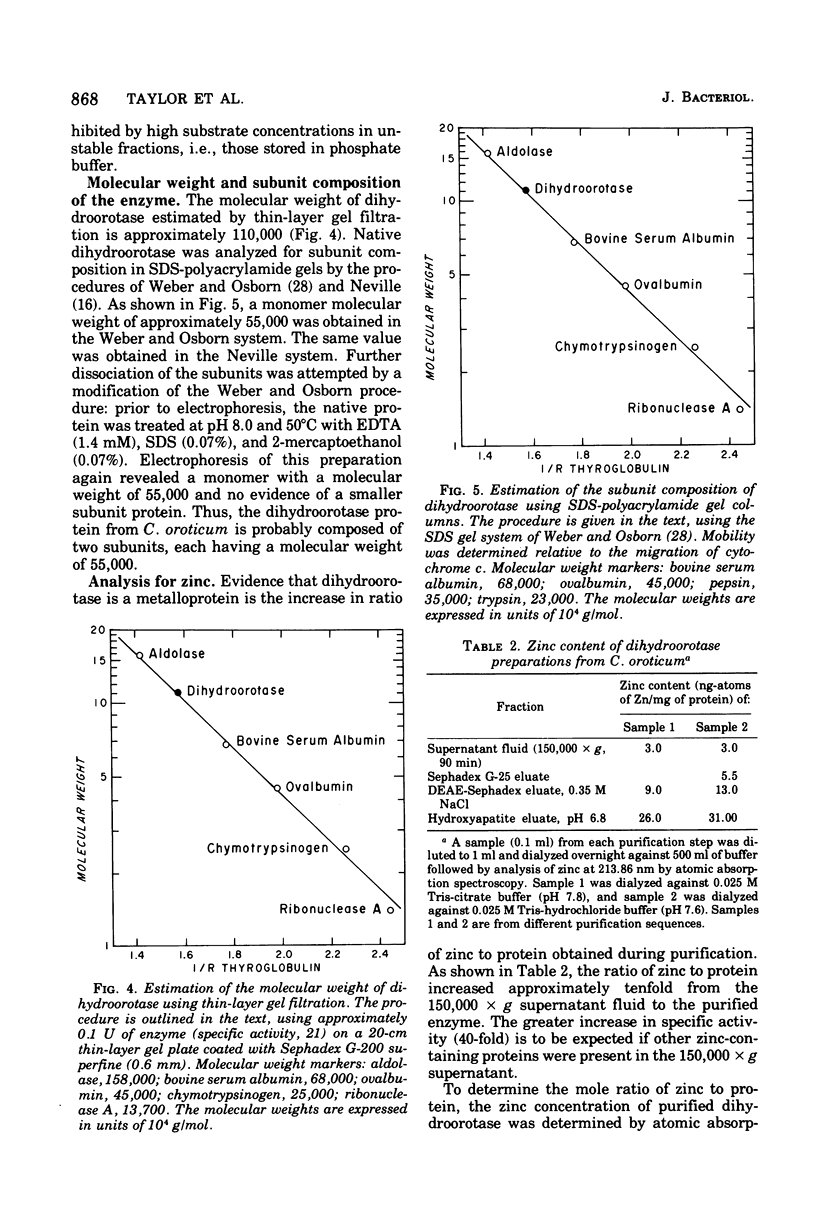
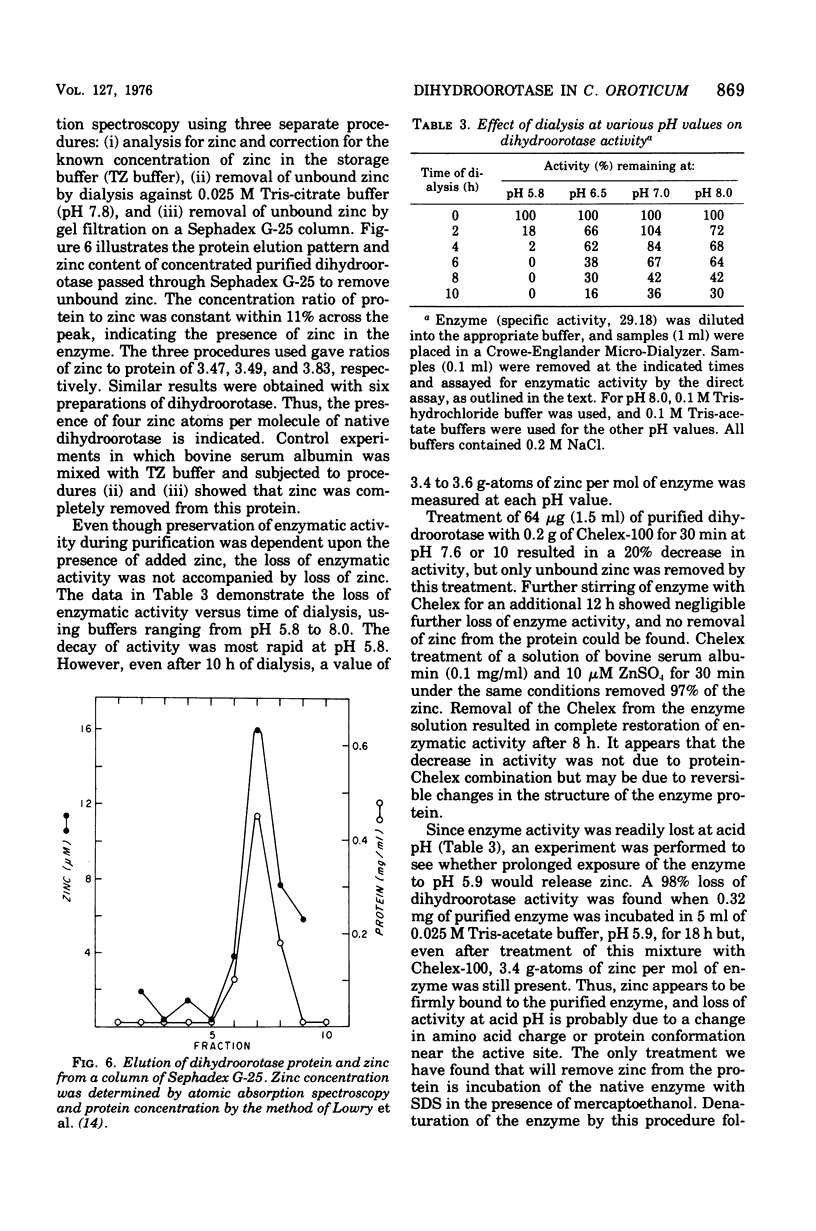




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- BRESNICK E., BLATCHFORD K. STUDIES ON DIHYDROOROTASE ACTIVITY IN PREPARATIONS FROM NOVIKOFF ASCITES HEPATOMA CELLS. Arch Biochem Biophys. 1964 Mar;104:381–386. doi: 10.1016/0003-9861(64)90479-5. [DOI] [PubMed] [Google Scholar]
- Brändén C. I., Eklund H., Nordström B., Boiwe T., Söderlund G., Zeppezauer E., Ohlsson I., Akeson A. Structure of liver alcohol dehydrogenase at 2.9-angstrom resolution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2439–2442. doi: 10.1073/pnas.70.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drum D. E., Harrison J. H., 4th, Li T. K., Bethune J. L., Vallee B. L. Structural and functional zinc in horse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1967 May;57(5):1434–1440. doi: 10.1073/pnas.57.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drum D. E., Vallee B. L. Differential chemical reactivities of zinc in horse liver alcohol dehydrogenase. Biochemistry. 1970 Oct 13;9(21):4078–4086. doi: 10.1021/bi00823a008. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HJERTEN S. "Molecular sieve" chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962 Sep;Suppl 1:147–151. [PubMed] [Google Scholar]
- Kennedy J. Dihydroorotase from rat liver: purification, properties and regulatory role in pyrimidine biosynthesis. Arch Biochem Biophys. 1974 Feb;160(2):358–365. [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A. Enzymatic synthesis and breakdown of a pyrimidine, orotic acid. I. Dihydroortic acid, ureidosuccinic acid, and 5-carboxymethylhydantoin. J Biol Chem. 1954 Apr;207(2):911–924. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazuś B., Buchowicz J. Purification and properties of dihydro-orotase from pea plants. Acta Biochim Pol. 1968;15(4):317–325. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- Pradhan T. K., Sander E. G. Noncompetitive inhibition by substituted sulfonamides of dihydroorotase from Zymobacterium oroticum. Life Sci. 1973 Dec 16;13(12):1747–1752. doi: 10.1016/0024-3205(73)90121-5. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S., LIEBERMAN I., KORNBERG A. The metabolism of orotic acid in aerobic bacteria. J Bacteriol. 1955 Mar;69(3):250–255. doi: 10.1128/jb.69.3.250-255.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E. G., Heeb M. J. Purification and properties of dihydroorotase from Escherichia coli B. Biochim Biophys Acta. 1971 Feb 10;227(2):442–452. doi: 10.1016/0005-2744(71)90075-1. [DOI] [PubMed] [Google Scholar]
- Sander E. G., Wright L. D., McCormick D. B. Evidence for function of a metal ion in the activity of dihydroorotase from Zymobacterium oroticum. J Biol Chem. 1965 Sep;240(9):3628–3630. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Taylor M. L., Taylor W. H., Eames D. F., Taylor C. D. Biosynthetic dihydroorotate dehydrogenase from Lactobacillus bulgaricus. J Bacteriol. 1971 Mar;105(3):1015–1027. doi: 10.1128/jb.105.3.1015-1027.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor C. D., Taylor M. L. Biosynthetic dihydroorotate dehydrogenase from Lactobacillus bulgaricus: partial characterization of the enzyme. J Bacteriol. 1974 Jul;119(1):98–105. doi: 10.1128/jb.119.1.98-105.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Eames D. F. Two functionally different dihydroorotic dehydrogenases in bacteria. J Bacteriol. 1966 Jun;91(6):2251–2256. doi: 10.1128/jb.91.6.2251-2256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Leisinger T. Dual role for N-2-acetylornithine 5-aminotransferase from Pseudomonas aeruginosa in arginine biosynthesis and arginine catabolism. J Bacteriol. 1975 Jun;122(3):799–809. doi: 10.1128/jb.122.3.799-809.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]