Abstract
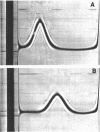
A nitroalkane-oxidizing enzyme was purified about 1,300-fold from a cell extract of Hansenula mrakii grown in a medium containing nitroethane as the sole nitrogen source by ammonium sulfate fractionation, diethylaminoethyl-cellulose column chromatography, hydroxyapatite column chromatography, and Bio-Gel P-150 column chromatography. The enzyme was shown to be homogeneous upon acrylamide gel electrophoresis and ultracentrifugation. The enzyme exhibits absorption maxima at 274, 370, 415, and 440 nm and a shoulder at 470 nm. Balance studies showed that 2 mol of 2-nitropropane is converted into an equimolar amount of acetone and nitrite with the consumption of 1 mol of oxygen. Hydrogen peroxide is not formed in the enzyme reaction. In addition to 2-nitropropane, 1-nitropropane and nitroethane are oxidatively dentrified by the enzyme, but nitromethane is inert to the enzyme. The nitroalkanes are not oxidized under anaerobic conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN H. C., VENNESLAND B. Purification and properties of dihydro-orotic dehydrogenase. J Biol Chem. 1958 Dec;233(6):1398–1406. [PubMed] [Google Scholar]
- Habig W. H., Keen J. H., Jakoby W. B. Glutathione S-transferase in the formation of cyanide from organic thiocyantes and as an organic nitrate reductase. Biochem Biophys Res Commun. 1975 May 19;64(2):501–506. doi: 10.1016/0006-291x(75)90349-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kido T., Yamamoto T., Soda K. Microbial assimilation of alkyl nitro compounds and formation of nitrite. Arch Microbiol. 1975 Dec 31;106(3):165–169. doi: 10.1007/BF00446519. [DOI] [PubMed] [Google Scholar]
- LITTLE H. N. Oxidation of nitroethane by extracts from Neurospora. J Biol Chem. 1951 Nov;193(1):347–358. [PubMed] [Google Scholar]
- LITTLE H. N. The oxidation of 2-nitropropane by extracts of pea plants. J Biol Chem. 1957 Nov;229(1):231–238. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Porter D. J., Voet J. G., Bright H. J. Direct evidence for carbanions and covalent N 5 -flavin-carbanion adducts as catalytic intermediates in the oxidation of nitroethane by D-amino acid oxidase. J Biol Chem. 1973 Jun 25;248(12):4400–4416. [PubMed] [Google Scholar]
- Porter D. J., Voet J. G., Bright H. J. Nitromethane. A novel substrate for D-amino acid oxidase. J Biol Chem. 1972 Mar 25;247(6):1951–1953. [PubMed] [Google Scholar]
- Soda K. Microdetermination of D-amino acids and D-amino acid oxidase activity with 3,methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1968 Oct 24;25(1):228–235. doi: 10.1016/0003-2697(68)90095-x. [DOI] [PubMed] [Google Scholar]