Abstract
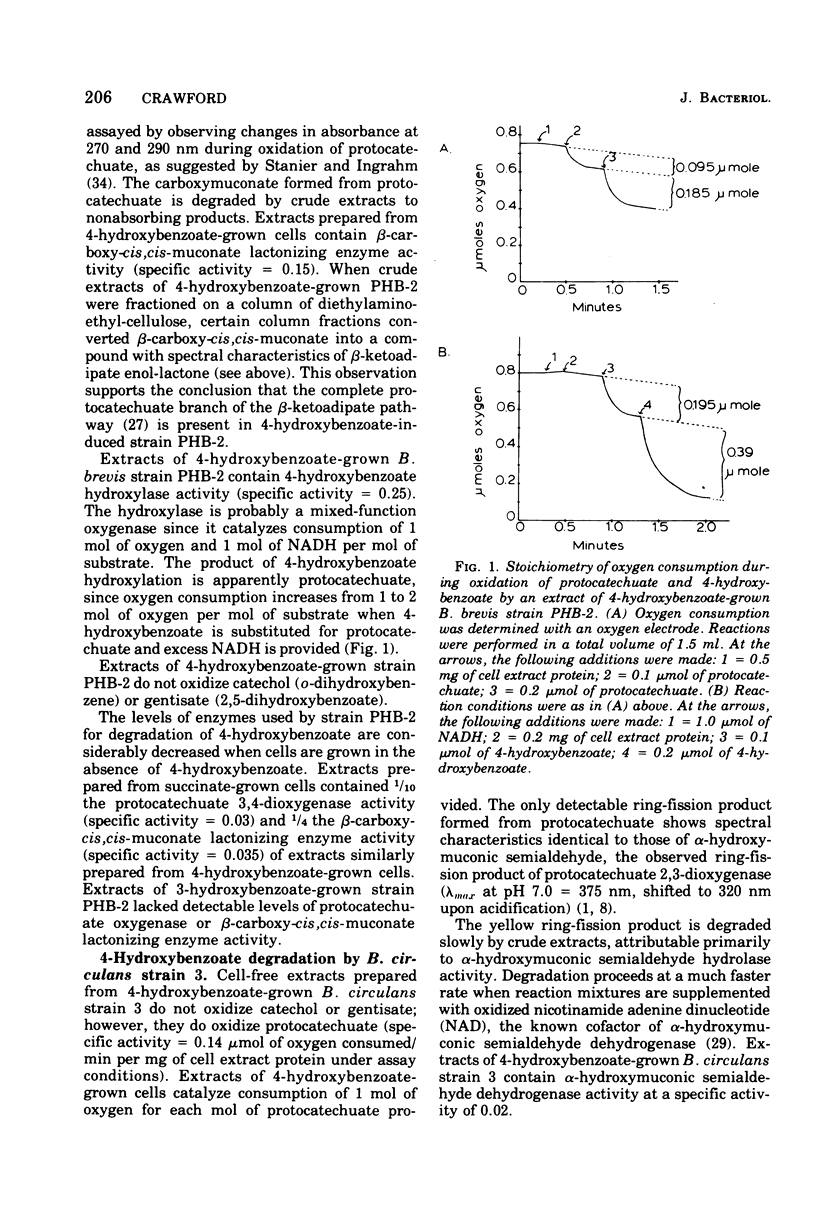
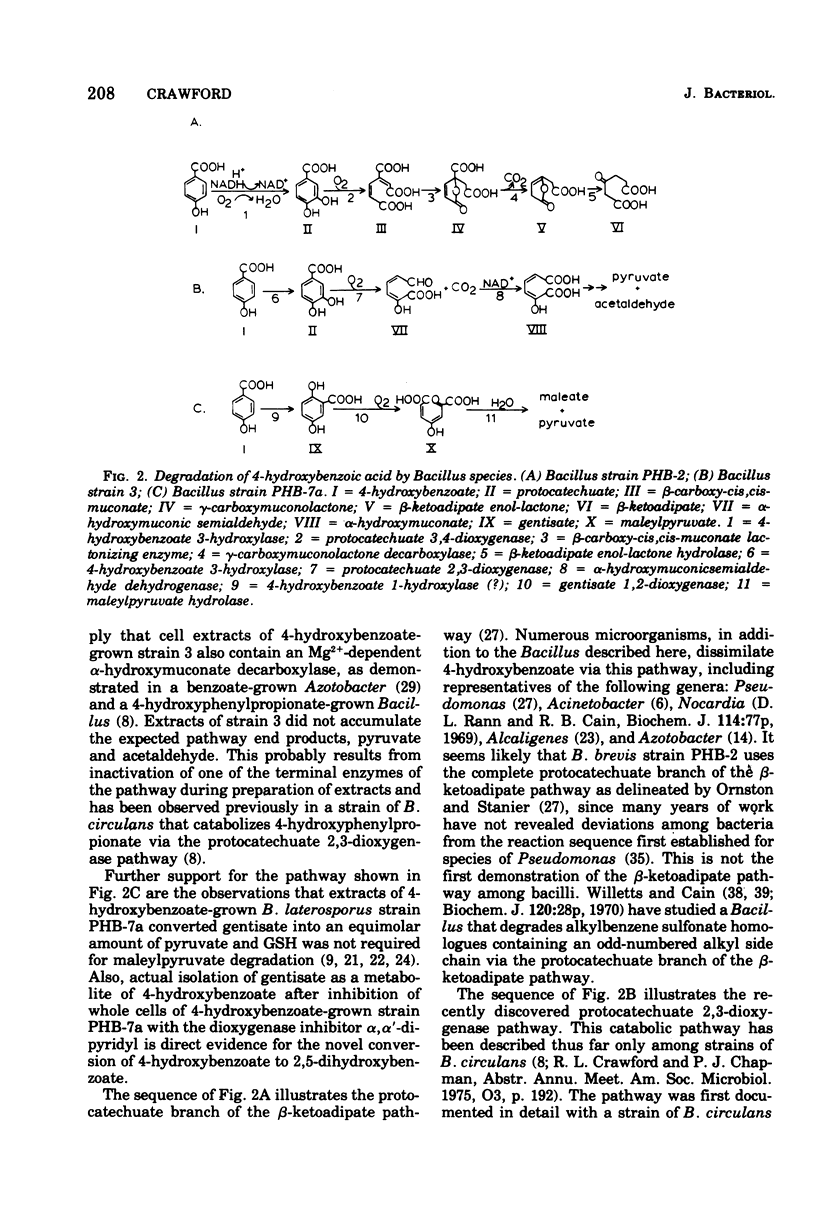
The pathways used by three bacterial strains of the genus Bacillus to degrade 4-hydroxybenzoate are delineated. When B. brevis strain PHB-2 is grown on 4-hydroxybenzoate, enzymes of the protocatechuate branch of the beta-ketoadipate pathway are induced. In contrast, B. circulans strain 3 contains high levels of the enzymes of the protocatechuate 2,3-dioxygenase pathway after growth on 4-hydroxybenzoate. B. laterosporus strain PHB-7a degrades 4-hydroxybenzoate by a novel reaction sequence. After growth on 4-hydroxybenzoate, strain PHB-7a contains high levels of gentisate oxygenase (EC 1.13.11.4) and maleylpyruvate hydrolase. Whole cells of strain PHB-7a (grown on 4-hydroxylbenzoate) accumulate 2,5-dihydroxybenzoate (gentisate) from 4-hydroxybenzoate when incubated in the presence of 1mM alpha,alpha'-dipyridyl. Thus, strain PHB-7a appears to convert 4-hydroxybenzoate to gentisate, which is further degraded by the glutathione-independent gentisic acid pathway. These pathway delineations provide evidence that Bacillus species are derived from a diverse evolutionary background.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouknight R. R., Sadoff H. L. Tryptophan catabolism in Bacillus megaterium. J Bacteriol. 1975 Jan;121(1):70–76. doi: 10.1128/jb.121.1.70-76.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell J. A. Metabolism of phenol and cresols by Bacillus stearothermophilus. J Bacteriol. 1975 Dec;124(3):1077–1083. doi: 10.1128/jb.124.3.1077-1083.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell J. A. The meta-cleavage of catechol by a thermophilic Bacillus species. Biochem Biophys Res Commun. 1974 Oct 8;60(3):934–941. doi: 10.1016/0006-291x(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Buswell J. A., Twomey D. G. Utilization of phenol and cresols by Bacillus stearothermophilus, strain PH24. J Gen Microbiol. 1975 Apr;87(2):377–379. doi: 10.1099/00221287-87-2-377. [DOI] [PubMed] [Google Scholar]
- Collinsworth W. L., Chapman P. J., Dagley S. Stereospecific enzymes in the degradation of aromatic compounds by pseudomonas putida. J Bacteriol. 1973 Feb;113(2):922–931. doi: 10.1128/jb.113.2.922-931.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Degradation of 3-hydroxybenzoate by bacteria of the genus Bacillus. Appl Microbiol. 1975 Sep;30(3):439–444. doi: 10.1128/am.30.3.439-444.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Hutton S. W., Chapman P. J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975 Mar;121(3):794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Novel pathway for degradation of protocatechuic acid in Bacillus species. J Bacteriol. 1975 Feb;121(2):531–536. doi: 10.1128/jb.121.2.531-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Dagley S. Catabolism of aromatic compounds by micro-organisms. Adv Microb Physiol. 1971;6(0):1–46. doi: 10.1016/s0065-2911(08)60066-1. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. THE PATHWAY OF NICOTINIC ACID OXIDATION BY A BACILLUS SPECIES. J Biol Chem. 1964 Jul;239:2285–2291. [PubMed] [Google Scholar]
- Hardisson C., Sala-Trepat J. M., Stanier R. Y. Pathways for the oxidation of aromatic compounds by Azotobacter. J Gen Microbiol. 1969 Nov;59(1):1–11. doi: 10.1099/00221287-59-1-1. [DOI] [PubMed] [Google Scholar]
- Hareland W. A., Crawford R. L., Chapman P. J., Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975 Jan;121(1):272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg E. Y., Kenyon G. L. Mandelic acid racemase from Pseudomonas putida. Purification and properties of the enzyme. Biochemistry. 1970 Oct 13;9(21):4029–4036. doi: 10.1021/bi00823a001. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: regulation of nicotinic acid and 6-hydroxynicotinic acid hydroxylases. J Bacteriol. 1972 Oct;112(1):392–397. doi: 10.1128/jb.112.1.392-397.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: source of oxygen atoms for the hydroxylation of nicotinic acid and 6-hydroxynicotinic acid. J Bacteriol. 1971 Nov;108(2):757–759. doi: 10.1128/jb.108.2.757-759.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: source of oxygen atoms for the hydroxylation of nicotinic acid and 6-hydroxynicotinic acid. J Bacteriol. 1971 Nov;108(2):757–759. doi: 10.1128/jb.108.2.757-759.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. Enzymic formation of D-malate. Biochem J. 1968 Dec;110(4):798–800. doi: 10.1042/bj1100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J. Gentisic acid and its 3- and 4-methyl-substituted homologoues as intermediates in the bacterial degradation of m-cresol, 3,5-xylenol and 2,5-xylenol. Biochem J. 1971 Mar;122(1):19–28. doi: 10.1042/bj1220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Dissimilation of aromatic compounds by Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):468–475. doi: 10.1128/jb.107.2.468-475.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK L. The enzymic oxidation of gentisic acid. Biochim Biophys Acta. 1959 Jul;34:117–123. doi: 10.1016/0006-3002(59)90239-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung P. T., Chapman P. J., Dagley S. Purification and properties of 4-hydroxy-2-ketopimelate aldolase from Acinetobacter. J Bacteriol. 1974 Oct;120(1):168–172. doi: 10.1128/jb.120.1.168-172.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Prasad C., Srinivasan V. R. Tryptophan catabolism during sporulation in Bacillus cereus. Biochem J. 1970 Sep;119(2):343–349. doi: 10.1042/bj1190343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Spokes J. R., Walker N. Chlorophenol and chlorobenzoic acid co-metabolism by different genera of soil bacteria. Arch Mikrobiol. 1974 Mar 4;96(2):125–134. doi: 10.1007/BF00590169. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Wallnöfer P., Engelhardt G. [The degradation of phenylamides by Bacillus sphaericus]. Arch Mikrobiol. 1971;80(4):315–323. [PubMed] [Google Scholar]
- Willetts A. J., Cain R. B. Microbial metabolism of alkylbenzene sulphonates. Bacterial metabolism of undecylbenzene-p-sulphonate and dodecylbenzene-p-sulphonate. Biochem J. 1972 Sep;129(2):389–402. doi: 10.1042/bj1290389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts A. J. Microbial metabolism of alkylbenzene sulphonates. The oxidation of key aromatic compounds by a Bacillus. Antonie Van Leeuwenhoek. 1974;40(4):547–559. doi: 10.1007/BF00403819. [DOI] [PubMed] [Google Scholar]


