Abstract
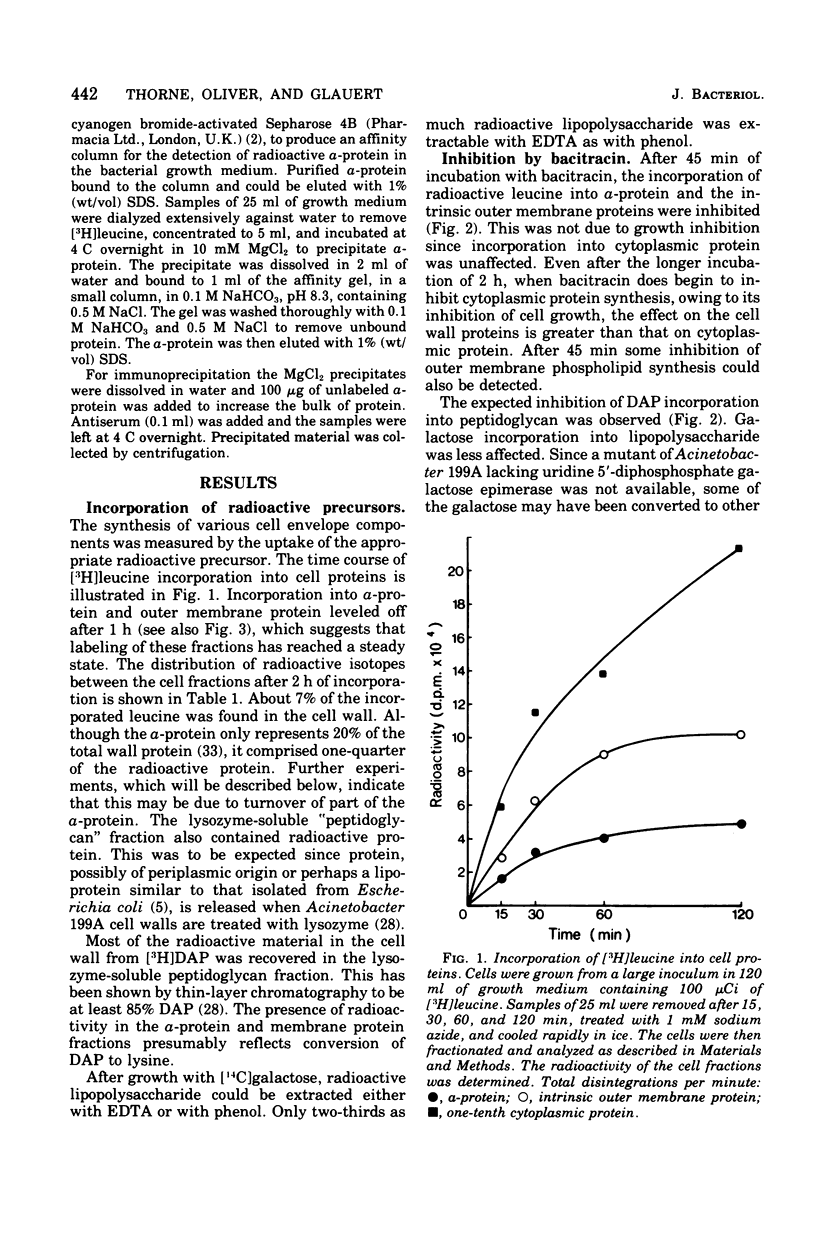
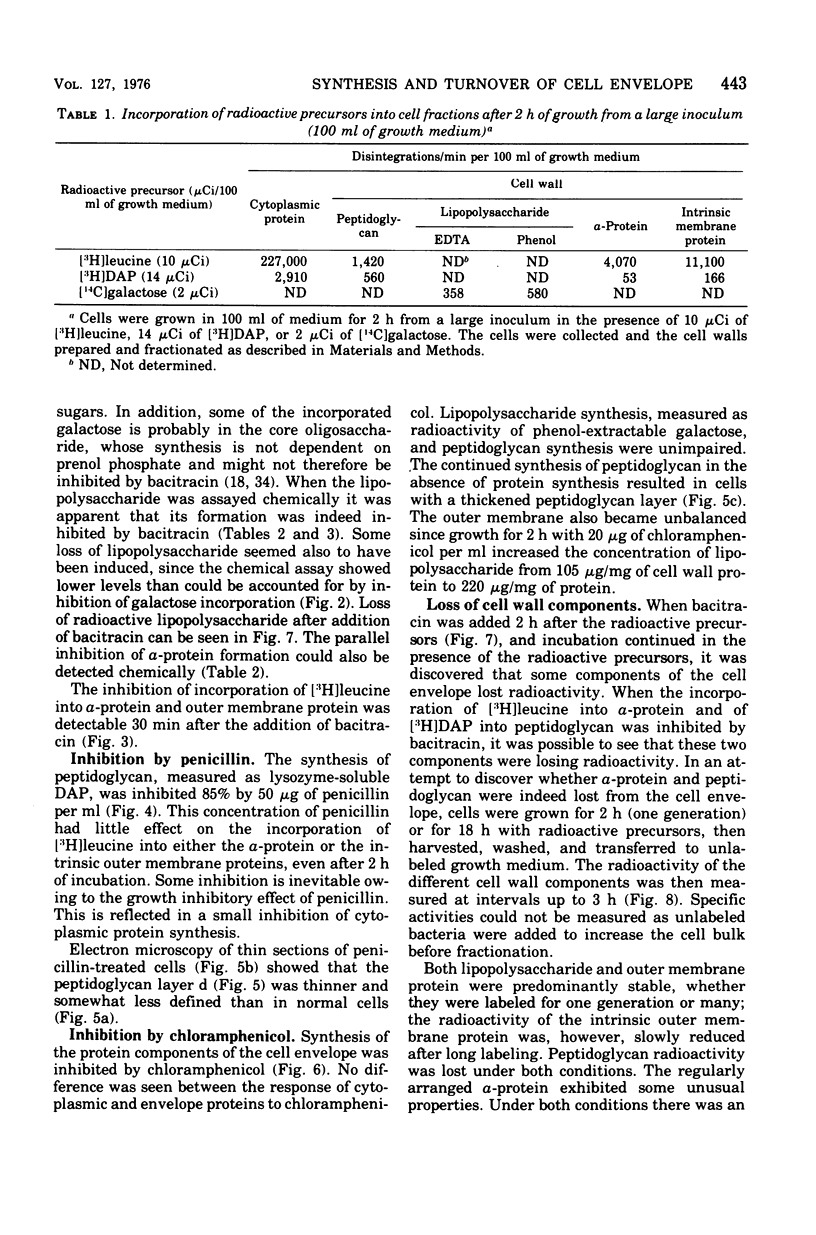
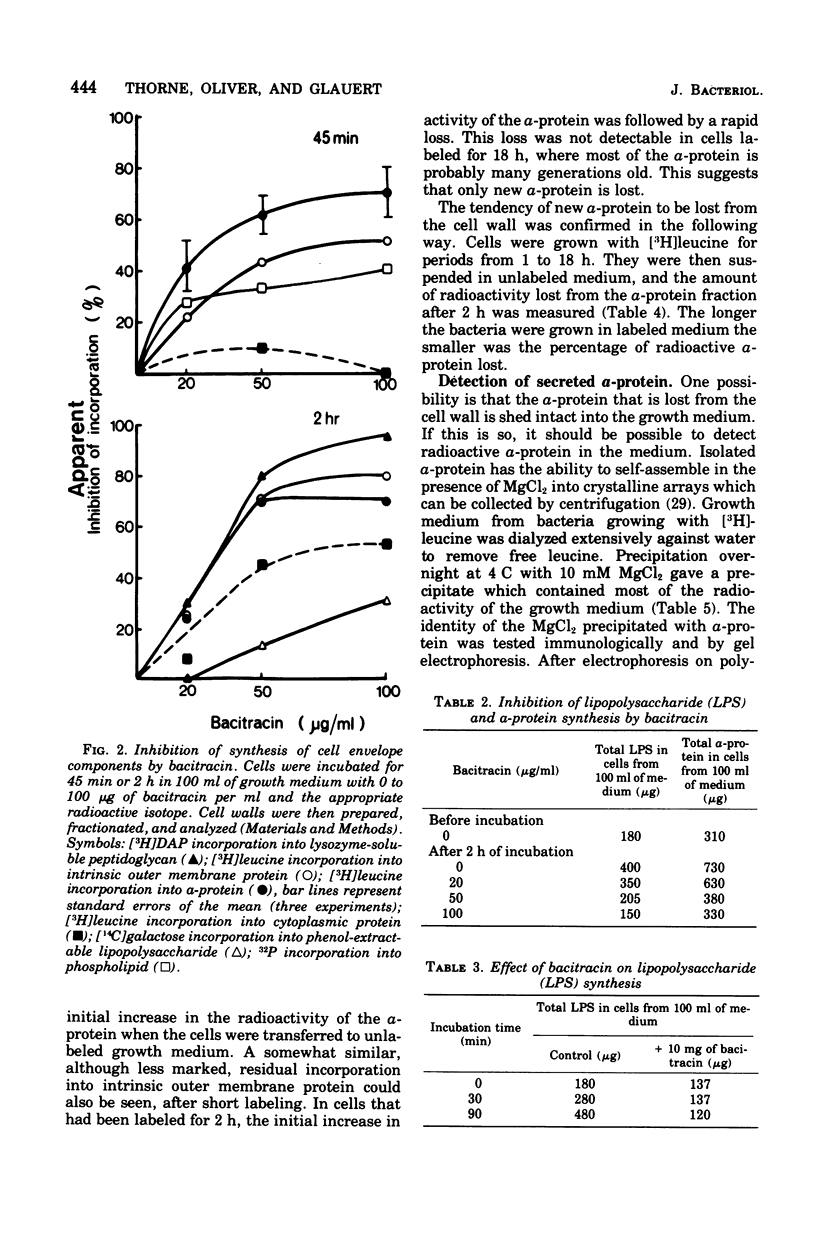
The formation of the components of the cell envelope of Acinetobacter sp. 199A was investigated by measuring the incorporation of [3H]leucine into protein, [14C]galactose into lipopolysaccharide, 32P into phospholipid, and [3H]diaminopimelic acid into peptidoglycan. Whereas the lipopolysaccharide and intrinsic protein of the outer membrane were stable, some of the regularly arranged surface protein, the alpha-protein, was lost into the growth medium. Only newly synthesized alpha-protein was lost. The peptidoglycan of the murein layer was also labile. Selective inhibition of the formation of individual components of the cell envelope with penicillin, chloramphenicol, and bacitracin showed that incorporation of protein into the outer membrane required the simultaneous formation of complete lipopolysaccharide. The converse was not true: protein synthesis was not required for lipopolysaccharide incorporation. Formation of the outer membrane and the murein layer proceeded independently.
Full text
PDF

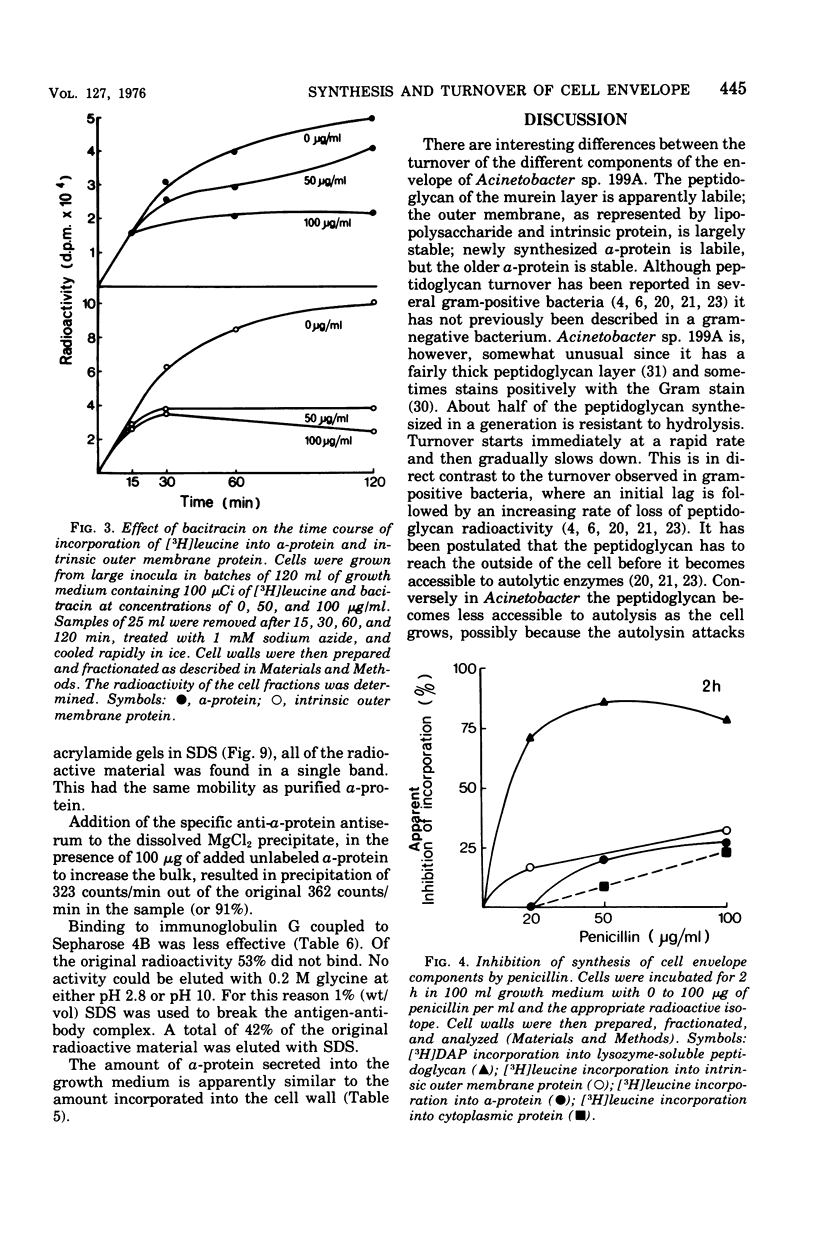
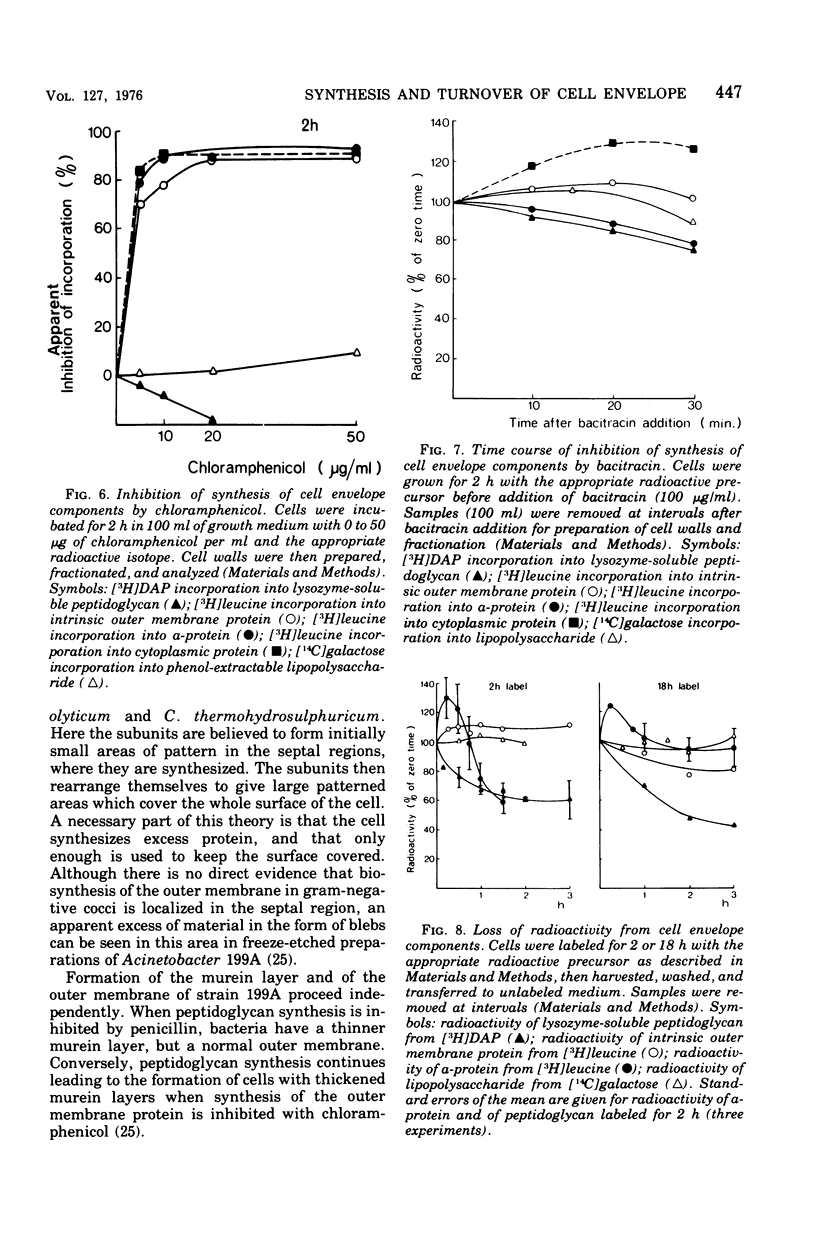
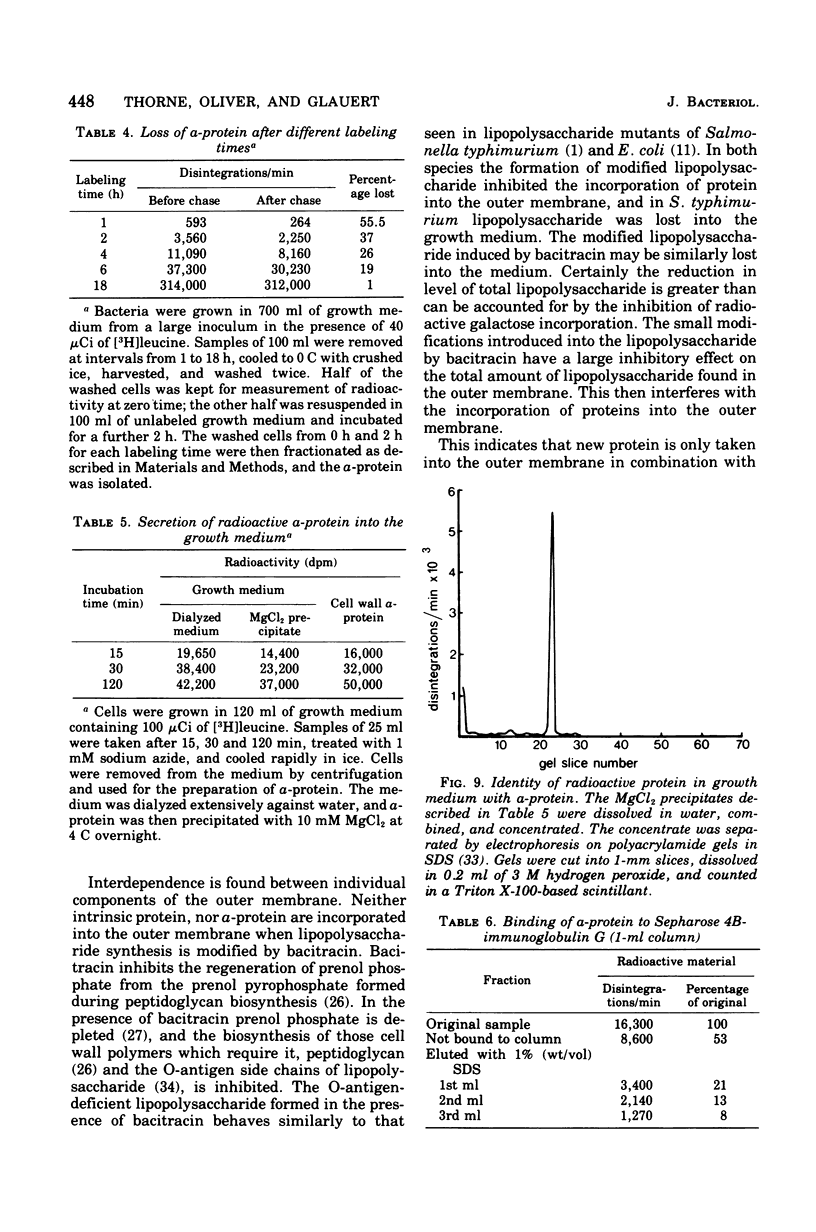


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Coyette J., Sayare M., Boothby D., Shockman G. D. Turnover of the cell wall peptidoglycan of Lactobacillus acidophilus. The presence of a fraction immune to turnover. J Biol Chem. 1975 Feb 25;250(4):1348–1353. [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Glaser L. On the mode of in vivo assembly of the cell wall of Bacillus subtilis. J Biol Chem. 1972 Feb 25;247(4):1180–1187. [PubMed] [Google Scholar]
- Movitz J. A study on the biosynthesis of protein A in Staphylococcus aureus. Eur J Biochem. 1974 Oct 1;48(1):131–136. doi: 10.1111/j.1432-1033.1974.tb03750.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Pooley H. M., Thurman P. F., Taylor C. Wall and membrane growth in bacilli and their mutants. Ann Microbiol (Paris) 1974 Sep;125 B(2):135–147. [PubMed] [Google Scholar]
- Sleytr U. B., Thornley M. J. Freeze-etching of the cell envelope of an Acinetobacter species which carries a regular array of surface subunits. J Bacteriol. 1973 Dec;116(3):1383–1397. doi: 10.1128/jb.116.3.1383-1397.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Strominger J. L. Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3223–3227. doi: 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J. Identification of prenol intermediates of wall biosynthesis in growing cells of Lactobacillus plantarum. J Bacteriol. 1973 Oct;116(1):235–244. doi: 10.1128/jb.116.1.235-244.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Glauert A. M. Chemical analysis of the outer membrane and other layers of the cell envelope of Acinetobacter sp. J Bacteriol. 1973 Oct;116(1):410–417. doi: 10.1128/jb.116.1.410-417.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J. A taxonomic study of Acinetobacter and related genera. J Gen Microbiol. 1967 Nov;49(2):211–257. doi: 10.1099/00221287-49-2-211. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M., Sleytr U. B. Isolation of outer membranes with an ordered array of surface subunits from Acinetobacter. J Bacteriol. 1973 Jun;114(3):1294–1308. doi: 10.1128/jb.114.3.1294-1308.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Thorne K. J., Glauert A. M. Detachment and chemical characterization of the regularly arranged subunits from the surface of an Acinetobacter. J Bacteriol. 1974 May;118(2):654–662. doi: 10.1128/jb.118.2.654-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]