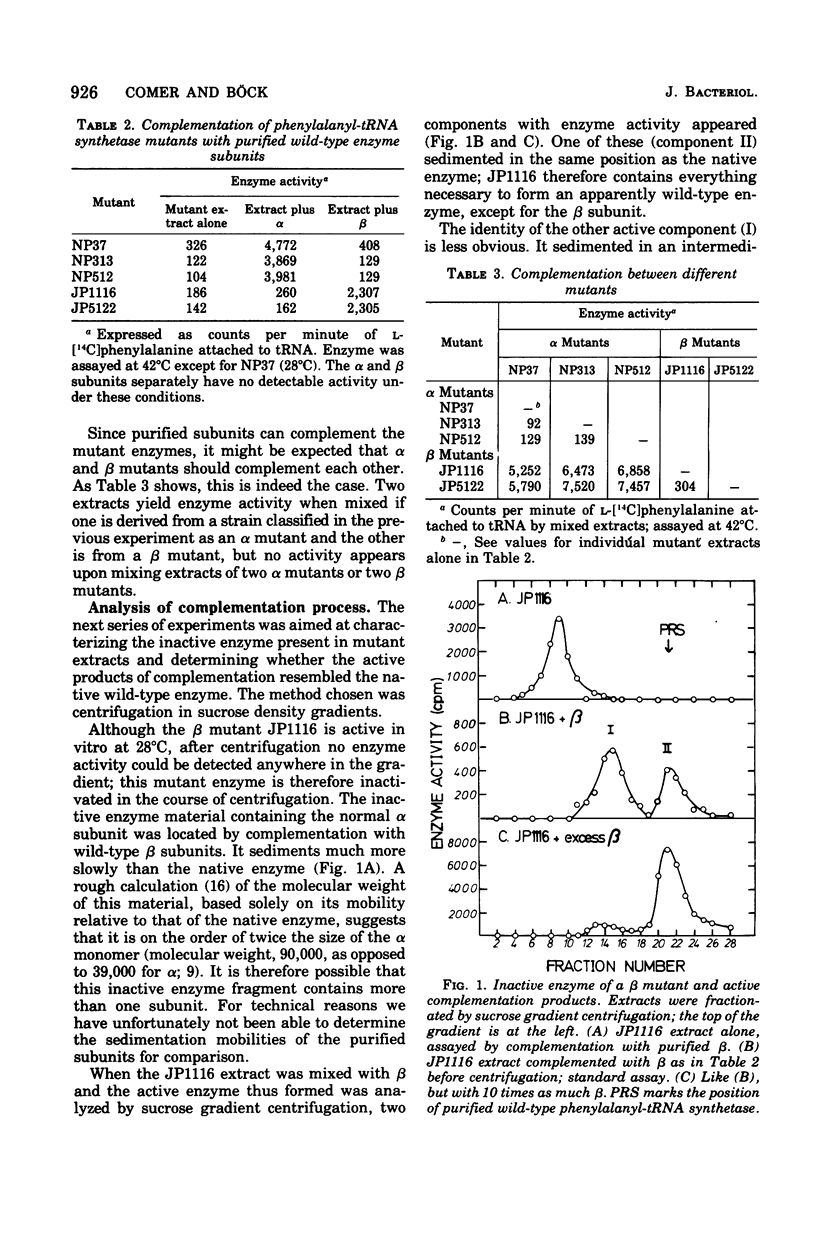
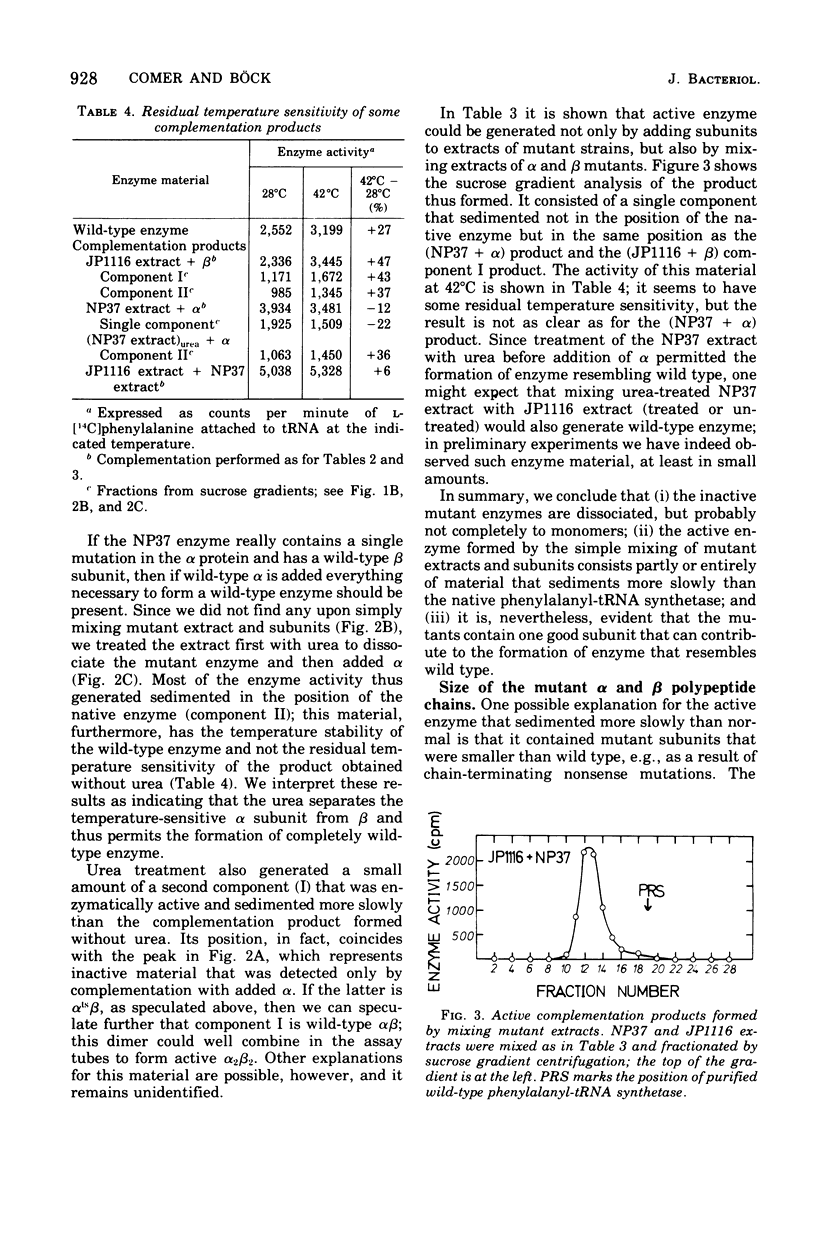
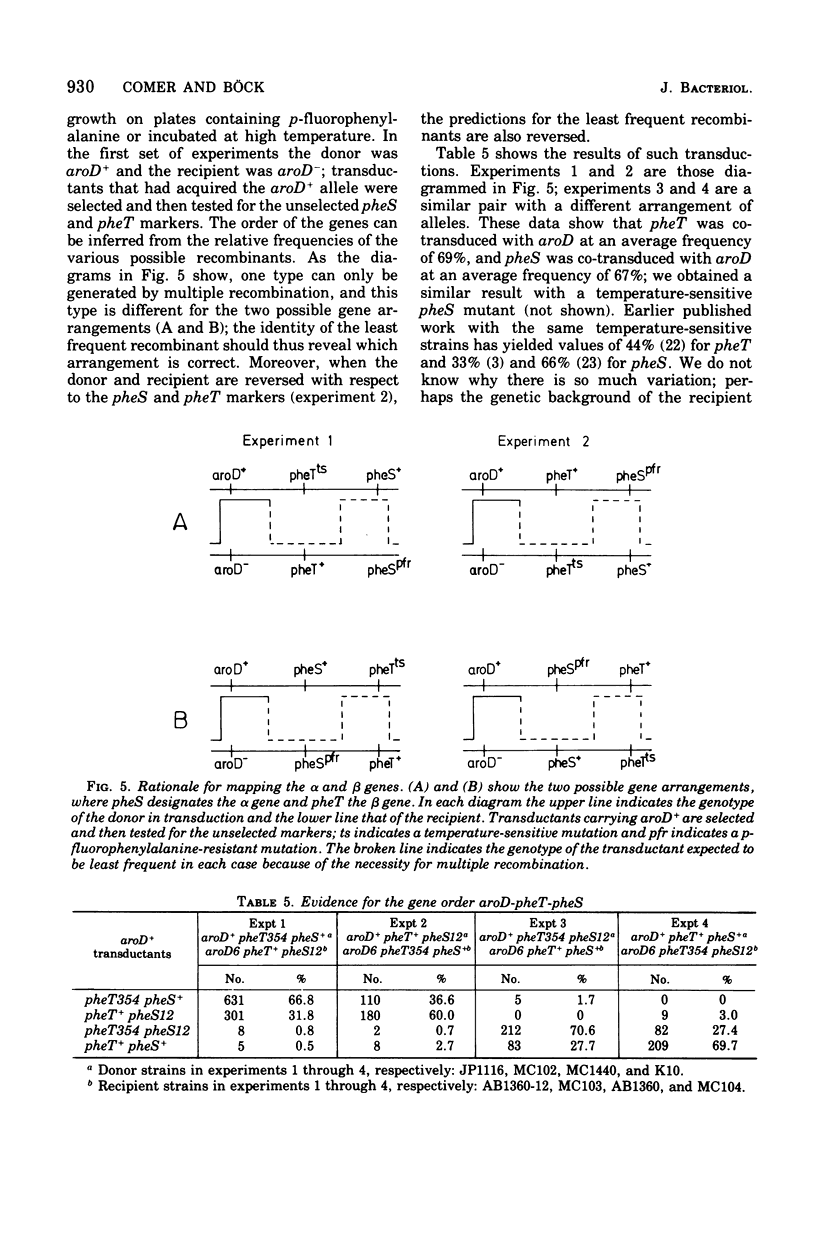
Abstract
The phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli is a tetramer that contains two different kinds of polypeptide chains. To locate the genes for the two polypeptides, we analyzed temperature-sensitive mutants with defective phenylalanyl-transfer ribonucleic acid synthetases to see which subunit was altered. The method was in vitro complementation; mutant cell extracts were mixed with purified separated alpha or beta subunits of the wild-type enzyme to generate an active hybrid enzyme. With three mutants, enzyme activity appeared when alpha was added, but not when beta was added: these are, therefore, assumed to carry lesions in the gene for the alpha subunit. Two other mutants gave the opposite response and are presumably beta mutants. Enzyme activity is also generated when alpha and beta mutant extracts are mixed, but not when two alpha or two beta mutant extracts are mixed. The inactive mutant enzymes appear to be dissociated, as judged by their sedimentation in sucrose density gradients, but the dissociation may be only partial. The active enzyme generated by complementation occurred in two forms, one that resembled the native wild-type enzyme and one that sedimented more slowly. Both alpha and beta mutants are capable of generating the native form, although alpha mutants require prior urea denaturation of the defective enzyme. With the mutants thus characterized, the genes for the alpha and beta subunits (designated pheS and heT, respectively) were mapped. The gene order, as determined by transduction is aroD-pps-pheT-pheS. The pheS and pheT genes are close together and may be immediately adjacent.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Genetic mapping of phenylalanyl-sRNA synthetase in Escherichia coli. Science. 1967 Jul 7;157(3784):78–79. doi: 10.1126/science.157.3784.78. [DOI] [PubMed] [Google Scholar]
- Böck A. Relation between subunit structure and temperature-sensitivity of mutant phenylalanyl RNA synthetases of Escherichia coli. Eur J Biochem. 1968 Apr;4(3):395–400. doi: 10.1111/j.1432-1033.1968.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Fayat G., Blanquet S., Dessen P., Batelier G., Waller J. P. The molecular weight and subunit composition of phenylalanyl-tRNA synthetase from Escherichia coli K-12. Biochimie. 1974;56(1):35–41. doi: 10.1016/s0300-9084(74)80353-6. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Isolation and partial characterization of Escherichia coli mutants with altered glycyl transfer ribonucleic acid synthetases. J Bacteriol. 1970 Apr;102(1):193–203. doi: 10.1128/jb.102.1.193-203.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. E., Creighton T. E., Baldwin R. L., Yanofsky C. Subunit structure of the tryptophan synthetase of Escherichia coli. J Mol Biol. 1966 Oct 28;21(1):71–82. doi: 10.1016/0022-2836(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Hanke T., Bartmann P., Hennecke H., Kosakowski H. M., Jaenicke R., Holler E., Böck A. L-phenylalanyl-tRNA synthetase of Escherichia coli K-10. A reinvestigation of molecular weight and subunit structure. Eur J Biochem. 1974 Apr 16;43(3):601–607. doi: 10.1111/j.1432-1033.1974.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Böck A. Altered alpha subunits in phenylalanyl-tRNA synthetases from p-fluorophenylalanine-resistant strains of Escherichis coli. Eur J Biochem. 1975 Jul 1;55(2):431–437. doi: 10.1111/j.1432-1033.1975.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O. Aminoacyl-tRNA synthetases: sone recent results and achievements. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):141–238. doi: 10.1002/9780470122853.ch5. [DOI] [PubMed] [Google Scholar]
- Kosakowski M. H., Neidhardt F. C., Böck A. Complementation in vitro of phenylalanyl-tRNA synthetases of Escherichia coli. Eur J Biochem. 1970 Jan;12(1):74–79. doi: 10.1111/j.1432-1033.1970.tb00822.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapointe J., Delcuve G. Thermosensitive mutants of Escherichia coli K-12 altered in the catalytic Subunit and in a Regulatory factor of the glutamy-transfer ribonucleic acid synthetase. J Bacteriol. 1975 May;122(2):352–358. doi: 10.1128/jb.122.2.352-358.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem D. L., Berg P. Glycyl-tRNA synthetase: an oligomeric protein containing dissimilar subunits. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1967–1974. doi: 10.1073/pnas.67.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepersberg A., Hennecke H., Engelhard M., Nass G., Böck A. Cross-reactivity of phenylalanyl-transfer ribonucleic acid ligases from different microorganisms. J Bacteriol. 1975 Dec;124(3):1482–1488. doi: 10.1128/jb.124.3.1482-1488.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. Mutants of Escherichia coli unable to make protein at 42 C. J Bacteriol. 1971 Nov;108(2):790–798. doi: 10.1128/jb.108.2.790-798.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. PfkB and pfkC loci of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1153–1161. doi: 10.1128/jb.122.3.1153-1161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



