Abstract
Vitamins are essential components of the human diet. By contrast, the malaria parasite Plasmodium falciparum and related apicomplexan parasites synthesise certain vitamins, de novo, either completely or in parts. The occurrence of the various biosynthesis pathways is specific to different apicomplexan parasites, emphasising their distinct requirements for nutrients and growth factors. The absence of vitamin biosynthesis from the human host implies that inhibition of the parasite pathways may be a way to interfere specifically with parasite development. However, the precise role of biosynthesis and potential uptake of vitamins for the overall regulation of vitamin homeostasis in the parasites needs to be established first. In this review Sylke Müller and Barbara Kappes focus mainly on the procurement of vitamin B1, B5 and B6 by Plasmodium and other apicomplexan parasites.
Keywords: Malaria, drug target, pantothenate, thiamine, pyridoxal phosphate, vitamins
Exploring the metabolism of protozoan parasites
The intracellular lifestyle of various protozoan parasites has advantages, but also can cause problems, for instance in terms of nutrient acquisition - particularly when considering the high growth rates of some parasitic protozoa. Plasmodium intraerythrocytic stages have solved some of these problems by inducing the new permeation pathway (NPP). The NPP allows transport of a variety of low molecular mass molecules and ions across the host cell membrane, with subsequent transit of these nutrients across the parasitophorous vacuole membrane into the parasite through transporters in their plasma membrane 1-3. P. falciparum growth in vitro absolutely depends on an external supply of pantothenate (vitamin B5), calcium and isoleucine, but the addition of other amino acids significantly improves parasite growth rates 4-6. Thus, minimal growth in vitro is achieved with relatively few external additions of nutrients to the growth medium, implying that some of the essential growth factors are either not important to sustain parasite growth, or that the parasites might be able to generate them in a sufficient amount to allow functional metabolism. Indeed, it was shown that Plasmodium synthesises vitamin B1 and B6 and it is well established that folate biosynthesis occurs in the parasites 7-10. The fact that the parasites can synthesise some of the metabolites known to be vitamins in humans (see Box 1) potentially makes them excellent targets for the development of new antimalarials. This has already been proven for folate metabolism which will not be discussed here because recent excellent reviews cover the role of this pathway for Plasmodium survival and its potential as drug target 8, 9. Here we will report and discuss the potential of vitamin B1, B5 and B6 biosynthesis/acquisition as new drug targets and current knowledge about these metabolic pathways in the related apicomplexans Toxoplasma gondii and Cryptosporidium will also be addressed.
Vitamin B1
Vitamin B1 is an essential nutrient for mammals (see Box 1), but plants, bacteria and fungi can synthesise it de novo 11. Mammals salvage thiamine (THI) from their diet and convert it into its active form, thiamine diphosphate (THI-PP), using thiamine diphosphokinase (TPK). The biosynthesis of thiamine in bacteria is well characterised and differs from the reactions in eukaryotes 12, 13. Thiamine phosphate (THI-P) is synthesised from 4-methyl-5-(2-phosphoethyl)-thiazole (THZ-P) and 4-amino-2-methyl-5-hydroxymethyl pyrimidine diphosphate (HMP-PP) (see Figure 1).
Figure 1. Vitamin B1 biosynthesis.
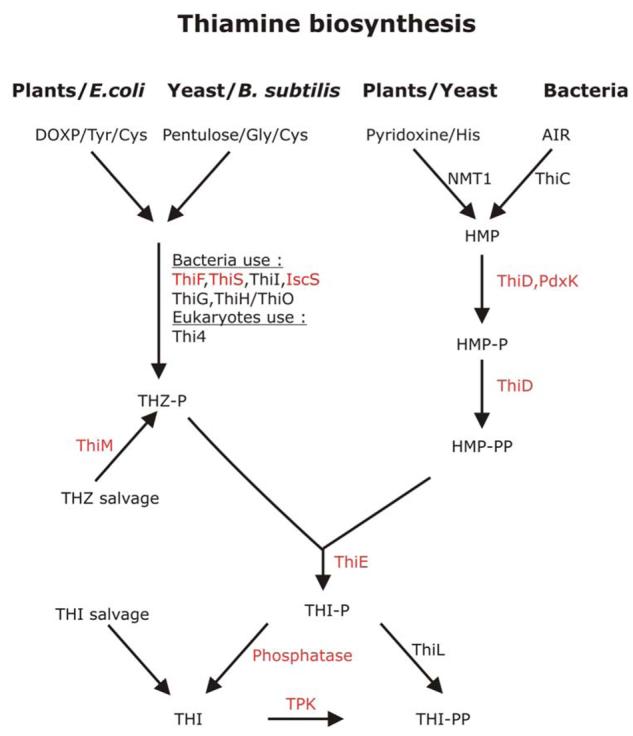
The left panel shows the biosynthesis of 4-methyl-5-(2-phosphoethyl)-thiazole (THZ-P). Plants and E. coli use 1-deoxyxylulose-5-phosphate (DOXP), tyrosine (Tyr) and cysteine (Cys) as precursors whereas Bacillus subtilis and yeast use a pentulose, glycine (Gly) and cysteine (Cys). In order to generate THZ-P, a number of enzymatic reactions are required and the best investigated systems are the bacterial pathways whereas the eukaryotic pathways are still poorly understood. In bacteria it is established that the ubiquitin-like protein ThiS-CoSH acts as sulphur carrier; ThiF catalyses the adenylation of ThiS; NifS or IscS transfer sulphur from cysteine to ThiS; in B. subtilis ThiO oxidises glycine and ThiG catalyses the formation of the thiazole phosphate ring 13, 69. The only enzyme shown to be involved in THZ-P biosynthesis in eukaryotes is Thi4 70, 71. On the right, the biosynthesis of 4-amino-2-methyl-5-hydroxymethyl pyrimidine diposphate (HMP–PP) is shown. Again different substrates are used in prokaryotes and eukaryotes. Bacteria generate HMP-PP from an intermediate of purine biosynthesis (AIR; 5-amino-imidazole ribonucleotide) whereas yeast uses pyridoxine and histidine (His) as precursors 72, 73. The enzymes that catalyse the reactions that lead to the formation of HMP-PP are ThiD (4-amino-2-methyl-5-hydroxymethyl pyrimidine kinase) and possibly pyridoxal kinase (PdxK). Red letters indicate that a gene has been identified in P. falciparum whereas black letters mean that orthologous genes have not been found in the Plasmodium genome databases. Abbreviations used: AIR, 5-amino-imidazole ribonucleotide; Cys, cysteine; Gly, glycine; DOXP, 1-deoxy-D-xylulose-5-phosphate; His, histidine; HMP, 4-amino-2-methyl-5-hydroxymethyl pyrimidine; HMP-P, 4-amino-2-methyl-5-hydroxymethyl pyrimidine phosphate; HMP-PP, 4-amino-2-methyl-5-hydroxymethyl pyrimidine diphosphate; NMT1, no message in thiamine; THZ, thiazole; THZ-P, 4-methyl-5-(2-phosphoethyl)-thiazole; THI, thiamine; THI-P, thiamine phosphate; THI-PP, thiamine pyrophosphate; ThiC, thiC gene product; ThiD, 4-amino-2-methyl-5-hydroxymethyl pyrimidine kinase; ThiE, thiamine phosphate synthase; ThiM, 4-methyl-5-β-hydroxyethylthiazole kinase TPK, thiamine diphosphokinase. This figure was generated following Bodzech and Ginsburg 14 and http://sites.huji.ac.il/malaria/.
Analyses of the P. falciparum genome revealed the presence of genes encoding proteins with similarities to bacterial and yeast thiamine biosynthesis enzymes (Table 1), such as 4-amino-2-methyl-5-hydroxymethyl pyrimidine phosphate (HMP)/HMP-P kinase (ThiD) 14. ThiD catalyses the phosphorylation of HMP and HMP-P, however, the specific activity for HMP-P is extremely low. The first phosphorylation step is also supported by the parasite's pyridoxal kinase 10. So far the precursors for HMP-biosynthesis were not identified in the parasites and it was suggested that the intraerythrocytic stages rely primarily on HMP uptake 10. In agreement with this, no genes encoding either ‘no message in thiamine’ (NMT1) or thiC gene product, the enzymes responsible for the formation of HMP in eukaryotes or prokaryotes, respectively, were identified in the parasite genome (http://sites.huji.ac.il/malaria/; Figure 1; Table 1) 11, 15. The importance of the ThiD reactions for parasite survival was investigated using the naturally occurring HMP analogue bacimethrin. In bacteria, bacimethrin is converted into 2'methoxythiamine which is subsequently phosphorylated to 2'methoxythiamine pyrophosphate and replaces thiamine diphosphate from its target proteins 16. These reactions appear to also occur in P. falciparum, however, bacimethrin had no adverse effect on parasite survival in vitro 10. These findings do not exclude that the HMP biosynthesis is of significance for Plasmodium survival because the lack of an antiplasmodial effect of bacimethrin could for instance be explained by poor uptake of the compound.
Table 1.
Genes potentially encoding proteins involved in vitamin biosynthesis and co-factor binding in apicomplexan parasitesa
| P. falciparum | T. gondii | C. parvum/hominis | Reference | |
|---|---|---|---|---|
| Vitamin B1 biosynthesis | ||||
| (A) HMP-biosynthesis | ||||
| 4-Amino-2-methyl-5-hydroxy methyl pyrimidine kinase (ThiD) |
yes (PFE1030c) | nob | no | 10, 14 |
| 4-Amino-2-methyl-5-hydroxy methyl pyrimidine kinase (ThiD) |
yes (PFE1030c) | no | no | 10, 14 |
| (B) Thiazole biosynthesis | ||||
| sulfur carrier protein ThiS | potential genes belonging to the ThiS/Urm/ubiquitine-like family were identified | c | ||
| ThiF | yes (PF13_0344) | yes (28.m00291) | yes (cgd2_1120) | c |
| ThiI | no | no | no | c |
| Cysteine desulfurase (IscS) | yes (MAL7P1.150) | yes (27.m00002) | yes (cgd4_3040/ Chro.40346) |
76, c |
| ThiO/ThiH | no | no | no | c |
| ThiG | no | no | no | c |
| Thi4 | no | no | no | c |
| (C) Thiazole salvage | ||||
| ThiM | yes (PFL1920c) | no | no | 10, 14 |
| (D) Thiamine-diphosphate biosynthesis | ||||
| Thiamine phosphate synthase (ThiE) |
yes (PFF0680c) | no | no | 10, 14 |
| Thiamine pyrophosphate kinase (TPK) |
yes (PFI1195c) | yes (33.m02662) | no | http://sites.huji.ac.il/malaria; c |
| Pantothenate and CoA biosynthesis | ||||
| Branched-chain amino acid transaminase |
maybe (PF14_0557) | yes (113.m01283) | ?d | c |
| 3-Methyl-2-oxobutanoate hydroxymethyltransferase |
no | yes (55.m04671) | no | c |
| 2-Dehydropantoate 2-reductase | no | yes (55.m04671) | no | c |
| Pantothenate synthetase | no | yes (57.m01763) | no | c |
| Pantothenate kinase (PanK) | yes (PF14_0200) | yes (551.m00221) | yes (cgd7_4950) | 31; http://sites.huji.ac.il/malaria; c |
| Phosphopantothenoylcysteine synthetase (PPCS) | yes (PF11_0036/ PFD0610) |
yes (641.m01494) | yes (cgd4_2250/ Chro.40256) |
31; http://sites.huji.ac.il/malaria; c |
| Phosphopantothenoylcysteine decarboxylase (PPCDC) | yes (MAL8P1.81) | yes (49.m00067) | yes (cgd4_2250) | 31; http://sites.huji.ac.il/malaria; c |
| Adenylyltransferase (PPAT) | yes (PF13_0159)/ (PF07_0018) |
yes (44.m02629) | yes (cgd7_3480/ Chro.70388) |
31; http://sites.huji.ac.il/malaria; c |
| De-phospho-CoA kinase (DPCK) | yes (PF14_0415) | yes (44.m02629) | yes (cgd2_380/Chro.20045) | 31; http://sites.huji.ac.il/malaria; c |
| Vitamin B6 biosynthesis/metabolism | ||||
| Pdx1 | yes (PFF1025c) | yes (46.m00006) | no | 7, 39, 40, c |
| Pdx2 | yes (PF11_0169) | yes (74.m00760) | no | 7, 39, 40, c |
| Pyridoxal kinase (PdxK) | yes (PFF0775w) | yes (113.m00756) | no | 40, c |
| Pyridoxal phosphate phosphatase | yes (PF07_0059) | yes (80.m02257) | no | c |
| Pyridoxamine/pyridoxine -phosphate oxidase (PdxH) |
??f (PF14_0570) | no | no | http://sites.huji.ac.il/malaria; c |
| Pyridoxal dehydrogenase /aldehyde oxidase |
yes (PF14_0088) | yes (25.m01796) | no | c |
| Biotin | ||||
| Biotin protein ligase | yes (PF14_0573/PF10_0409) | yes (80.m02241) | yes (cgd2_2890/Chro.20303) | c |
Blast searches (TBLASTN and BLASTN) were performed in ApiDB.org (www.ApiDB.org) using bacterial or plant homologous of the respective genes; numbers in brackets give gene identifiers of gene loci in ApiDB.org. Potential genes were used to search the Swissprot database to corroborate the results.
No – No gene/protein with convincing similarity was identified
unpublished observations from a by the authors
“?” – Gene possibly present but similarity does not allow clear annotation or their predicted amino acid sequences have been annotated differently
“??” – Gene present on http://sites.huji.ac.il/malaria, but questioned by the authors of this review
Despite the fact that most of the genes encoding proteins that are involved in THZ-P biosynthesis are not conserved or could not be identified in the Plasmodium (see Table 1), it was suggested that the parasites are able to generate the metabolite using yet unknown enzymatic reactions 10. In addition to a potential biosynthesis of THZ-P, the presence of the gene encoding 4-methyl-5-β-hydroxyethylthiazole kinase (ThiM) suggests that the metabolite is salvaged by the parasites 10, 14. HMP-PP and THZ-P are merged into thiamine phosphate (THI-P) by thiamine phosphate synthase (ThiE). A gene encoding this protein was also identified in the parasite genome (Table 1)14. Its deduced amino acid sequence revealed similarity to ThiE from other organisms only in the N-terminal 235 of the 538 amino acids, whereas the C-terminus does not show any similarity to other proteins of known function 10. To become the active co-factor, thiamine monophosphate needs to be dephosphorylated and subsequently activated by thiamine diphosphokinase (TPK) to form thiamine diphosphate. A specific phosphatase acting on thiamine monophosphate was not identified but a gene potentially encoding an ortholog of TPK appears to be present (Table 1).
In addition to the reactions described above, it is likely that thiamine is taken up by the parasite 10. So far, the precise contributions of biosynthesis and uptake to the overall vitamin B1 homeostasis of the parasite are not clear. Yet, the lack of these pathways in the mammalian host and the fact that bacimethrin acts in a similar way on parasite ThiD as it does on the bacterial counterpart suggests that thiamine biosynthesis in Plasmodium might be exploitable for the design of new antimalarials 17.
Genome analyses suggest that the related apicomplexan parasites T. gondii and Cryptosporidium parvum/hominis cannot synthesise vitamin B1 – genes encoding proteins of the pathways described above seem to be absent from these parasite genomes (Table 1). A gene potentially encoding TPK was identified in the genome of T. gondii, supporting the hypothesis that, like their human host, they probably fully rely on thiamine uptake. No clear orthologs could be found in the Crytosporidium (unpubl. observations), leaving the question how Cryptosporidium acquires this essential co-factor (Table 1). However, the lack of obvious orthologs in both parasites does not necessarily mean the lack of this pathway because it might be very distinct from those characterised in other organisms so far and therefore was not recognised to date.
Pantothenate (vitamin B5) and CoA-biosynthesis
Pantothenate and pantotheine are precursors of the essential co-factor coenzyme A (CoA) (see Box 1). CoA is synthesised from aspartate and ketovalerate in bacteria, fungi and plants via the formation of pantothenic acid. Animals and some pathogenic bacteria cannot generate pantothenate, thus lacking the first part of the CoA-biosynthesis pathway. They therefore rely on pantothenate uptake from their diet. T. gondii possesses genes encoding proteins which potentially synthesise pantothenate which are lacking in Plasmodium and Cryptosporidium (Figure 2; Table 1). This is in agreement with the finding that pantothenate supplementation is beneficial for growth of malaria parasites 4, 18. Mammals take up pantothenate via a family of Na+:pantothenate symporters 19, whereas, in P. falciparum, it is taken up by a H+:pantothenate symporter 20. The Plasmodium-specific vitamin B5 uptake system differs from that of the host not only with respect to its cation dependence, but also by its affinity for pantothenate. This transporter represents a low affinity uptake system as opposed to the high affinity transport system characterised in other organisms 21, 22. By contrast, T. gondii contains a gene (locus 541.m01156) with a high degree of similarity to the mammalian Na+ -dependent multivitamin symporter (MVT). These symporters are often found to be responsible for the transport of biotin and potentially lipoic acid as well as pantothenate 23. From the gene sequence it is impossible to predict its primary function and thus, it is possible that this predicted T. gondii MVT not only confers the ability to take up pantothenate but also is important for biotin uptake especially as the parasites are able to generate pantothenate de novo.
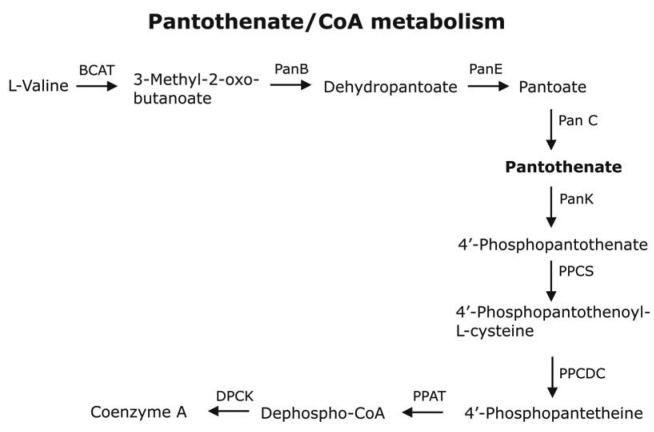
Figure 2. Vitamin B5/CoA biosynthesis.
Genes encoding proteins potentially synthesising pantothenate from valine and aspartate were identified in T. gondii when searching the ApiDB database (www.ApiDB.org). P. falciparum and C. parvum/hominis do not possess the ability to generate vitamin B5. All three apicomplexans contain genes encoding for the enzymes necessary for CoA biosynthesis. In T. gondii PPAT and DPCK form a bifunctional protein whereas in Plasmodium and Cryptosporidium these reactions are catalysed by distinct enzymes. Abbreviations used: BCAT, branched-chain amino acid transaminase; PanB, 3-methyl-2-oxobutanoate hydroxymethyltransferase; PanE, 2-dehydropantoate 2-reductase; PanC, pantothenate synthase; PanK, pantothenate kinase; PPCS, phosphopantothenoyl synthase; PPCDC, phosphopantothenoyl cysteine decarboxylase; PPAT, pantetheine-phosphate adenylyltransferase; DPCK, de-phospho-CoA kinase.
Once inside the parasite, pantothenate is transformed into CoA via 5 enzymatic steps (see Figure 2; Table 1). The first is catalysed by pantothenate kinase (PanK). Humans contain 4 distinct PanKs whereas in P. falciparum only one activity was identified. The kinetic parameters of the P. falciparum PanK are quite distinct from the mammalian counterpart with a Km for pantothenate in the nM range showing its high specificity for the substrate 20, 24. In bacteria and mammals, PanK regulates CoA-biosynthesis 24-26, and mutations in PanK2, one of the four human PanKs, results in the development of pantothenate kinase-associated neurodegeneration 27, 28. In Drosophila melanogaster, a PanK termed ‘fumble’ is not only important for CoA-biosynthesis, but also for progression through the cell cycle by actively associating with the metaphase and telophase spindle during cell division 29. These data suggest an important role for PanK also in malaria and other apicomplexan parasites which makes it an interesting target for future drug design. Indeed, recent work has identified a number of pantothenate analogues that show antimalarial activity and act through inhibition of the parasite PanK 6, 30. In the second step, 4'-phosphopantothenate is conjugated to cysteine via phosphopantothenoylcysteine synthetase (PPCS) (Figure 2). P. falciparum appears to possess two genes encoding potential homologues of eukaryotic ATP-dependent PPCS (Table 1). They are annotated as hypothetical proteins and their authenticity and functionality is yet to be determined. This is in contrast to T. gondii and C. parvum/hominis, where only 1 gene potentially encoding PPCS was identified (Table 1, Figure 2). The following step of the CoA-biosynthesis requires the decarboxylation of phosphopanthenoylcysteine, by phosphopanthenoylcysteine decarboxylase (PPCDC). In Plasmodium, T. gondii and Cryptosporidium, genes encoding orthologs of eukaryotic PPCDC have been identified (Table 1) 31, 32. Apicomplexan CoA-biosynthesis, therefore, is more similar to that found in mammals, because bacteria contain a bifunctional PPCS/PPCDC (Table 1) 31, 32. The following two steps of the CoA-biosynthesis are catalysed by a bifunctional protein in mammals – pantetheine phosphate adenylyltransferase/de-phospho-CoA kinase (PPAT/DPCK) 31. In contrast, these steps are catalysed by separate enzymes in the malaria parasite. It was suggested that the lack of sequence similarity between the Plasmodium and host PPAT has some potential for drug development 31, 32 (Figure 2, Table 1). Similarly Cyrptosporidium possesses two genes potentially catalysing these last two reactions of CoA biosynthesis whereas in T. gondii a single gene encoding a polypeptide similar to the mammalian protein was identified (Table 1, Figure 2).
Vitamin B6
The term ‘vitamin B6’ collectively refers to the vitamers pyridoxal, pyridoxine, pyridoxamine, and their respective phosphate esters (Figure 3). The metabolically active form is pyridoxal-5′-phosphate (PLP) (see Box 1). Proteobacteria synthesise vitamin B6 via the 1-desoxyxylulose 5-phosphosphate (DOXP)-dependent pathway 33, whereas other bacteria and eukaryotes generate PLP using Pdx1 and Pdx2, which form a typical class I glutamine amidotransferase (GATase). In addition PLP can be acquired by salvage which requires the activity of a pyridoxine/pyridoxamine oxidase (PdxH) and a pyridoxal kinase (PdxK) 33, 34. The GATase formed by Pdx1 and Pdx2 acts as a PLP synthase using glutamine, ribose-5-phosphate (or ribulose-5-phosphate) and glyceraldeheyde-3-phosphate (or dihydroxyacetone phosphate) to synthesise PLP 35, 36. Crystal structures of two bacterial PLP synthase complexes were recently solved leading to a better understanding of the reaction mechanism of this enzyme complex and offering great opportunities for future drug development not only for antibacterials but also antimalarials 37, 38.
Figure 3. Vitamin B6 metabolism.
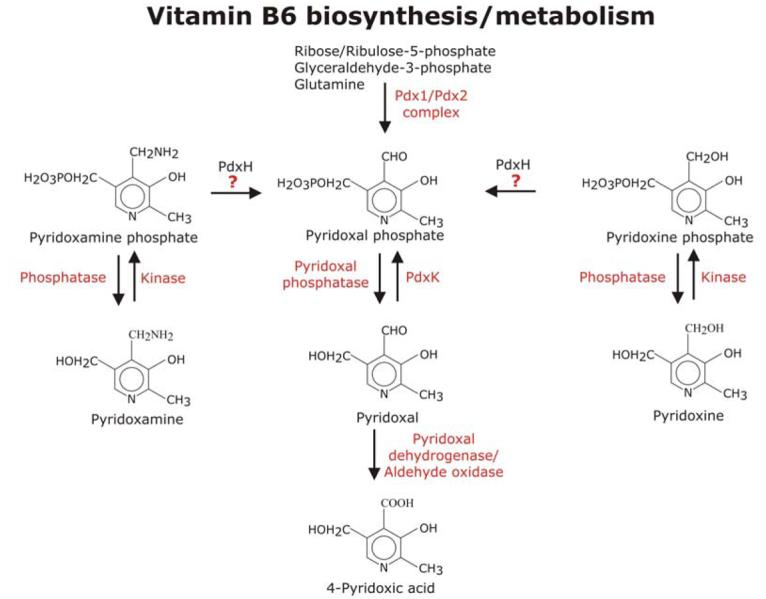
The expression vitamin B6 comprises pyridoxal, pyridoxine and pyridoxamine and their phosphate esters. In eukaryotes such as fungi and plants and most bacteria (except the γ-subdivision of proteobacteria including E. coli) pyridoxal-phosphate (PLP) is synthesised by the enzyme complex Pdx1/Pdx2 from ribose or ribulose-5-phosphate, glyceraldehyde-3-phosphate and glutamine. Crystal structures of both proteins have been solved and they reveal that Pdx1 forms a doughnut-like hexameric structure, which binds the glutamine amidotransferase Pdx2 to form the PLP-synthase complex 7, 37, 71, 74, 75. Regulation of PLP levels is crucial and can be achieved in several ways. PLP is supplied by biosynthesis, oxidation from pyridoxamine phosphate or pyridoxine phosphate as well as from pyridoxal by phosphorylation. Whether apicomplexan parasites can convert pyridoxamine phosphate and pyridoxine phosphate into PLP is not clear, because a gene encoding an ortholog of pyridoxmaine/pyridoxine phosphate oxidase (PdxH) was not unambiguously identified in the Plasmodium genome (question mark in Figure). Therefore this figure deviates from that published on http://sites.huji.ac.il/malaria/. Thus it is possible that PLP levels in apicomplexans may solely depend on PLP biosynthesis, pyridoxal uptake and phosphorylation as well as on the degree of PLP catabolism. Abbreviations used: PdxH, pyridoxamine/pyridoxine phosphate oxidase; PdxK, pyridoxal kinase.
Single genes encoding Pdx1 and Pdx2 have been identified in P. falciparum and are also present in T. gondii [39, 40] (see Table 1). The Plasmodium proteins form a functional PLP synthase complex with parasite-specific properties and substrate specificities 7. Both proteins are expressed throughout the intraerythrocytic and gametocyte development of P. falciparum, suggesting that these parasitic stages possess a functional vitamin B6 biosynthesis of the DOXP-independent type 7. In addition, PdxK has been described in Plasmodium and a gene encoding a potential ortholog is as also present in T. gondii [40] (see Table 1). The Plasmodium metabolic map of vitamin B6 (http://sites.huji.ac.il/malaria) predicts the presence of a potential pyridoxine/pyridoxamine oxidase (PF14_0570). However, the predicted gene product has the highest similarity to apolipoprotein-A1 binding protein albeit it also has some similarity with a predicted pyridoxine/pyridoxamine oxidase from rice. These ambiguous results emphasise that often it is impossible to reliably predict gene product functions without experimental evidence. The potential lack of pyridoxine/pyridoxamine oxidase in the malaria parasite needs therefore to be experimentally confirmed before making final assumptions about how the parasites maintain their crucial PLP homeostasis although the potential lack of the oxidase could make this parasite pathway particularly vulnerable to inhibition.
Biotin
Generally, bacteria, plants and some fungi are able to synthesise biotin, but animals have to obtain it through their diet (see Box 1). Biotin is covalently attached to biotin-dependent proteins such as acetyl-CoA carboxylase by a biotin-protein ligase (BPL). In a first step, these ligases activate biotin to form a biotinyl-AMP intermediate before biotin is ligated to a specific lysine residue of the acceptor protein 41. Plasmodium parasites lack the ability to synthesise biotin, but they possess at least one biotin-dependent protein, acetyl-CoA carboxylase, representing the first step of fatty acid biosynthesis in the apicoplast. Inhibition of this protein in T. gondii by aryloxyphenoxypropionate herbicides has an adverse effect on parasite growth 42. Searching the Plasmodium genome database revealed two orthologs of genes potentially encoding BPLs (Table 1). In contrast, T. gondii and Cryptosporidum only possess one gene encoding BPL (Table 1). The apicomplexan gene products have similarity to the bacterial biotin operon repressor BirA that was also shown to act as transcriptional regulator in bacteria, yeast and mammals by sensing of intracellular biotin levels via the biotinyl-AMP bound to BPL 43, 44. It would be highly interesting to investigate the hypothesis that biotin concentrations within the parasite are sensed by a similar mechanism.
Vitamin status of the host and its effect on Plasmodium development
An infection with Plasmodium causes an immune response resulting in the activation of macrophages leading to the production and release of reactive oxygen species (ROS). The vitamins C (L-ascorbic acid) and E (α-tocopherol) (see Box 1) play pivotal roles in protection against oxidative stress. It was postulated that the increased concentrations of both vitamins in Plasmodium-infected cells in a mouse model is the consequence of the increased oxidative stress that the animal encounters when its immune system is activated by the malaria infection 45-48. This simultaneous increase in concentration of both vitamins in erythrocytes apparently offered an enhanced protection against lipid peroxidation where lipid peroxides are detoxified by vitamin E which itself is then reduced by the water-soluble ascorbate. The resulting dehydroascorbate is recycled enzymatically or non-enzymatically in a glutathione-dependent way 48. The increase of vitamin E and C concentrations in Plasmodium-infected erythrocytes of rodents led to the suggestion that the parasites are able to synthesise the two vitamins. However, scrutiny of the genome did not support this suggestion – we could not find genes encoding proteins involved in the biosynthesis of ascorbate or tocopherol. Interestingly, vitamin E or vitamin C deficiencies have a protective effect in malaria patients apparently because the lack of this antioxidant renders the parasites more vulnerable to the damage by ROS 45. Indeed, it was postulated that ascorbic acid not only acts as an antioxidant but also has strong pro-oxidant features resulting in the generation of free radicals in the presence of oxygen and free transition metals according to the Fenton reaction 49.
There is evidence that β-carotene and vitamin A have protective effects against malaria 50, 51. Malaria infection seems to be accompanied by decreases of vitamin A concentrations in the serum from ≥ 120 mmol/L to ≤ 70 μmol/L 52, 53, and, in rodent models, there appears to be an inverse correlation between parasitemia and vitamin A concentrations in the host. Providing vitamin A supplements partially protected against malaria infection, particularly in immunologically naïve patients 50. This was suggested to be mediated by an increased clearance of parasitized erythrocytes and a reduced pro-inflammatory cytokine response, which is generally high in malaria patients. Vitamin A supplementation in a randomised and controlled trial showed a marked benefit with ∼70 % lower parasite densities in young children 51. One of the underlying mechanisms was found to be an upregulation of the phagocytotic receptor CD36, and the downregulation of cytokines such as TNFα by binding of 9-cis-retinoic acid to the peroxisome proliferator-activated receptor γ (PPARγ) or retinoid-x-receptor 50, 54. How the vitamin deficiency is caused in the first place is not entirely clear, and the suggestion that the parasites take up the vitamin and therefore remove it from its host could not be substantiated 55.
Vitamin D3 is pivotal for calcium homeostasis, but also exerts a number of other functions, which are mediated through its receptor – vitamin D3 receptor (VDR) belonging to the family of nuclear hormone receptors 56, 57. Genes involved in the generation of vitamin D3 have been characterised in mammals, but none of those appears to be present in Plasmodium (www.PlasmoDB.org; unpubl. observations); neither is a gene encoding VDR. In the context of infectious diseases, vitamin D3 immunomodulatory functions are of particular interest 58. It has been shown that VDR is expressed in antigen-presenting cells (e.g. macrophages and dendritic cells) and expression is inducible in lymphocytes. However, there is no clear evidence that vitamin D3 contributes in a beneficial or adverse way to a malaria infection 59.
One way in which vitamin D3 might affect Plasmodium is through its involvement in phospholipid metabolism and signalling pathways 60. Vitamin D3 and analogues have pronounced inhibitory effects on P. falciparum erythrocytic late stage development possibly because the phospholipid biosynthesis pathways of the parasite is affected by these compounds 61. Inhibition of phospholipid biosynthesis by other classes of inhibitors (for instance choline analogues) has been followed up extensively 62, 63 and it is likely that these inhibitors will be developed as new drugs against malaria in the near future 64. Thus the activity of vitamin D3 analogues merits further attention.
Conclusions
This review has concentrated primarily on those growth factors that are likely to be required for the development of Plasmodium and other apicomplexan parasites such as vitamin B1, B5 and B6, and which are absolutely necessary as dietary additions in humans. The finding that some vitamins are generated fully or partially by apicomplexans is of great interest with respect to the potential of these parasite-specific pathways as drug targets. Indeed the suitability of CoA biosynthesis as target for antimalarials as a concept has been shown as pantothenate analogues act as inhibitors of P. falciparum viability probably through PanK inhibition 6, 30, 65. ThiD, one of the enzymes involved in thiamine biosynthesis of bacteria, is the target of the naturally occurring HMP analogue bacimethrin and Wrenger and colleagues showed that bacimethrin is metabolised by the parasite ThiD protein in a similar way suggesting that it acts similarly in the parasites 10. Unfortunately this could not be shown unambiguously because bacimethrin had no effect on parasite viability in vitro. This is disappointing but might be due to poor uptake of the inhibitor into the parasite, which certainly could be improved by medicinal chemistry 10. The occurrence of vitamin B6 biosynthesis in apicomplexa and the recently available structural information of two bacterial PLP synthase complexes will aid to identify strategies for the design of inhibitors against the parasite PLP synthase given their similarity and the presumed similar mode of action between bacterial and parasite proteins 37, 38. Inhibitors could either be substrate analogues, reaction intermediates or peptidomimetics interfering with protein-protein interaction that is absolutely required for PLP synthase activity 7.
The data available about some of the vitamin and co-factor biosynthesis pathways to date are extremely promising and support the idea that these pathways have indeed potential for disease control against apicomplexa.
Acknowledgements
The authors would like to thank Professor G.H. Coombs for critical reading of the manuscript. SM is a Wellcome Senior Research Fellow (GR061173AIA). This work was supported by the European Commission (Grant VITBIOMAL-012158).
Box 1: Role of vitamins described in this review
| Vitamins are micronutrients that are essential in small quantities for the normal functioning of the human body. All natural vitamins are organic food substances found solely in plants, fungi, bacteria and animals. Generally, humans cannot synthesize vitamins, so they must be supplied in the diet. There are two types of vitamins: fat-soluble and water-soluble. Fat soluble vitamins are stored in the body, primarily in the liver, and include vitamins A, D, E and K. Water-soluble vitamins, which include the B vitamins and vitamin C, are, with the exception of vitamin B12, not stored by the body and need constant replacement 66-68. | |
Vitamin B1 (Thiamine)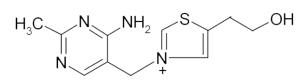 Thiamine diphosphate, the metabolically important form, is a co-factor of α-keto acid dehydrogenase complexes and the glycine cleavage system as well as enzymes like transketolase and pyruvate decarboxylase. | |
| essential for |
|
| clinical deficiency |
|
Vitamin B5 (Panthothenic acid) Pantothenic acid is the pre-cursor of coenzyme-A (CoA). CoA acts as acyl-chain carrier in numerous important cellular functions. | |
| essential for |
|
| clinical deficiency |
|
Vitamin B6 complex  The vitamin B6 complex consists of pyridoxamine, pyridoxine, pyridoxal and the related phosphateesters. The active form is pyridoxal phosphate (PLP). | |
| essential for |
|
| clinical deficiency |
|
Vitamin H or B7 (biotin) Biotin functions as the carboxyl carrier for biotin-dependent carboxylases. In humans there are only five biotin-dependent carboxylases. | |
| essential for |
|
| clinical deficiency |
|
Vitamin C Almost all animals and plants synthesize ascorbate, but primates (including humans) need an external source of vitamin C. | |
| essential for |
|
| clinical deficiency |
|
Vitamin A (Retinol) The term ‘vitamin A’ refers to a group of fat-soluble compounds known as the ‘retinoids’. | |
| essential for |
|
| clinical deficiency |
|
Vitamin D (calciferol) The term vitamin D refers to a group of five chemically related compounds (D1-D5). Vitamin D3, also known as cholecalciferol, is a form of vitamin D that is made by in the skin the human body, when 7-dehydrocholesterol reacts with ultraviolet light (UVB; 290 to 315 nm). Vitamin D is not a true vitamin since human skin can create vitamin D in some circumstance. It may be better described as a conditional vitamin. | |
| essential for |
|
| clinical deficiency |
|
Vitamin E (d-α-tocopherol) Vitamin E is a generic term for a group of eight chemically-similar compounds sharing the tocopherol and tocotrienol structures, which are lipid-soluble. | |
| essential for |
|
| clinical deficiency |
|
References
- 1.Desai SA, et al. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- 2.Kirk K. Membrane transport in the malaria-infected erythrocyte. Physiol Rev. 2001;81:495–537. doi: 10.1152/physrev.2001.81.2.495. [DOI] [PubMed] [Google Scholar]
- 3.Saliba KJ, Kirk K. Nutrient acquisition by intracellular apicomplexan parasites: staying in for dinner. Int J Parasitol. 2001;31:1321–1330. doi: 10.1016/s0020-7519(01)00258-2. [DOI] [PubMed] [Google Scholar]
- 4.Divo AA, et al. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J Protozool. 1985;32:59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, et al. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci U S A. 2006;103:8840–8845. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saliba KJ, et al. Provitamin B5 (pantothenol) inhibits growth of the intraerythrocytic malaria parasite. Antimicrob Agents Chemother. 2005;49:632–637. doi: 10.1128/AAC.49.2.632-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gengenbacher M, et al. Vitamin B6 biosynthesis by the malaria parasite Plasmodium falciparum: biochemical and structural insights. J Biol Chem. 2006;281:3633–3641. doi: 10.1074/jbc.M508696200. [DOI] [PubMed] [Google Scholar]
- 8.Nzila A, et al. Comparative folate metabolism in humans and malaria parasites (part II): activities as yet untargeted or specific to Plasmodium. Trends Parasitol. 2005;21:334–339. doi: 10.1016/j.pt.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nzila A, et al. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol. 2005;21:292–298. doi: 10.1016/j.pt.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrenger C, et al. Vitamin B1 de novo synthesis in the human malaria parasite Plasmodium falciparum depends on external provision of 4-amino-5-hydroxymethyl-2-methylpyrimidine. Biol Chem. 2006;387:41–51. doi: 10.1515/BC.2006.007. [DOI] [PubMed] [Google Scholar]
- 11.Begley TP, et al. Thiamin biosynthesis in prokaryotes. Arch Microbiol. 1999;171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 12.Morett E, et al. Systematic discovery of analogous enzymes in thiamin biosynthesis. Nat Biotechnol. 2003;21:790–795. doi: 10.1038/nbt834. [DOI] [PubMed] [Google Scholar]
- 13.Settembre E, et al. Structural biology of enzymes of the thiamin biosynthesis pathway. Curr Opin Struct Biol. 2003;13:739–747. doi: 10.1016/j.sbi.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Bozdech Z, Ginsburg H. Data mining of the transcriptome of Plasmodium falciparum: the pentose phosphate pathway and ancillary processes. Malar J. 2005;4:17. doi: 10.1186/1475-2875-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- 16.Reddick JJ, et al. The mechanism of action of bacimethrin, a naturally occurring thiamin antimetabolite. Bioorg Med Chem Lett. 2001;11:2245–2248. doi: 10.1016/s0960-894x(01)00373-0. [DOI] [PubMed] [Google Scholar]
- 17.Zilles JL, et al. Action of the thiamine antagonist bacimethrin on thiamine biosynthesis. J Bacteriol. 2000;182:5606–5610. doi: 10.1128/jb.182.19.5606-5610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brackett S, et al. The relation between pantothenic acid and Plasmodium gallinaceum infections in the chicken and the activity of analogues of pantothenic acid. J. Parasitol. 1946;32:453–462. [PubMed] [Google Scholar]
- 19.Prasad PD, Ganapathy V. Structure and function of mammalian sodium-dependent multivitamin transporter. Curr Opin Clin Nutr Metab Care. 2000;3:263–266. doi: 10.1097/00075197-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Saliba KJ, Kirk K. H+-coupled pantothenate transport in the intracellular malaria parasite. J Biol Chem. 2001;276:18115–18121. doi: 10.1074/jbc.M010942200. [DOI] [PubMed] [Google Scholar]
- 21.Germinario RJ, Waller JR. Transport of pantothenic acid in Lactobacillus plantarum. Can J Microbiol. 1977;23:922–930. doi: 10.1139/m77-136. [DOI] [PubMed] [Google Scholar]
- 22.Stolz J, Sauer N. The fenpropimorph resistance gene FEN2 from Saccharomyces cerevisiae encodes a plasma membrane H+-pantothenate symporter. J Biol Chem. 1999;274:18747–18752. doi: 10.1074/jbc.274.26.18747. [DOI] [PubMed] [Google Scholar]
- 23.Zempleni J. Uptake, localization, and noncarboxylase roles of biotin. Annu Rev Nutr. 2005;25:175–196. doi: 10.1146/annurev.nutr.25.121304.131724. [DOI] [PubMed] [Google Scholar]
- 24.Saliba KJ, et al. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J Biol Chem. 1998;273:10190–10195. doi: 10.1074/jbc.273.17.10190. [DOI] [PubMed] [Google Scholar]
- 25.Rock CO, et al. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J Biol Chem. 2000;275:1377–1383. doi: 10.1074/jbc.275.2.1377. [DOI] [PubMed] [Google Scholar]
- 26.Rock CO, et al. Role of feedback regulation of pantothenate kinase (CoaA) in control of coenzyme A levels in Escherichia coli. J Bacteriol. 2003;185:3410–3415. doi: 10.1128/JB.185.11.3410-3415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayflick SJ. Pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome) J Neurol Sci. 2003;207:106–107. doi: 10.1016/s0022-510x(02)00433-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YM, et al. Biochemical properties of human pantothenate kinase 2 isoforms and mutations linked to pantothenate kinase-associated neurodegeneration. J Biol Chem. 2006;281:107–114. doi: 10.1074/jbc.M508825200. [DOI] [PubMed] [Google Scholar]
- 29.Afshar K, et al. fumble encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics. 2001;157:1267–1276. doi: 10.1093/genetics/157.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saliba KJ, Kirk K. CJ-15,801, a fungal natural product, inhibits the intraerythrocytic stage of Plasmodium falciparum in vitro via an effect on pantothenic acid utilisation. Mol Biochem Parasitol. 2005;141:129–131. doi: 10.1016/j.molbiopara.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Genschel U. Coenzyme A biosynthesis: reconstruction of the pathway in archaea and an evolutionary scenario based on comparative genomics. Mol Biol Evol. 2004;21:1242–1251. doi: 10.1093/molbev/msh119. [DOI] [PubMed] [Google Scholar]
- 32.Pinney JW, et al. metaSHARK: software for automated metabolic network prediction from DNA sequence and its application to the genomes of Plasmodium falciparum and Eimeria tenella. Nucleic Acids Res. 2005;33:1399–1409. doi: 10.1093/nar/gki285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittenhuber G. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J Mol Microbiol Biotechnol. 2001;3:1–20. [PubMed] [Google Scholar]
- 34.Yang Y, et al. Identification of the pdxK gene that encodes pyridoxine (vitamin B6) kinase in Escherichia coli K-12. FEMS Microbiol Lett. 1996;141:89–95. doi: 10.1111/j.1574-6968.1996.tb08368.x. [DOI] [PubMed] [Google Scholar]
- 35.Burns KE, et al. Reconstitution and biochemical characterization of a new pyridoxal-5'-phosphate biosynthetic pathway. J Am Chem Soc. 2005;127:3682–3683. doi: 10.1021/ja042792t. [DOI] [PubMed] [Google Scholar]
- 36.Tambasco-Studart M, et al. Vitamin B6 biosynthesis in higher plants. Proc Natl Acad Sci U S A. 2005;102:13687–13692. doi: 10.1073/pnas.0506228102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strohmeier M, et al. Structure of a bacterial pyridoxal 5′-phosphae synthase complex. Proc Natl Acad Sci U S A. 2006;103:19284–19289. doi: 10.1073/pnas.0604950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zein F, et al. Structural Insights into the Mechanism of the PLP Synthase Holoenzyme from Thermotoga maritima. Biochemistry. 2006;45:14609–14620. doi: 10.1021/bi061464y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassera MB, et al. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2004;279:51749–51759. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 40.Wrenger C, et al. Analysis of the vitamin B6 biosynthesis pathway in the human malaria parasite Plasmodium falciparum. J Biol Chem. 2005;280:5242–5248. doi: 10.1074/jbc.M412475200. [DOI] [PubMed] [Google Scholar]
- 41.Bagautdinov B, et al. Crystal structures of biotin protein ligase from Pyrococcus horikoshii OT3 and its complexes: structural basis of biotin activation. J Mol Biol. 2005;353:322–333. doi: 10.1016/j.jmb.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 42.Zuther E, et al. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1999;96:13387–13392. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckett D. The Escherichia coli biotin regulatory system: a transcriptional switch. J Nutr Biochem. 2005;16:411–415. doi: 10.1016/j.jnutbio.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Pirner HM, Stolz J. Biotin sensing in Saccharomyces cerevisiae is mediated by a conserved DNA element and requires the activity of biotin-protein ligase. J Biol Chem. 2006;281:12381–12389. doi: 10.1074/jbc.M511075200. [DOI] [PubMed] [Google Scholar]
- 45.Stocker R, et al. Oxidative stress and protective mechanisms in erythrocytes in relation to Plasmodium vinckei load. Proc Natl Acad Sci U S A. 1985;82:548–551. doi: 10.1073/pnas.82.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stocker R, et al. Antioxidants in plasma from mice infected with Plasmodium vinckei. Biochem Biophys Res Commun. 1986;134:152–158. doi: 10.1016/0006-291x(86)90540-1. [DOI] [PubMed] [Google Scholar]
- 47.Stocker R, et al. Protection of vitamin E from oxidation by increased ascorbic acid content within Plasmodium vinckei-infected erythrocytes. Biochim Biophys Acta. 1986;876:294–299. doi: 10.1016/0005-2760(86)90287-0. [DOI] [PubMed] [Google Scholar]
- 48.Stocker R, et al. Possible mechanisms responsible for the increased ascorbic acid content of Plasmodium vinckei-infected mouse erythrocytes. Biochim Biophys Acta. 1986;881:391–397. doi: 10.1016/0304-4165(86)90031-0. [DOI] [PubMed] [Google Scholar]
- 49.Winter RW, et al. Potentiation of an antimalarial oxidant drug. Antimicrob Agents Chemother. 1997;41:1449–1454. doi: 10.1128/aac.41.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serghides L, Kain KC. Mechanism of protection induced by vitamin A in falciparum malaria. Lancet. 2002;359:1404–1406. doi: 10.1016/S0140-6736(02)08360-5. [DOI] [PubMed] [Google Scholar]
- 51.Shankar AH, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet. 1999;354:203–209. doi: 10.1016/S0140-6736(98)08293-2. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez Uribe MC, et al. Plasma retinol concentration according to pubertal maturation in school children and adolescents of Medellin, Colombia. Eur J Clin Nutr. 2004;58:456–461. doi: 10.1038/sj.ejcn.1601828. [DOI] [PubMed] [Google Scholar]
- 53.Rosales FJ, et al. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am J Clin Nutr. 2000;71:1582–1588. doi: 10.1093/ajcn/71.6.1582. [DOI] [PubMed] [Google Scholar]
- 54.Serghides L, Kain KC. Peroxisome proliferator-activated receptor gamma-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-alpha secretion by monocytes/macrophages. J Immunol. 2001;166:6742–6748. doi: 10.4049/jimmunol.166.11.6742. [DOI] [PubMed] [Google Scholar]
- 55.Mizuno Y, et al. In-vitro uptake of vitamin A by Plasmodium falciparum. Ann Trop Med Parasitol. 2003;97:237–243. doi: 10.1179/000349803235001723. [DOI] [PubMed] [Google Scholar]
- 56.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 57.Haussler MR, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 58.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Newens K, et al. Plasma 25-hydroxyvitamin D does not vary over the course of a malarial infection. Trans R Soc Trop Med Hyg. 2006;100:41–44. doi: 10.1016/j.trstmh.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 60.Boyan BD, et al. 1,25-(OH)2D3 modulates growth plate chondrocytes via membrane receptor-mediated protein kinase C by a mechanism that involves changes in phospholipid metabolism and the action of arachidonic acid and PGE2. Steroids. 1999;64:129–136. doi: 10.1016/s0039-128x(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 61.Vial HJ, et al. Inhibition of the in vitro growth of Plasmodium falciparum by D vitamins and vitamin D-3 derivatives. Mol Biochem Parasitol. 1982;5:189–198. doi: 10.1016/0166-6851(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 62.Salom-Roig XJ, et al. Dual molecules as new antimalarials. Comb Chem High Throughput Screen. 2005;8:49–62. doi: 10.2174/1386207053328219. [DOI] [PubMed] [Google Scholar]
- 63.Vial HJ, et al. Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc Natl Acad Sci U S A. 2004;101:15458–15463. doi: 10.1073/pnas.0404037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicolas O, et al. Pharmacological properties of a new antimalarial bisthiazolium salt, T3, and a corresponding prodrug, TE3. Antimicrob Agents Chemother. 2005;49:3631–3639. doi: 10.1128/AAC.49.9.3631-3639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spry C, et al. A class of pantothenic acid analogs inhibits Plasmodium falciparum pantothenate kinase and represses the proliferation of malaria parasites. Antimicrob Agents Chemother. 2005;49:4649–4657. doi: 10.1128/AAC.49.11.4649-4657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson J. Vitamins, minerals and supplements: part three. Community Pract. 2005;78:407–408. [PubMed] [Google Scholar]
- 67.Thompson J. Vitamins, minerals and supplements: part two. Community Pract. 2005;78:366–368. [PubMed] [Google Scholar]
- 68.Thompson J. Vitamins, minerals and supplements. Community Pract. 2005:24–26. 2005. [PubMed] [Google Scholar]
- 69.Park JH, et al. Biosynthesis of the thiazole moiety of thiamin pyrophosphate (vitamin B1) Biochemistry. 2003;42:12430–12438. doi: 10.1021/bi034902z. [DOI] [PubMed] [Google Scholar]
- 70.Chatterjee A, et al. Thiamin biosynthesis in eukaryotes: characterization of the enzyme-bound product of thiazole synthase from Saccharomyces cerevisiae and its implications in thiazole biosynthesis. J Am Chem Soc. 2006;128:7158–7159. doi: 10.1021/ja061413o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jurgenson CT, et al. Structural insights into the function of the thiamin biosynthetic enzyme Thi4 from Saccharomyces cerevisiae. Biochemistry. 2006;45:11061–11070. doi: 10.1021/bi061025z. [DOI] [PubMed] [Google Scholar]
- 72.Tazuya K, et al. Incorporation of histidine into the pyrimidine moiety of thiamin in Saccharomyces cerevisiae. Biochim Biophys Acta. 1989;990:73–79. doi: 10.1016/s0304-4165(89)80014-5. [DOI] [PubMed] [Google Scholar]
- 73.Zeidler J, et al. Biosynthesis of vitamin B1 in yeast. Derivation of the pyrimidine unit from pyridoxine and histidine. Intermediacy of urocanic acid. J Am Chem Soc. 2003;125:13094–13105. doi: 10.1021/ja030261j. [DOI] [PubMed] [Google Scholar]
- 74.Bauer JA, et al. Three-dimensional structure of YaaE from Bacillus subtilis, a glutaminase implicated in pyridoxal-5′-phosphate biosynthesis. J Biol Chem. 2004;279:2704–2711. doi: 10.1074/jbc.M310311200. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, et al. A new arrangement of (beta/alpha)8 barrels in the synthase subunit of PLP synthase. J Biol Chem. 2005;280:27914–27923. doi: 10.1074/jbc.M503642200. [DOI] [PubMed] [Google Scholar]
- 76.Ellis KE, et al. Nifs and Sufs in malaria. Mol Microbiol. 2001;41:973–981. doi: 10.1046/j.1365-2958.2001.02588.x. [DOI] [PubMed] [Google Scholar]