Abstract
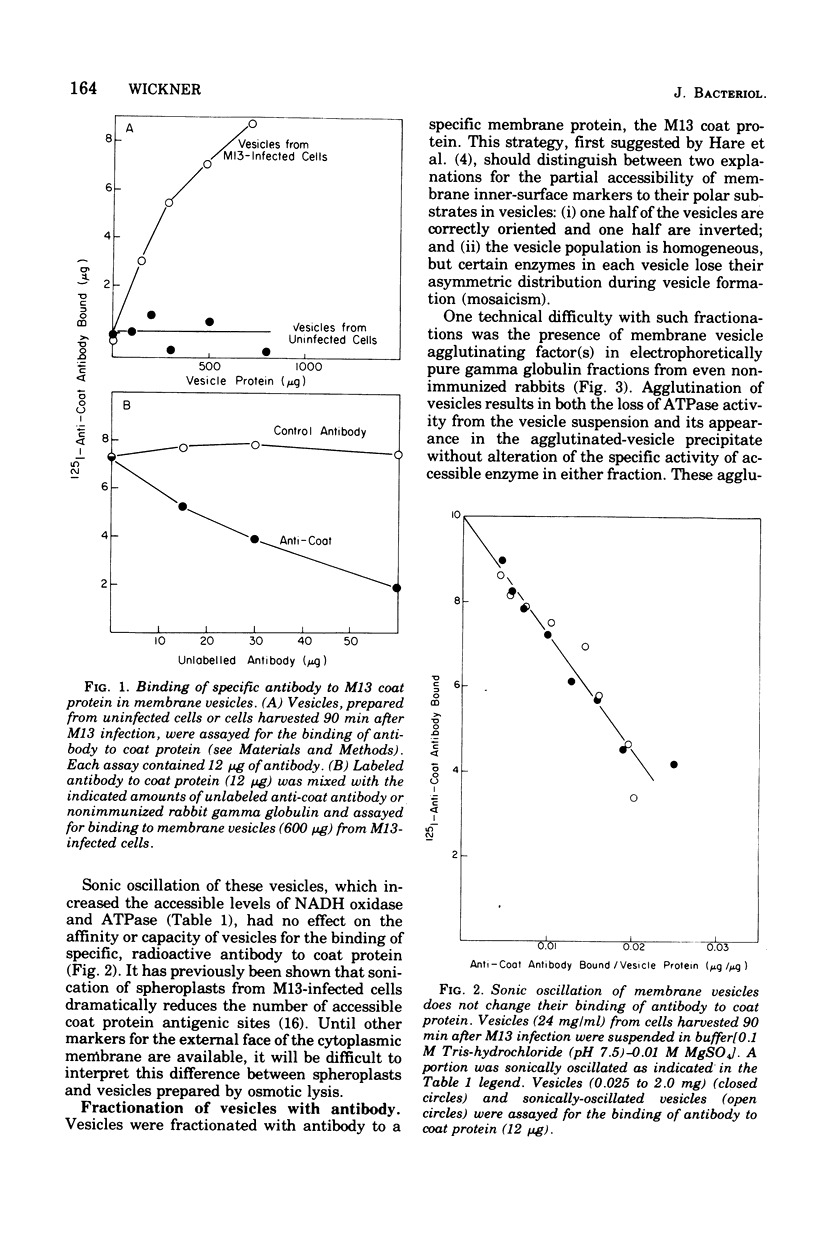
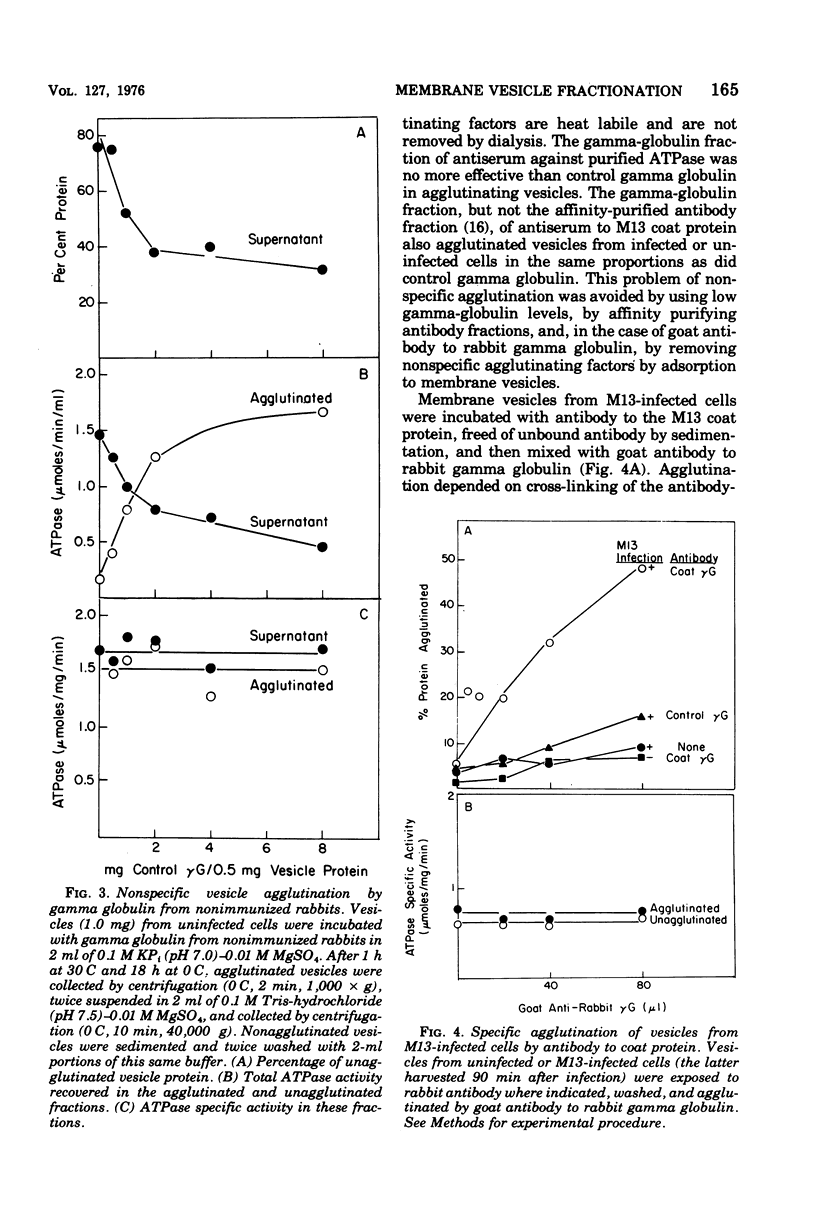
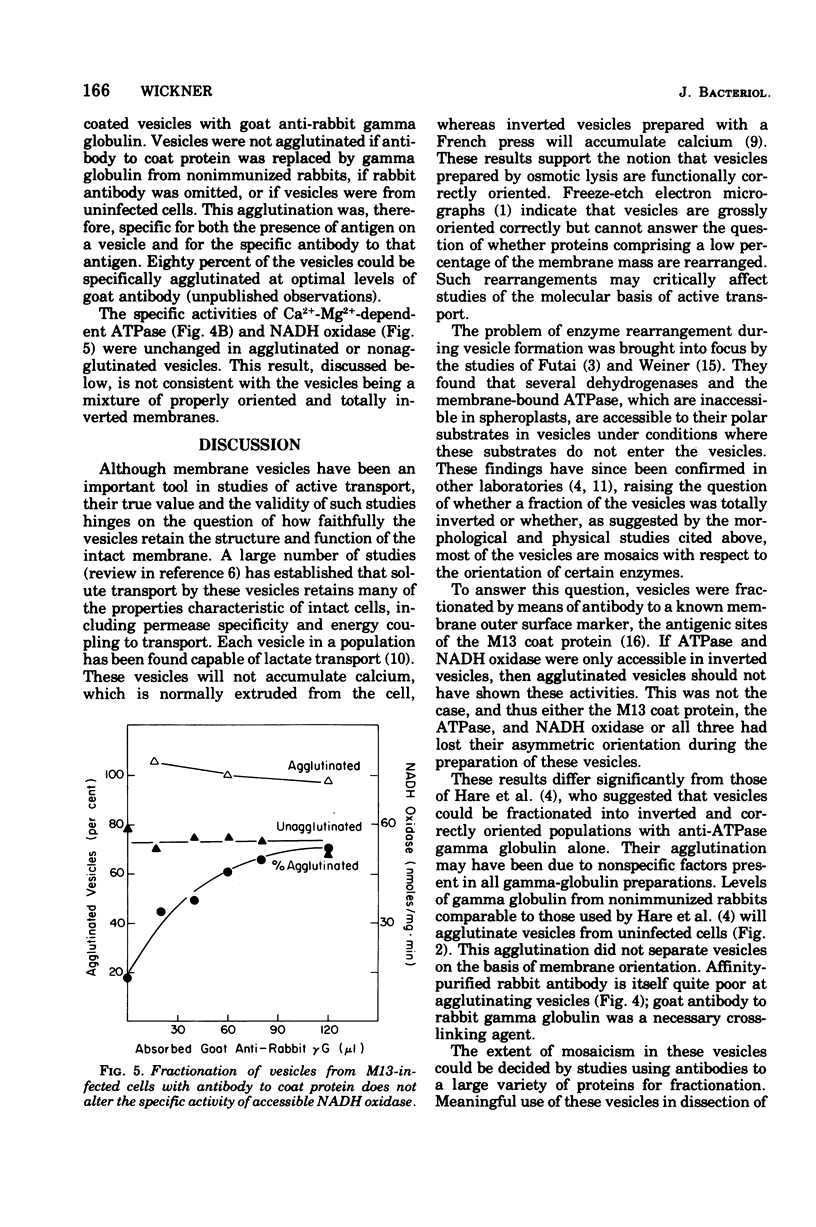
Membrane vesicles were prepared by osmotic lysis of spheroplasts from M13-infected Escherichia coli. Reduced nicotinamide adenine dinucleotide (NADH) oxidase (reduced NAD: oxidoreductase, EC 1.6.99.3) and Mg2+-Ca2+-activated adenosine triphosphatase (ATP phosphohydrolase, EC 3.6.1.3), which are normally localized to the inner surface of the cytoplasmic membrane, were 50% acceesible to their polar substrates in these vesicles. The major coat protein of coliphage M13 is also bound to the cytoplasmic membrane (prior to phage assembly) but with its antigenic sites exposed to the exterior of the cell. Antibody to M13 coat protein was used to fractionate membrane vesicles. Neither agglutinated nor unagglutinated vesicles had altered NADH oxidase and adenosine triphosphatase specific activities. This is inconsistent with such vesicles being a mixture of correctly oriented and completely inverted membrane sacs and suggests that NADH oxidase, adenosine triphosphatase, M13 coat protein, or all three proteins rearrange during vesicle preparation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., McClees J. S. Active transport of calcium in inverted membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5042–5046. doi: 10.1073/pnas.71.12.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kaczorowski G., Fisher J., Walsh C. T., Silverstein S. C. Determination of the absolute number of Escherichia coli membrane vesicles that catalyze active transport. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5032–5036. doi: 10.1073/pnas.71.12.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Cashman J. S. Abortive infection of Escherichia coli with the bacteriophage f1: cytoplasmic membrane proteins and the f1 DNA-gene 5 protein complex. Virology. 1973 Sep;55(1):20–38. doi: 10.1016/s0042-6822(73)81005-0. [DOI] [PubMed] [Google Scholar]
- Weiner J. H. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J Membr Biol. 1974;15(1):1–14. doi: 10.1007/BF01870078. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. doi: 10.1073/pnas.72.12.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]


