Abstract
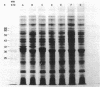
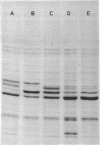
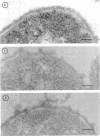
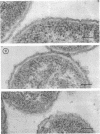
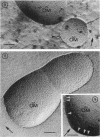
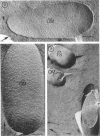
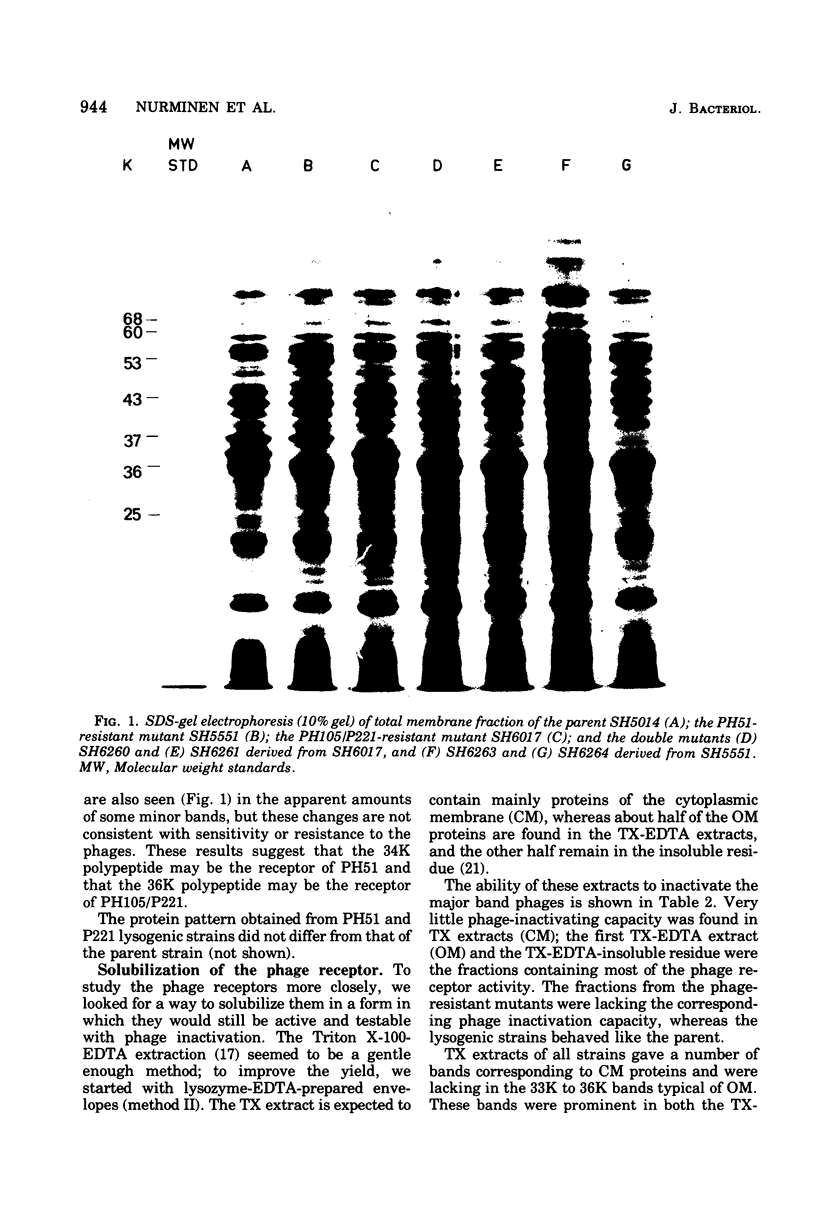
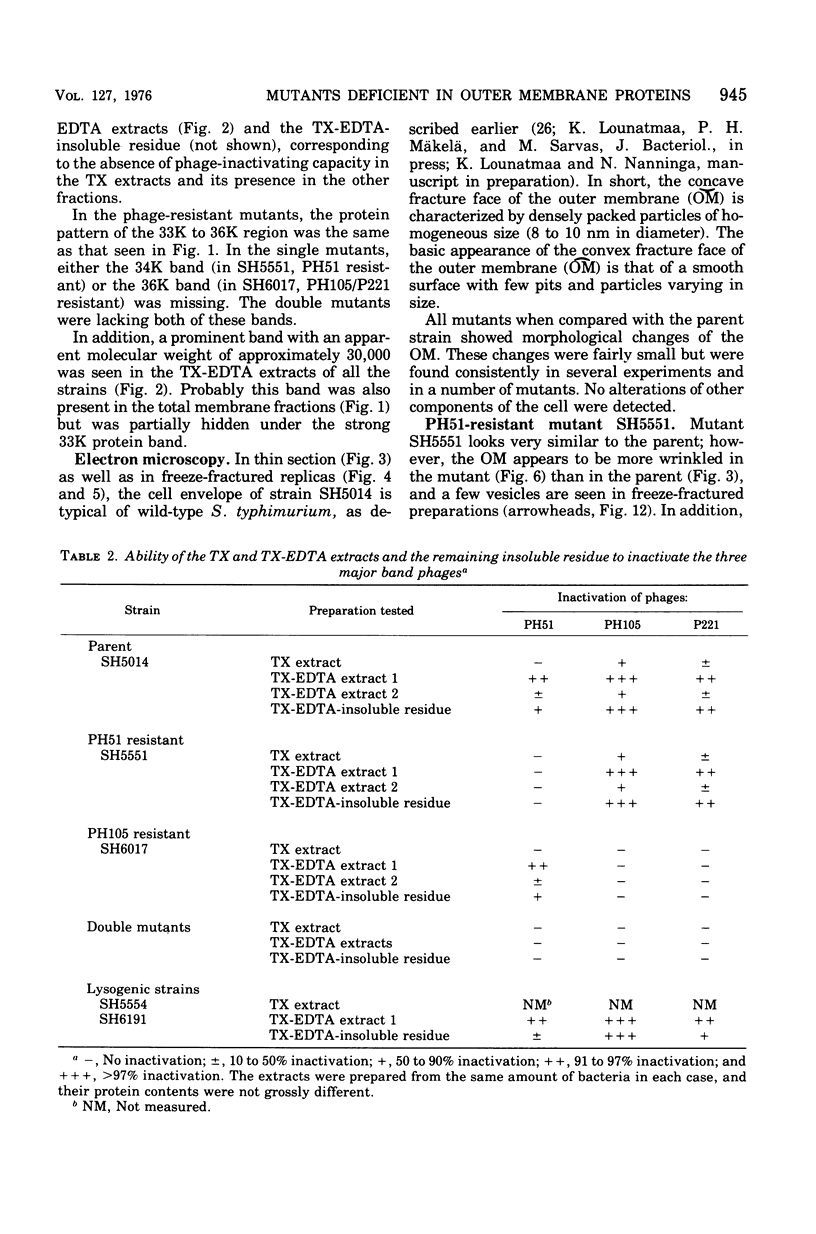
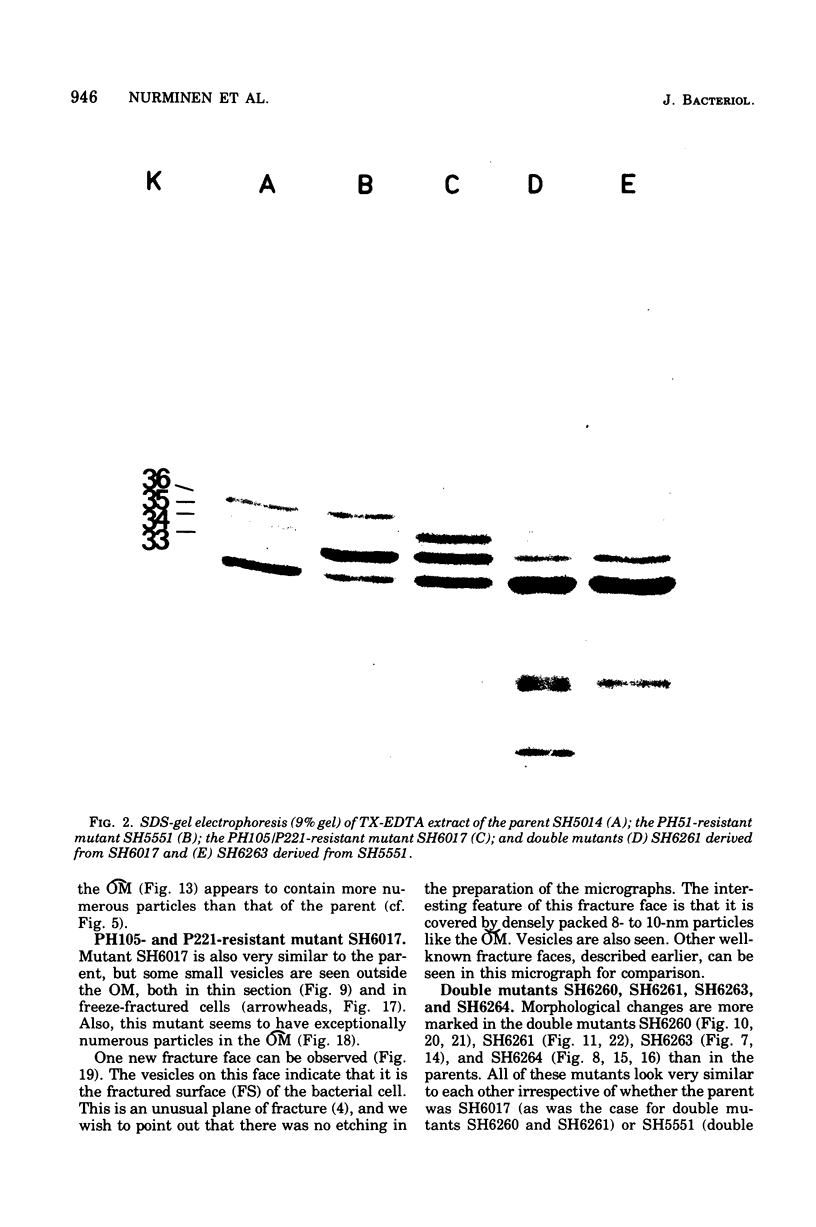
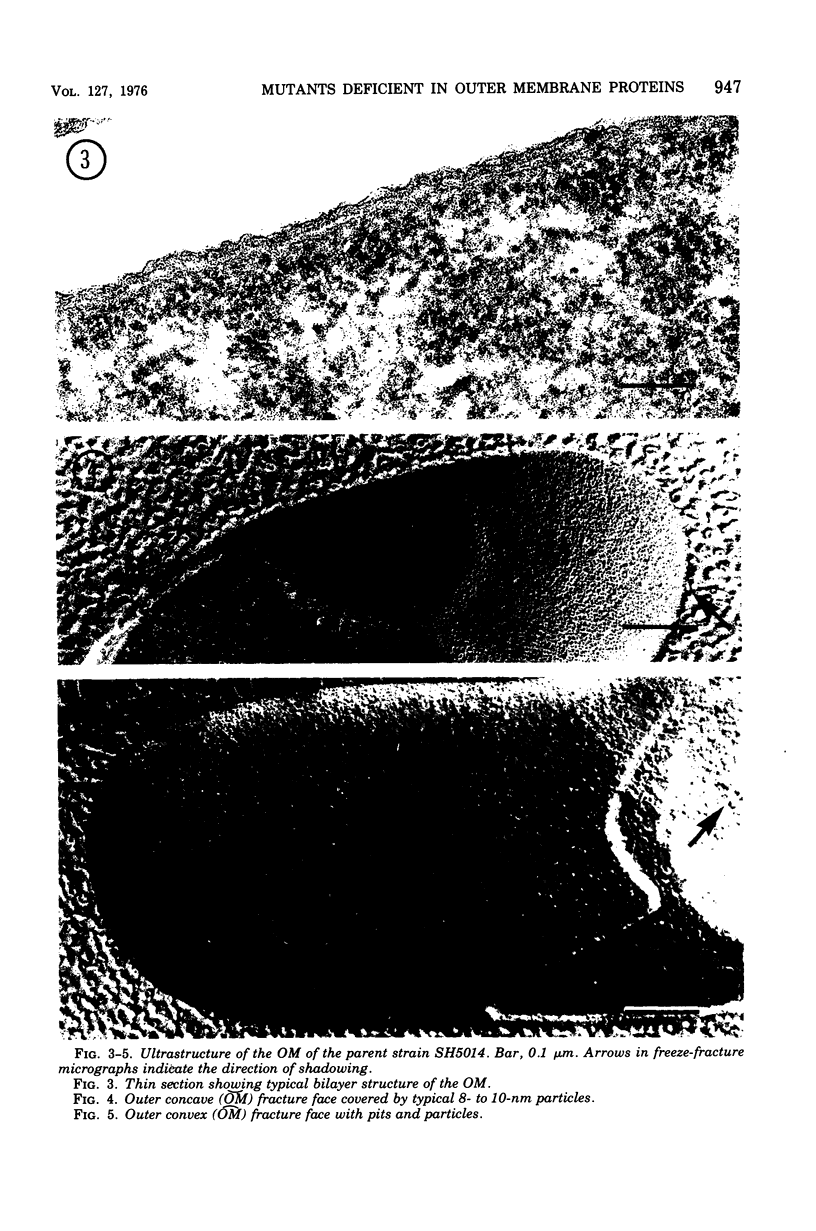
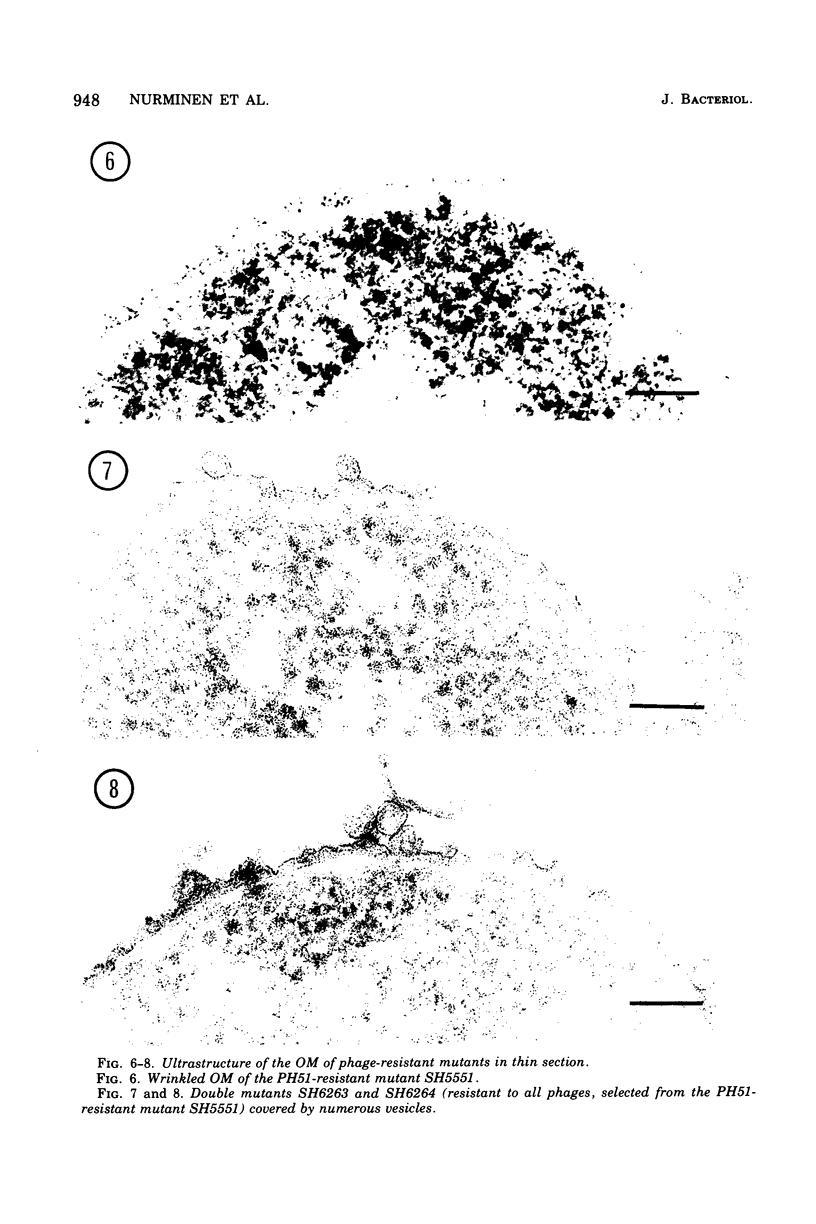
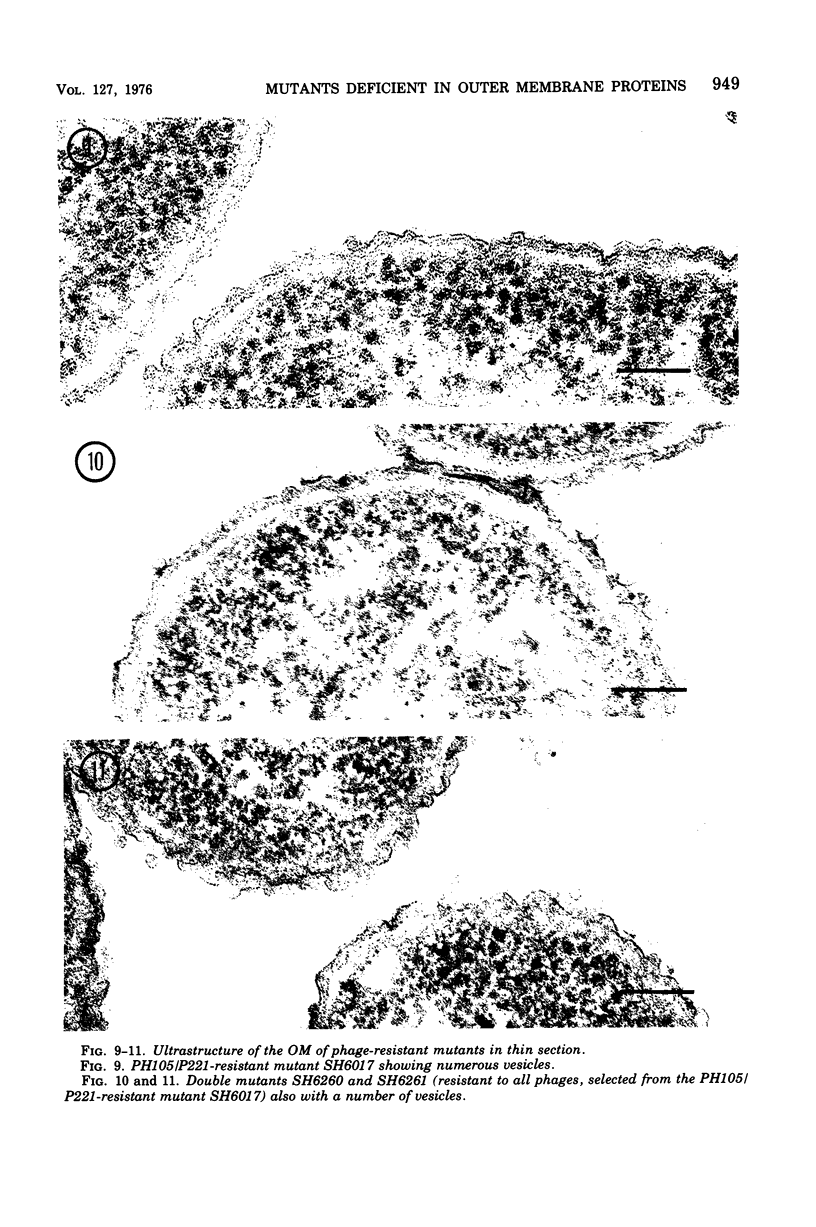
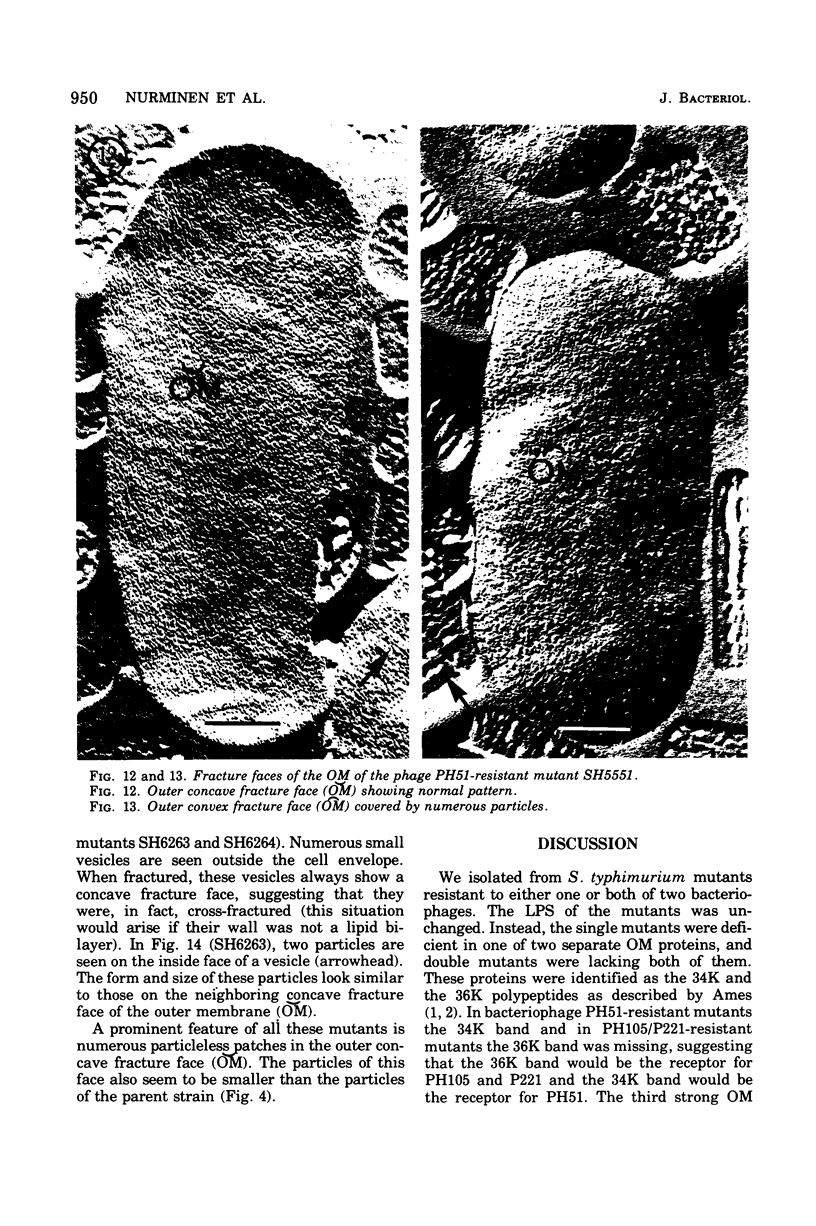
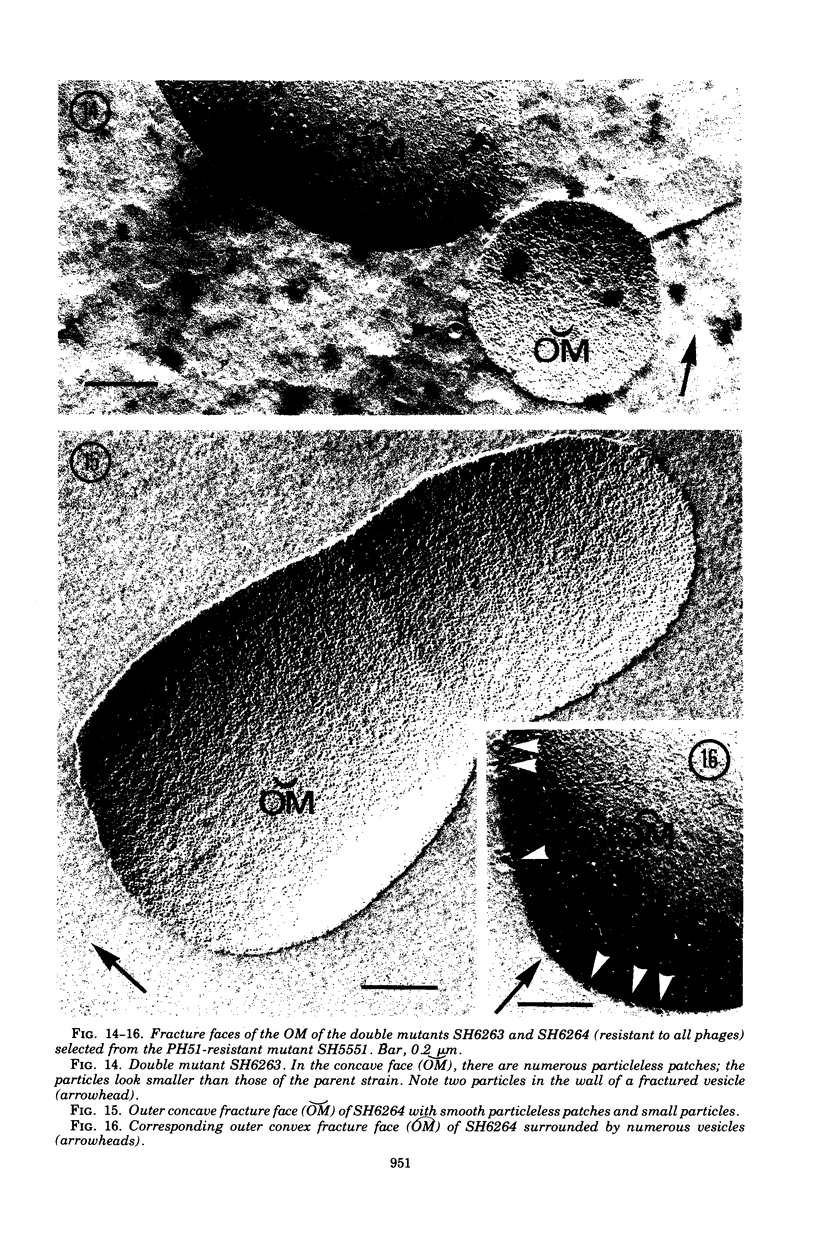
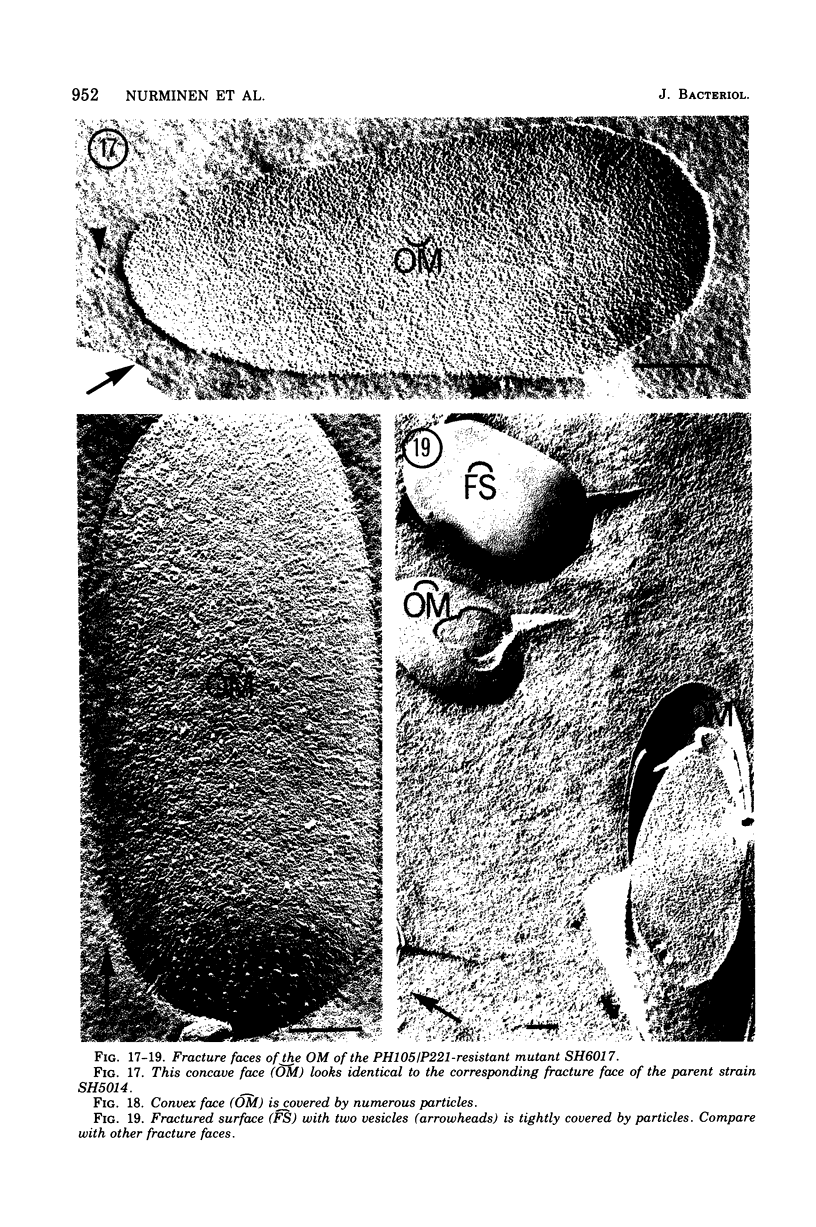
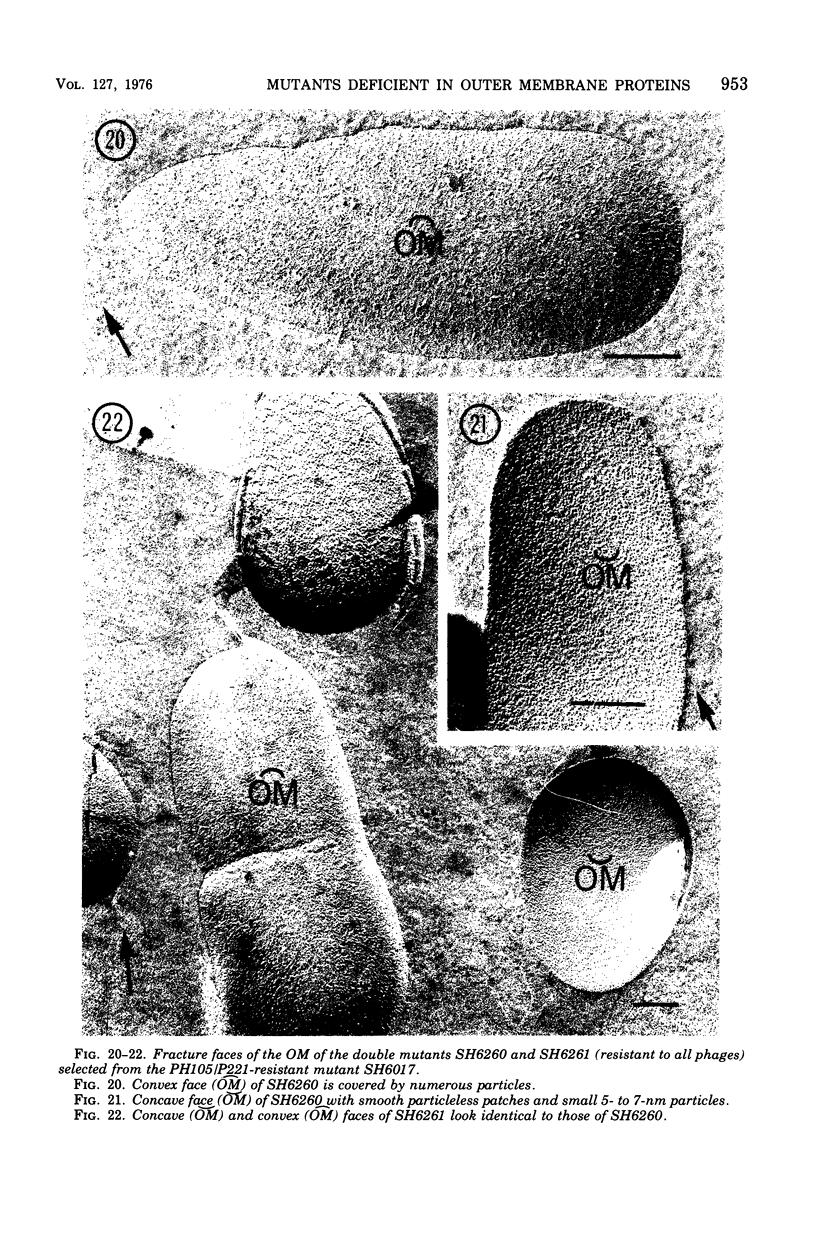
Mutants resistant to bacteriophages (P221 and PH105 or PH51) were isolated from a rfa strain of Salmonella typhimurium. They were found deficient in separate 33,000- to 36,000-dalton band proteins (major band proteins). Double mutants derived from both types of mutants were deficient in both of the bands. The growth behavior of all the mutants was normal. The outer membrane of the mutants appeared to be more wrinkled than normal and formed vesicles in many of the mutants. In freeze-fractured cells, changes were seen in the outer membrane (particleless patches in the concave fracture face, the particles themselves being smaller than normal). These changes were more marked in the double mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Koplow J., Goldfine H. Alterations in envelope structure of heptose-deficient mutants of Escherichia coli as revealed by freeze-etching. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5145–5149. doi: 10.1073/pnas.72.12.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. ES18, a general transducing phage for smooth and nonsmooth Salmonella typhimurium. Virology. 1970 Nov;42(3):621–632. doi: 10.1016/0042-6822(70)90308-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Jahkola M., Lüderitz O. A new gene cluster rfe concerned with the biosynthesis of Salmonella lipopolysaccharide. J Gen Microbiol. 1970 Jan;60(1):91–106. doi: 10.1099/00221287-60-1-91. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Wachstum und Lipopolysaccharid (O-Antigen)-Gehalt von Salmonellen bei Züchtung auf Agarnährböden. Zentralbl Bakteriol Orig. 1966 Jun;200(2):241–259. [PubMed] [Google Scholar]
- Schmidt G., Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 3. Typisierung von S. minnesota-Mutanten mittels chemischer Agenzien. Zentralbl Bakteriol Orig. 1969 Dec;212(1):88–96. [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO N., ANDERSON T. F. Genomic masking and recombination between serologically unrelated phages P22 and P221. Virology. 1961 Aug;14:430–439. doi: 10.1016/0042-6822(61)90334-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto N. The origin of bacteriophage P221. Virology. 1967 Nov;33(3):545–547. doi: 10.1016/0042-6822(67)90132-8. [DOI] [PubMed] [Google Scholar]