Abstract
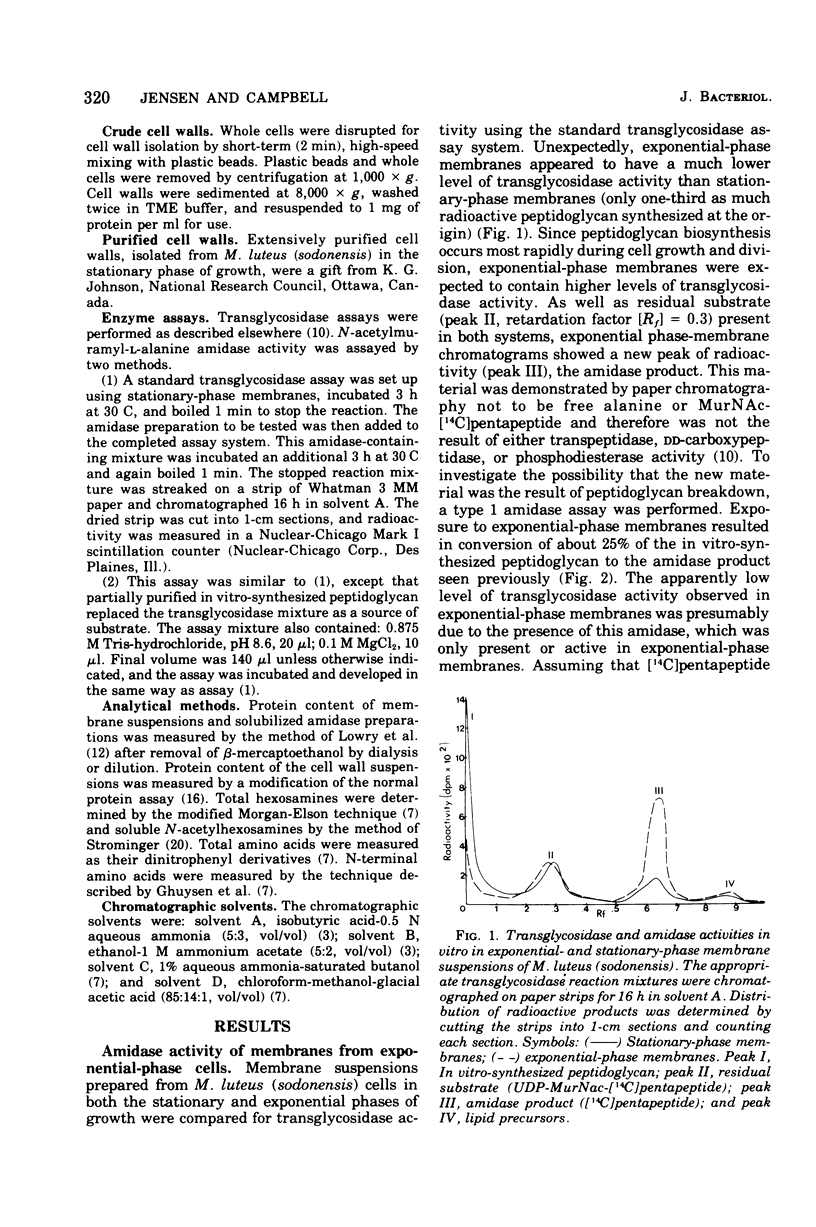
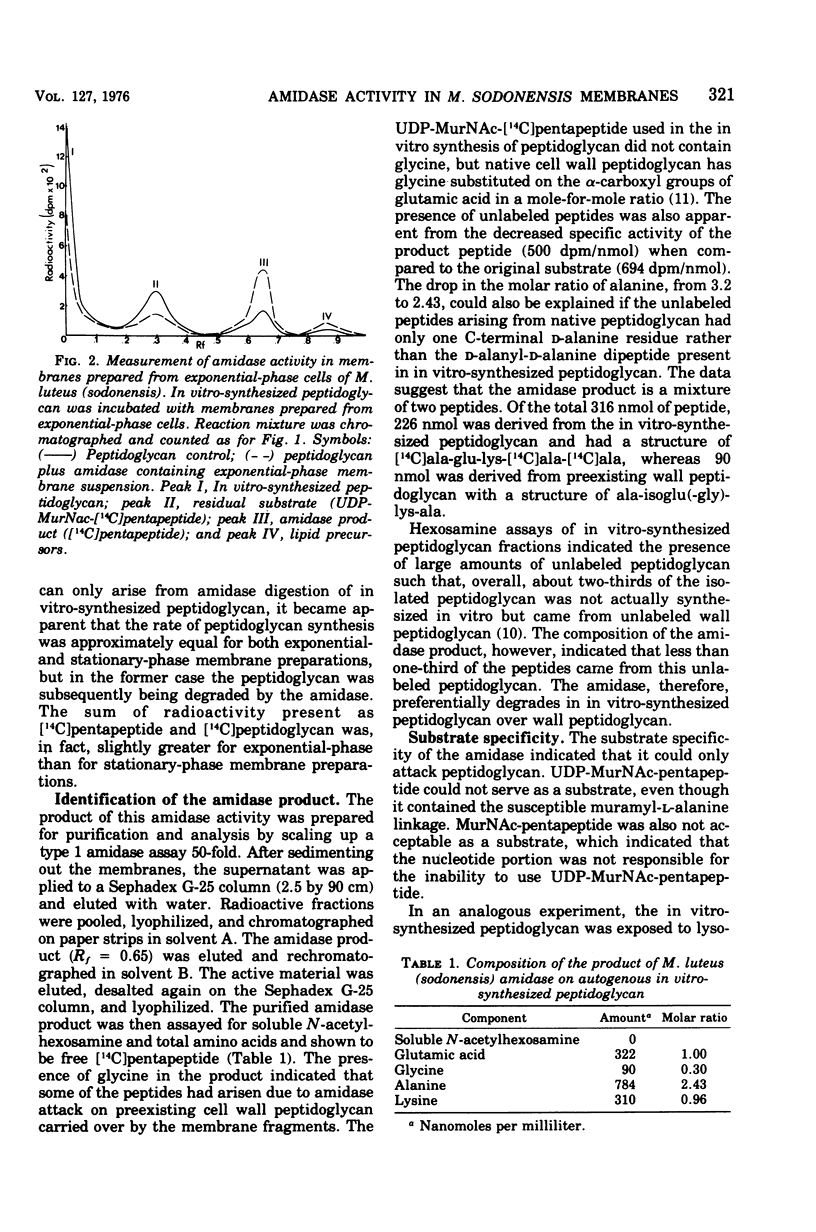
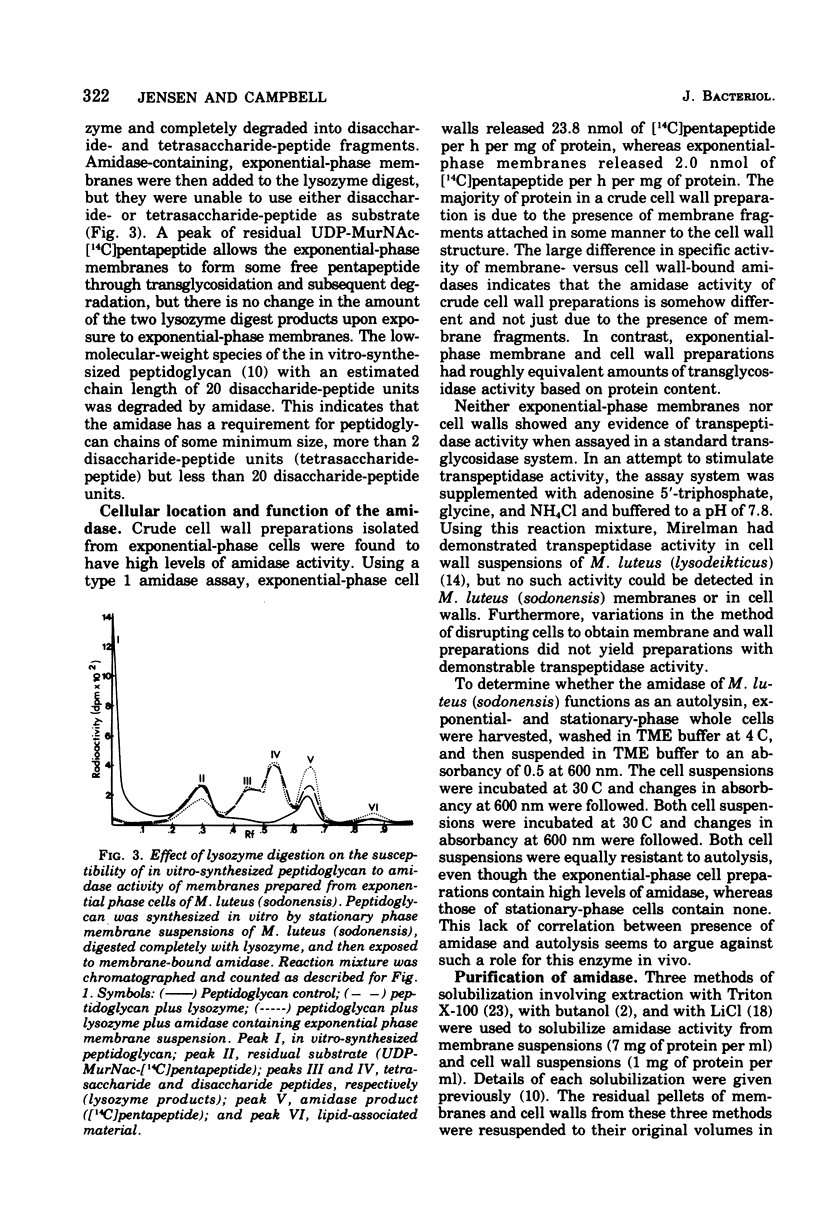
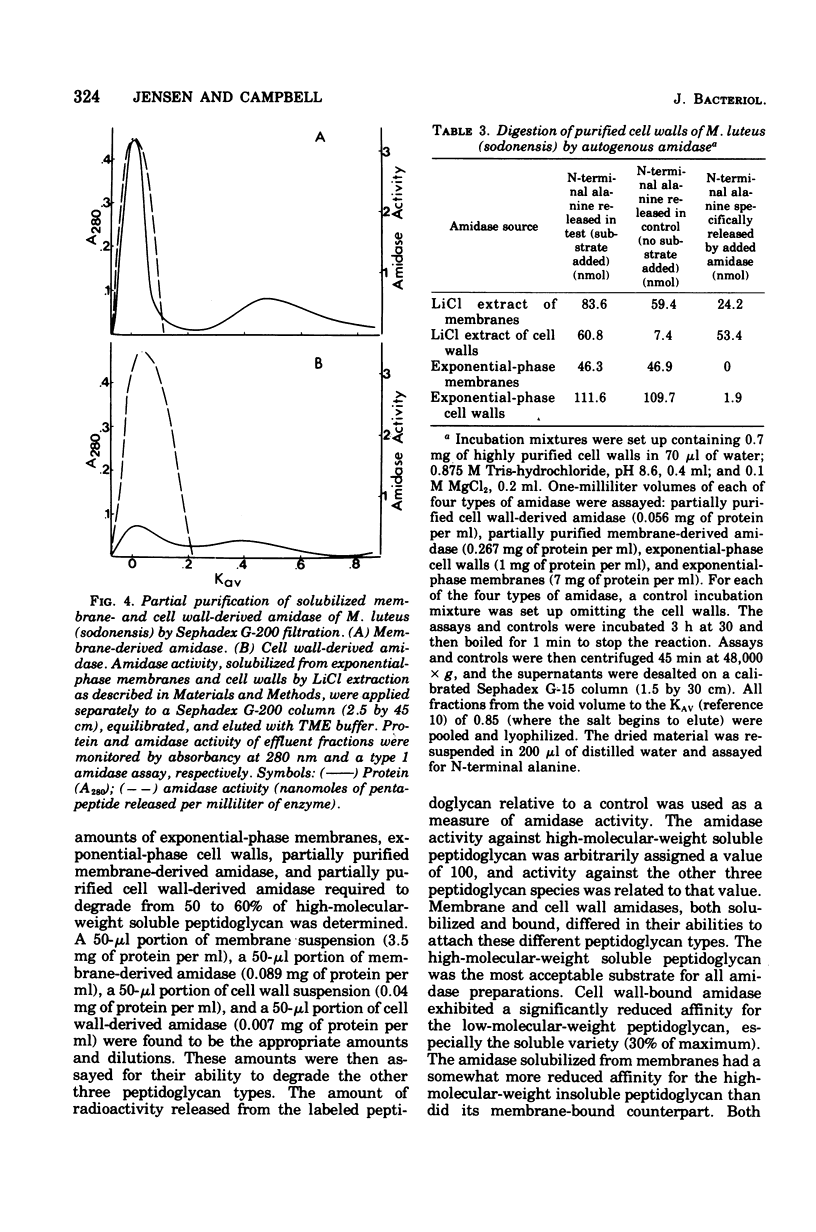
Membrane suspensions prepared from Micrococcus luteus (sodonensis) in both the exponential and stationary phases of growth contained a transglycosidase activity capable of synthesizing linear peptidoglycan. Exponential-phase membranes also contained an N-acetylmuramyl-L-alanine amidase activity which degraded the peptidoglycan as it was formed. The product of this amidase was purified and found to be free pentapeptide. The amidase was specific for peptidoglycan and could not attack lower-molecular-weight substrates even though the susceptible bond was present. Crude cell wall preparations isolated from exponential-phase cells also contained high levels of amidase. This cell wall-bound amidase would preferentially degrade in vitro-synthesized peptidoglycan over its own cell wall. Amidase activity could be solubilized from both cell walls and membranes by Triton X-100 treatment, butanol extraction, or LiCl extraction. Both membrane- and cell wall-derived amidases, solubilized by LiCl extraction, appeared to be of high molecular weight (greater than 150,000). Once solubilized, these wall- and membrane-derived amidases could attack the cross-bridged peptidoglycan of purified native cell walls, whereas bound amidases could not.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett H. J. D-alanine carboxypeptidases of Bacillus stearothermophilus: solubilisation of particulate enzymes and mechanism of action of penicillin. Biochim Biophys Acta. 1973 Apr 28;304(2):332–352. doi: 10.1016/0304-4165(73)90252-3. [DOI] [PubMed] [Google Scholar]
- Bordet C., Perkins H. R. Iodinated vancomycin and mucopeptide biosynthesis by cell-free preparations from Micrococcus lysodeikticus. Biochem J. 1970 Oct;119(5):877–883. doi: 10.1042/bj1190877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Shockman G. D. Some properties of the autolytic N-acetylmuramidase of Lactobacillus acidophilus. J Bacteriol. 1973 Apr;114(1):34–41. doi: 10.1128/jb.114.1.34-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Glaser L. Bacterial cell surface polysaccharides. Annu Rev Biochem. 1973;42:91–112. doi: 10.1146/annurev.bi.42.070173.000515. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Jensen S. E., Campbell J. N. Peptidoglycan biosynthesis in Micrococcus luteus (sodonensis): transglycosidase and phosphodiesterase activities in membrane preparations. J Bacteriol. 1976 Jul;127(1):309–318. doi: 10.1128/jb.127.1.309-318.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Campbell J. N. Effect of growth conditions on peptidoglycan structure and susceptibility to lytic enzymes in cell walls of Micrococcus sodonensis. Biochemistry. 1972 Jan 18;11(2):277–286. doi: 10.1021/bi00752a020. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mirelman D., Bracha R. Effect of penicillin on the in vivo formation of the D-alanyl-L-alanine peptide cross-linkage in cell walls of Micrococcus luteus. Antimicrob Agents Chemother. 1974 Jun;5(6):663–666. doi: 10.1128/aac.5.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Biosynthesis of peptidoglycan by a cell wall preparation of Staphylococcus aureus and its inhibition by penicillin. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1909–1917. doi: 10.1016/0006-291x(72)90069-1. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Shaw D. R., Park J. T. Nature and origins of phosphorus compounds in isolated cell walls of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):239–244. doi: 10.1128/jb.107.1.239-244.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L. Microbial uridine-5'-pyrophosphate N-acetylamino sugar compounds. I. Biology of the penicillin-induced accumulation. J Biol Chem. 1957 Jan;224(1):509–523. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of the murein. Biochem Biophys Res Commun. 1967 Sep 27;28(6):965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. Relation of detergent HLB number to solubilization and stabilization of D-alanine carboxypeptidase from Bacillus subtilis membranes. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2997–3001. doi: 10.1073/pnas.70.10.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]


