Abstract
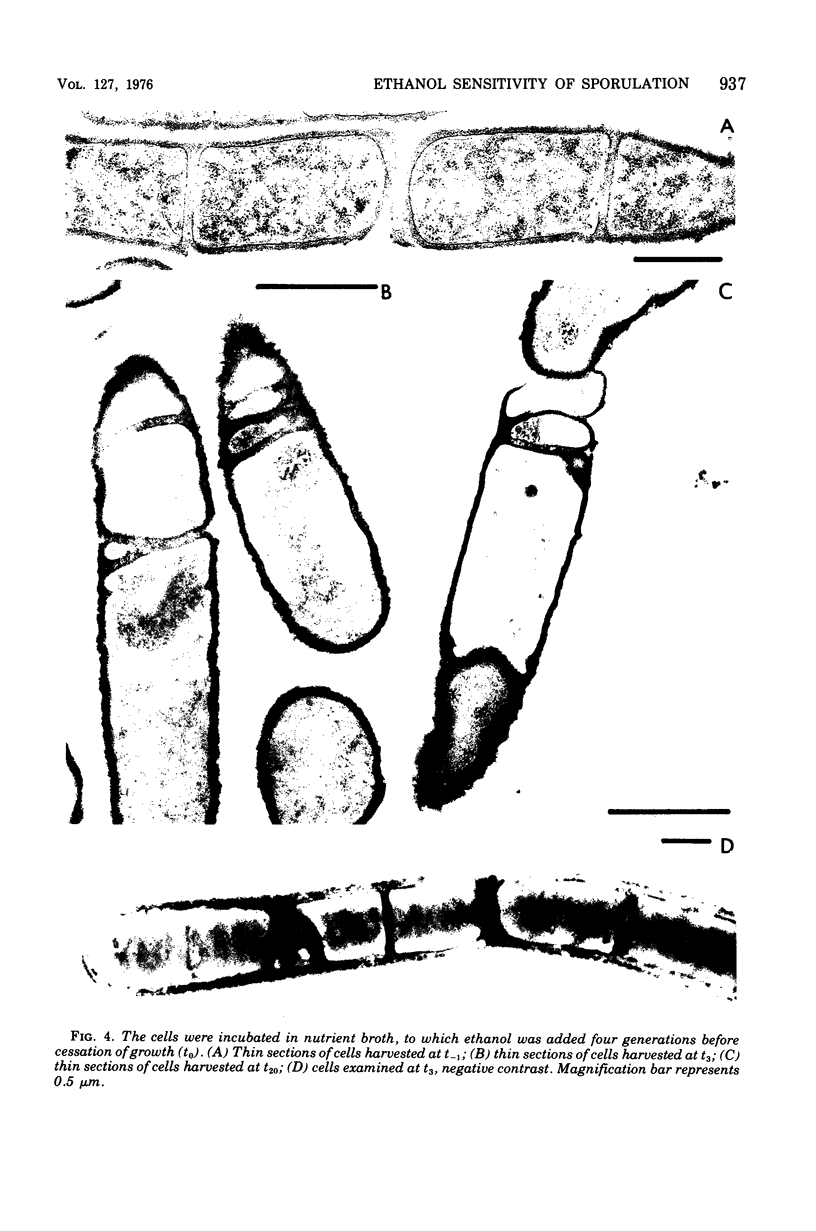
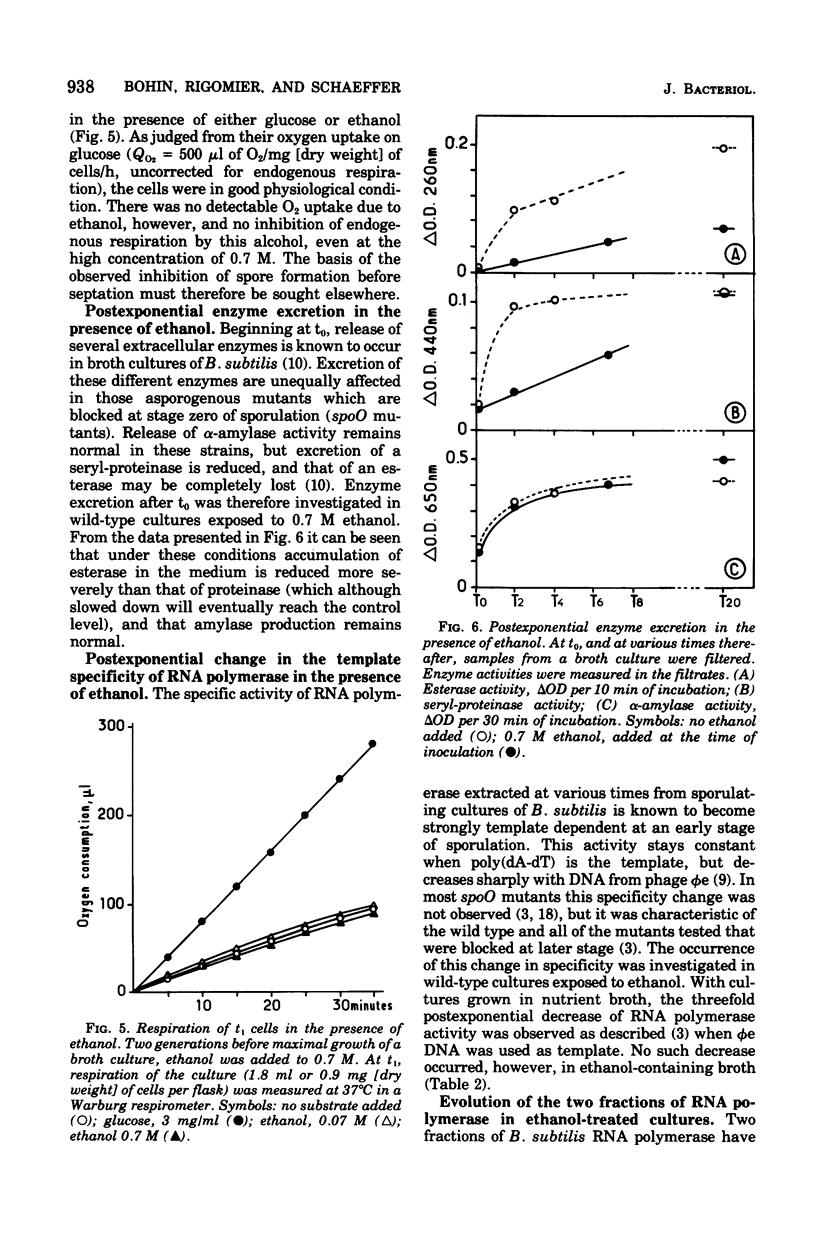
The growth rate of Bacillus subtilis is lowered but the final cell yield is unchanged when certain concentrations of ethanol are present in the culture medium. At the concentration allowing growth at half-maximal rate, practically no spores are formed. Blockage of spore formation generally occurs at stage 0-I. Sensitivity to ethanol of the capacity to form spores is limited, in a nonsynchronized culture, to a period of at most 45 min around t1. Postexponential events such as excretion of certain enzymes and modification of ribonucleic acid polymerase are altered or suppressed in the presence of ethanol, possibly as the results of a physical change upon the cell membrane. In effect, ethanol is turning wild-type cells into phenocopies of spoO mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohin J. P., Bohin A., Schaeffer P. Increased nitrate reductase A activity as a sign of membrane alteration in early blocked asporogenous mutants of Bacillus subtilis. Biochimie. 1976;58(1-2):99–108. doi: 10.1016/s0300-9084(76)80360-4. [DOI] [PubMed] [Google Scholar]
- Brevet J. Direct assay for sigma factor activity and demonstration of the loss of this activity during sporulation in Bacillus subtilis. Mol Gen Genet. 1974 Feb 6;128(3):223–221. doi: 10.1007/BF00267111. [DOI] [PubMed] [Google Scholar]
- Brevet J., Sonenshein A. L. Template specificity of ribonucleic acid polymerase in asporogenous mutants of Bacillus subtilis. J Bacteriol. 1972 Dec;112(3):1270–1274. doi: 10.1128/jb.112.3.1270-1274.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Fried V. A., Novick A. Organic solvents as probes for the structure and function of the bacterial membrane: effects of ethanol on the wild type and an ethanol-resistant mutant of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):239–248. doi: 10.1128/jb.114.1.239-248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guespin-Michel J. E. Phenotypic reversion in some early blocked sporulation mutants of Bacillus subtilis: isolation and phenotype identification of partial revertants. J Bacteriol. 1971 Oct;108(1):241–247. doi: 10.1128/jb.108.1.241-247.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S. J., Vannier F. S. Temperature-sensitive initiation of chromosome replication in a mutant of Bacillus subtilis. J Bacteriol. 1973 May;114(2):474–484. doi: 10.1128/jb.114.2.474-484.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Michel J. F., Millet J. Physiological studies on early-blocked sporulation mutants of Bacillus subtilis. J Appl Bacteriol. 1970 Mar;33(1):220–227. doi: 10.1111/j.1365-2672.1970.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Orrego C., Kerjan P., Manca de Nadra M. C., Szulmajster J. Ribonucleic acid polymerase in a thermosensitive sporulation mutant (ts-4) of Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):636–647. doi: 10.1128/jb.116.2.636-647.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Rigomier D., Lubochinsky B. Métabolisme des phospholipides chez des mutants asporogènes de Bacillus subtilis au cours de la croissance exponentielle. Ann Microbiol (Paris) 1974 Sep;125 B(2):295–303. [PubMed] [Google Scholar]
- Ryter A., Aubert J. P. Etude autoradiographique de la synthèse de l'ADN aucours de la sporulation de Bacillus subtilis. Ann Inst Pasteur (Paris) 1969 Nov;117(5):601–611. [PubMed] [Google Scholar]
- SZULMAJSTER J., SCHAEFFER P. [Augmentation of the DPNH-oxidase activity during sporulation of Bacillus subtilis]. C R Hebd Seances Acad Sci. 1961 Jan 4;252:220–222. [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Losick R. RNA polymerase mutants blocked in sporulation. Nature. 1970 Aug 29;227(5261):906–909. doi: 10.1038/227906a0. [DOI] [PubMed] [Google Scholar]
- Taber H., Freese E. Sporulation properties of cytochrome a-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1004–1011. doi: 10.1128/jb.120.3.1004-1011.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



