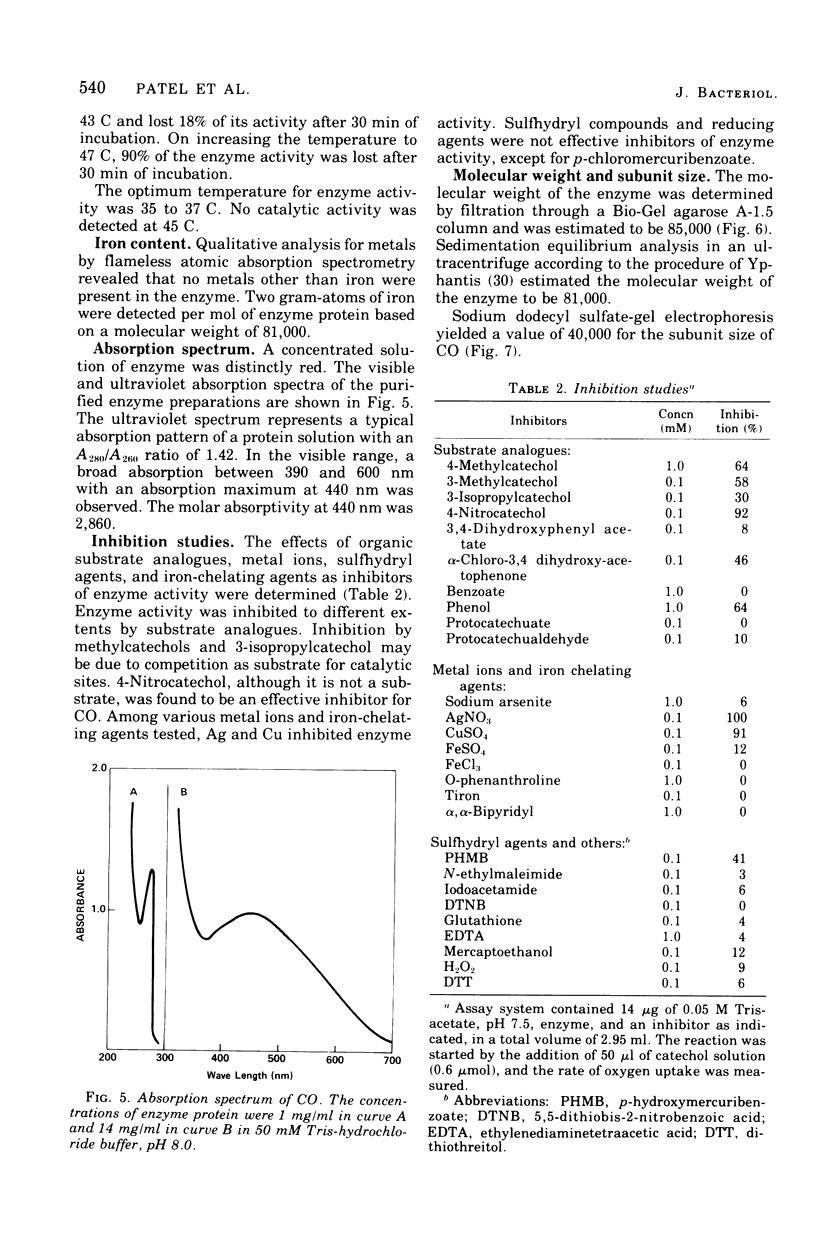
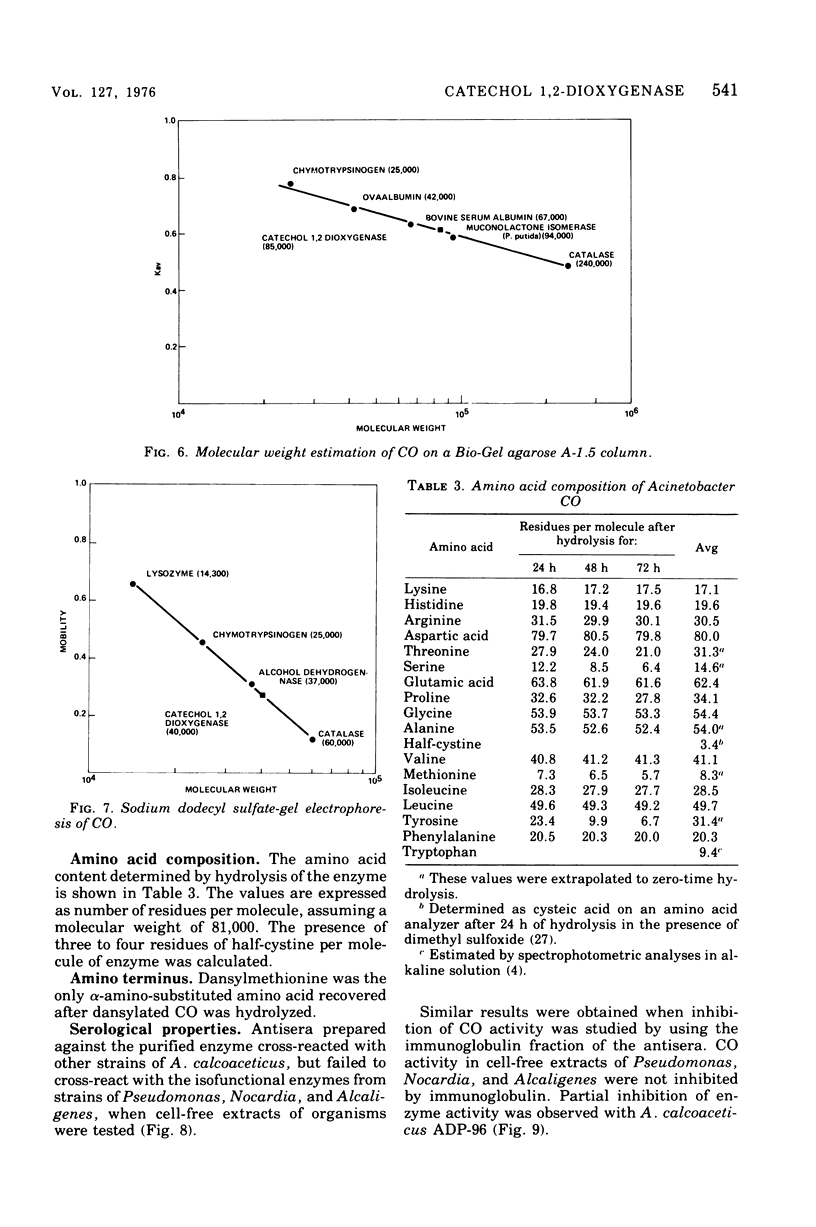
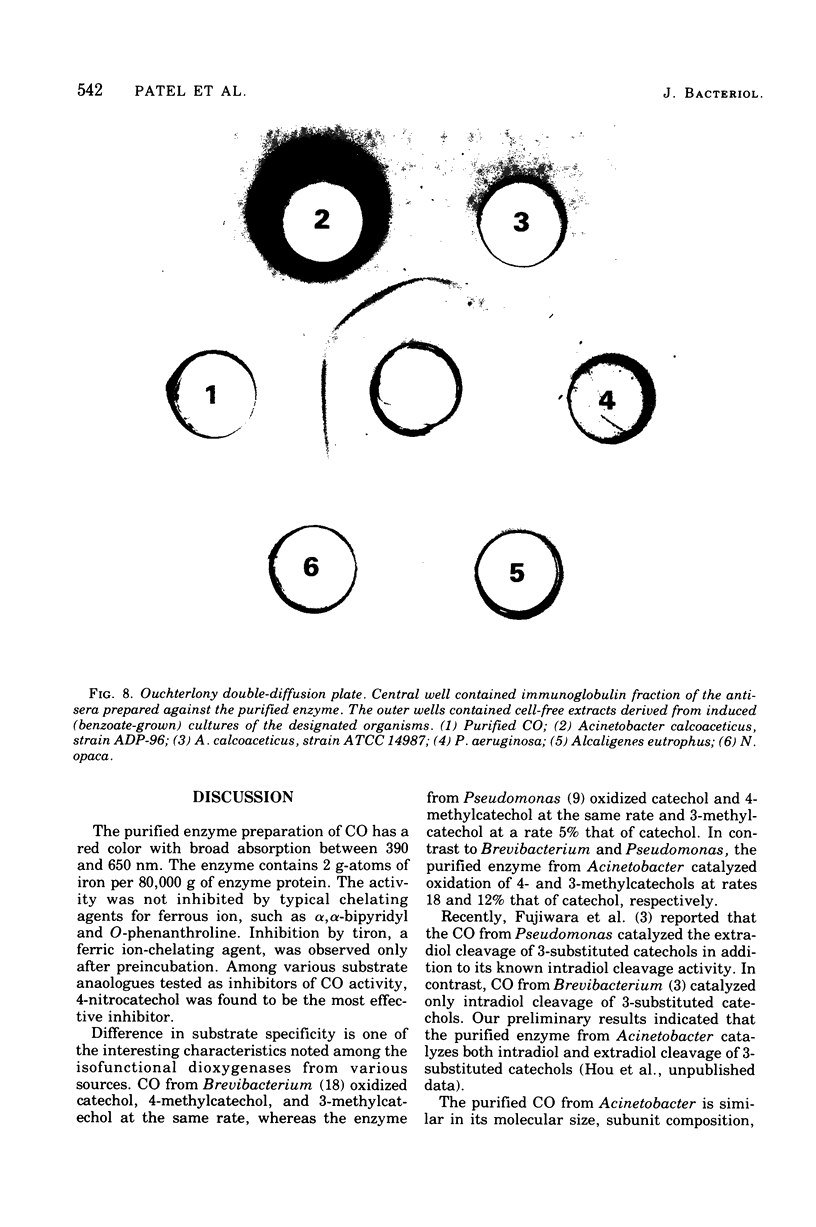
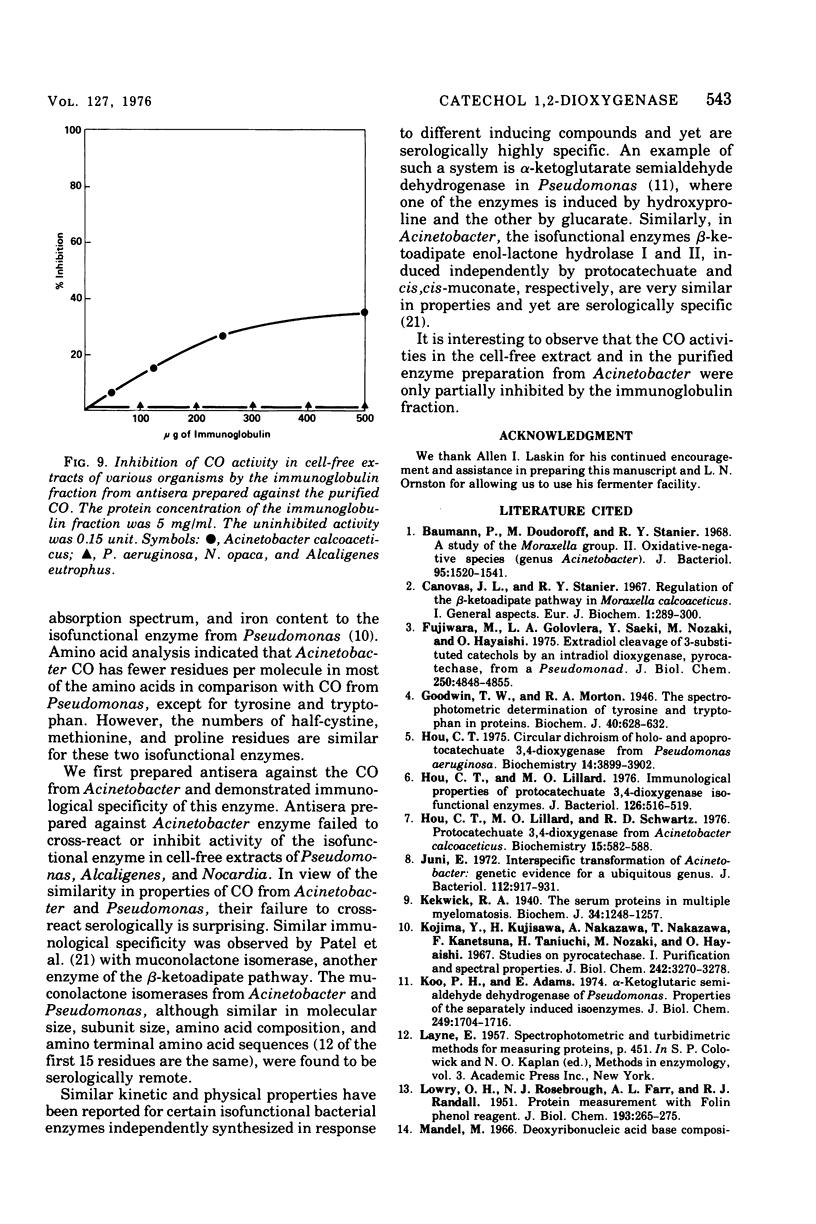
Abstract
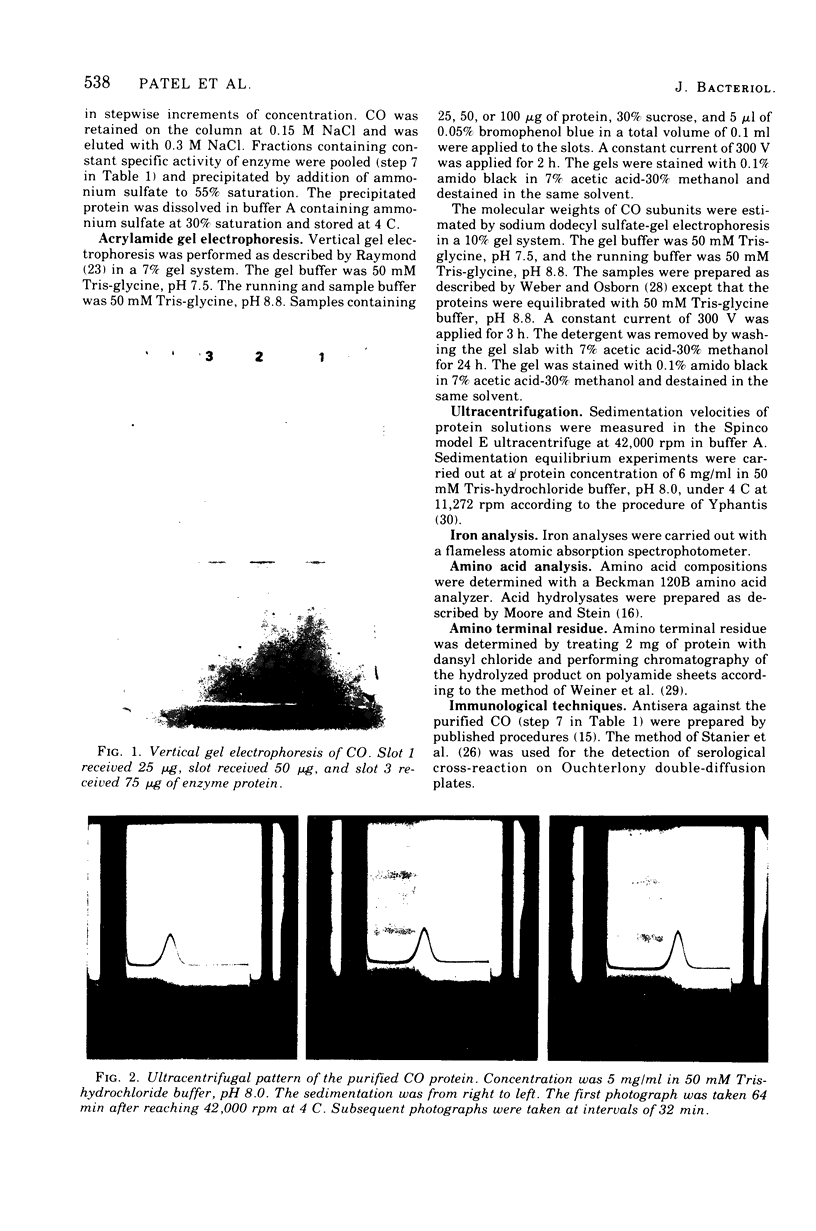
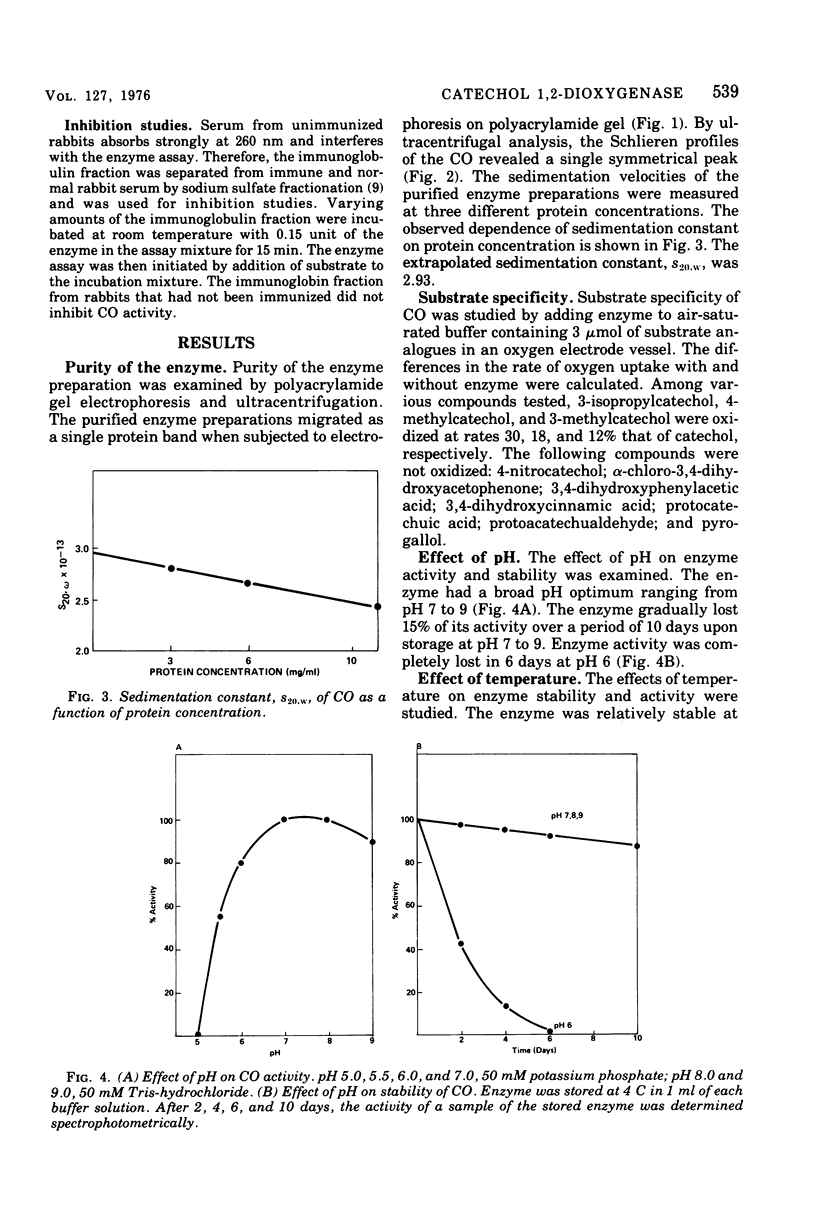
Procedures for the purification of catechol 1,2-dioxygenase from extracts of Acinetobacter calcoaceticus strain ADP-96 are described. The purified enzyme was homogeneous as judged by ultracentrifugation and acrylamide gel electrophoresis. The enzyme contained 2 g-atoms of iron per mol of protein. The enzyme had a broad substrate specificity and catalyzed the oxidation of catechol, 4-methylcatechol, 3-methylcatechol, and 3-isopropyl catechol. The activity of the enzyme was inhibited by heavy metals, sulfhydryl inhibitors, and substrate analogues. The molecular weight of the enzyme was 85,000 as estimated by filtration on Bio-Gel agarose and 81,000 as estimated by sedimentation equilibrium analysis. The subunit size determined by sodium dodecyl sulfate-gel electrophoresis was 40,000. The amino terminal amino acid was methionine. The amino acid composition and spectral properties of 1,2-dioxygenase are also presented. Antisera prepared against the purified enzyme cross-reacted and inhibited enzyme activity in crude extracts from the other strain of A. calcoaceticus, but failed to cross-react and inhibit isofunctional enzyme from organisms of the genera Pseudomonas, Alcaligenes, and Nocardia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Golovleva L. A., Saeki Y., Nozaki M., Hayaishi O. Extradiol cleavage of 3-substituted catechols by an intradiol dioxygenase, pyrocatechase, from a Pseudomonad. J Biol Chem. 1975 Jul 10;250(13):4848–4855. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T. Circular dichroism of holo- and apoprotocatechuate 3,4-dioxygenase from Pseudomonas aeruginosa. Biochemistry. 1975 Aug 26;14(17):3899–3902. doi: 10.1021/bi00688a025. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Lillard M. O. Immunological properties of protocatechuate 3, 4-dioxygenase isofunctional enzymes. J Bacteriol. 1976 Apr;126(1):516–519. doi: 10.1128/jb.126.1.516-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Lillard M. O., Schwartz R. D. Protocatechuate 3, 4-dioxygenase from Acinetobacter calcoaceticus. Biochemistry. 1976 Feb 10;15(3):582–588. doi: 10.1021/bi00648a020. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Fujisawa H., Nakazawa A., Nakazawa T., Kanetsuna F., Taniuchi H., Nozaki M., Hayaishi O. Studies on pyrocatechase. I. Purification and spectral properties. J Biol Chem. 1967 Jul 25;242(14):3270–3278. [PubMed] [Google Scholar]
- Koo P. H., Adams E. Alpha-ketoglutaric semialdehyde dehydrogenase of Pseudomonas. Properties of the separately induced isoenzymes. J Biol Chem. 1974 Mar 25;249(6):1704–1716. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. I. Properties of cis,cis-muconate-lactonizing enzyme and muconolactone isomerase from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3523–3530. doi: 10.1021/bi00742a027. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA H., INOUE H., TAKEDA Y. CHARACTERISTICS OF CATECHOL OXYGENASE FROM BREVIBACTERIUM FUSCUM. J Biochem. 1963 Jul;54:65–74. doi: 10.1093/oxfordjournals.jbchem.a127748. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Kojima Y., Taniuchi H. Purification and properties of pyrocatechase from Pseudomonas fluorescens. Biochim Biophys Acta. 1967 Oct 23;147(2):189–199. doi: 10.1016/0005-2795(67)90398-4. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Patel R. N., Mazumdar S., Ornston L. N. Beta-ketoadipate enol-lactone hydrolases I and II from Acinetobacter calcoaceticus. J Biol Chem. 1975 Aug 25;250(16):6567–6567. [PubMed] [Google Scholar]
- Patel R. N., Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. IV. Muconolactone isomerase from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1974 Dec 10;249(23):7410–7419. [PubMed] [Google Scholar]
- RAYMOND S. A convenient apparatus for vertical gel electrophoresis. Clin Chem. 1962 Sep-Oct;8:455–470. [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N. Quantitative microdetermination of total cysteine plus cystine in proteins. Anal Biochem. 1971 Mar;40(1):200–208. doi: 10.1016/0003-2697(71)90093-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zaborsky O. R., Ogletree J., Hou C. T. Circular dichroism of protocatechuate 3,4-dioxygenase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1975 Mar 28;386(1):18–25. doi: 10.1016/0005-2795(75)90241-x. [DOI] [PubMed] [Google Scholar]