Abstract
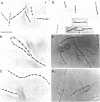
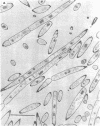
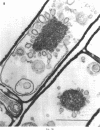
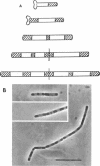
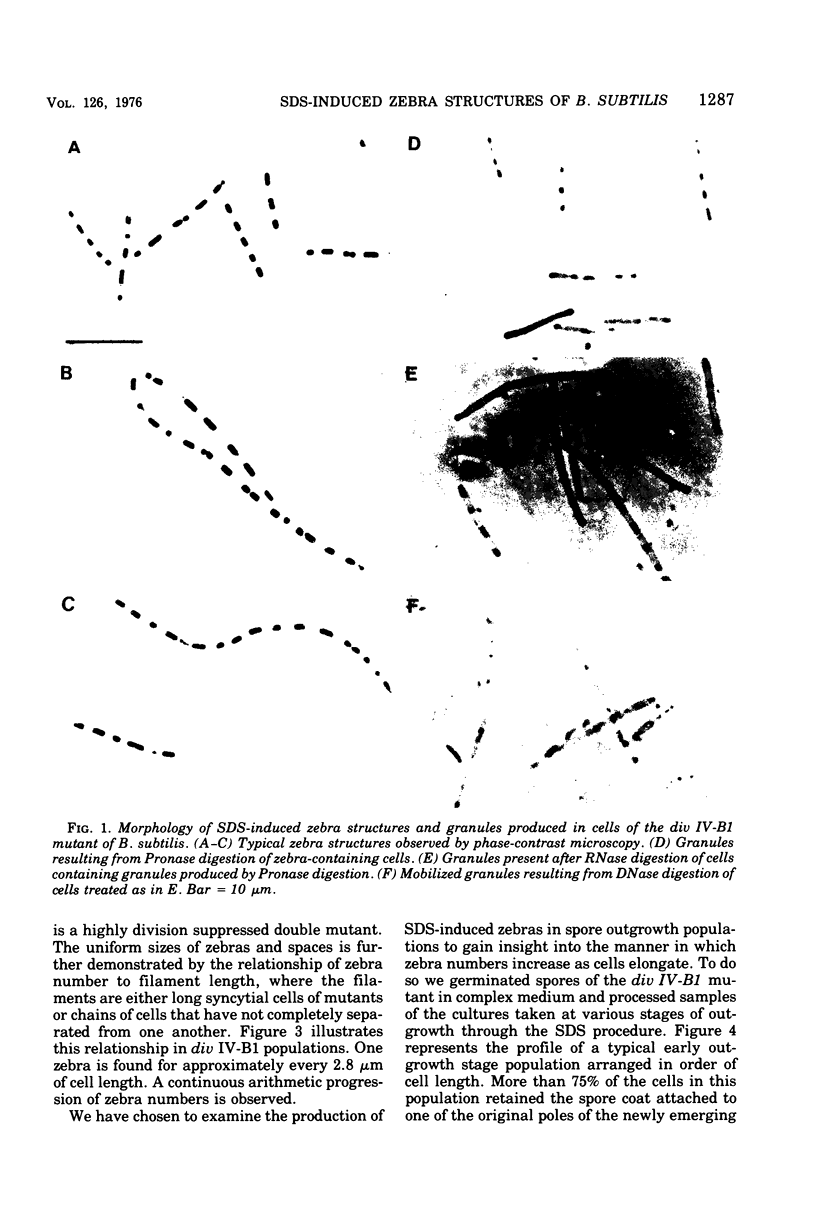
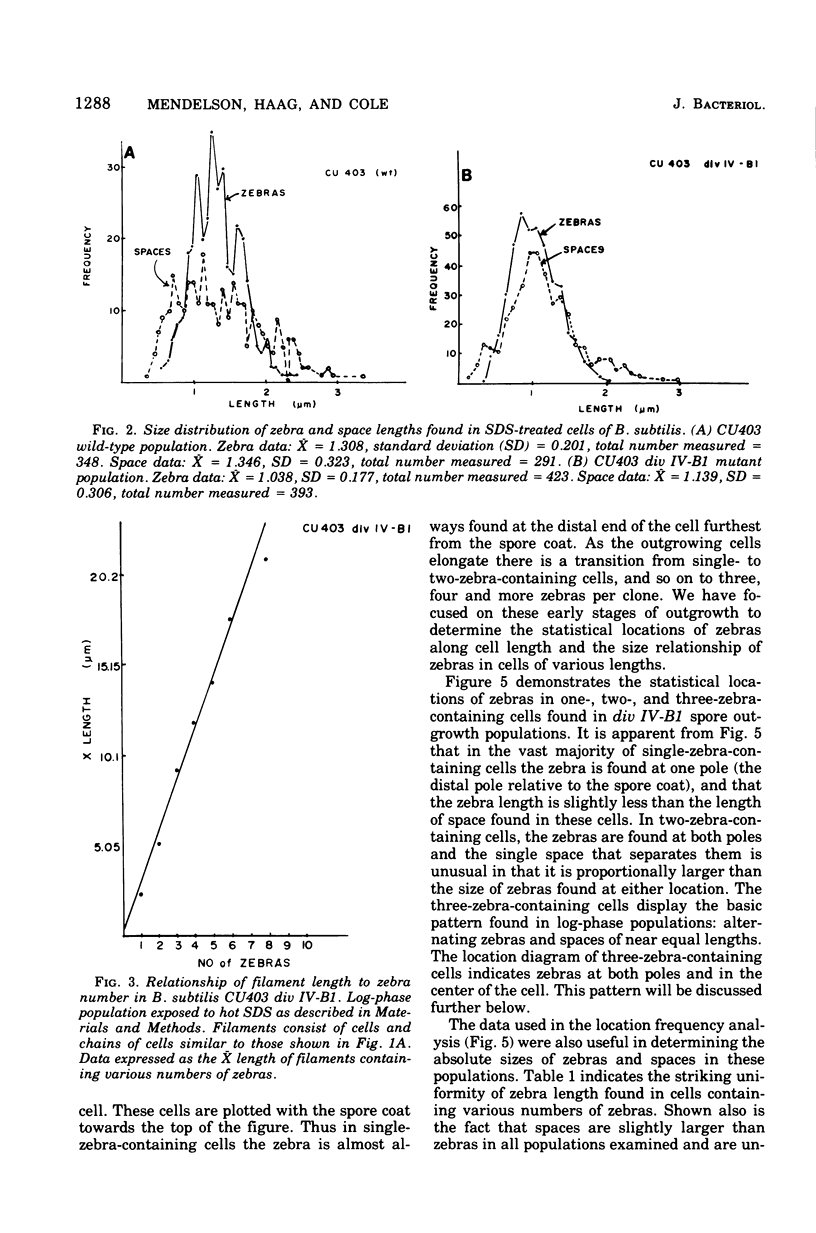
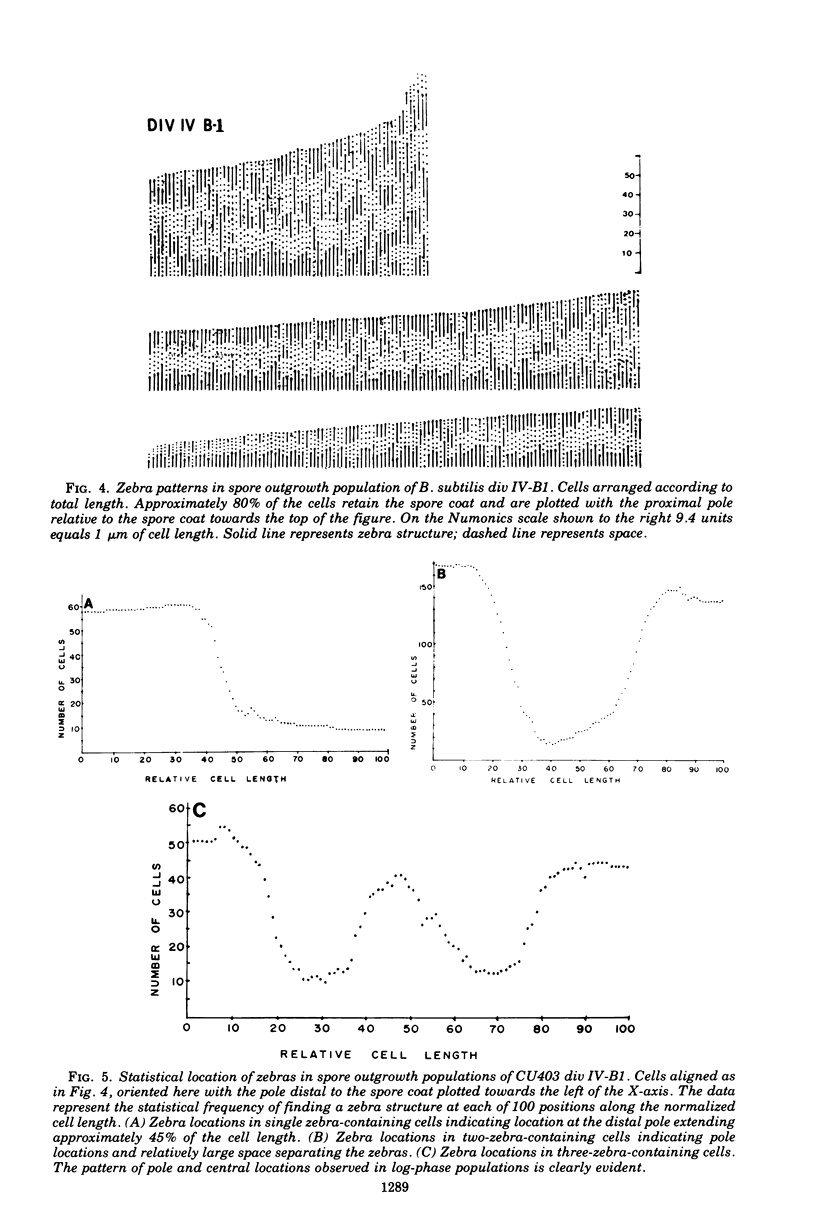
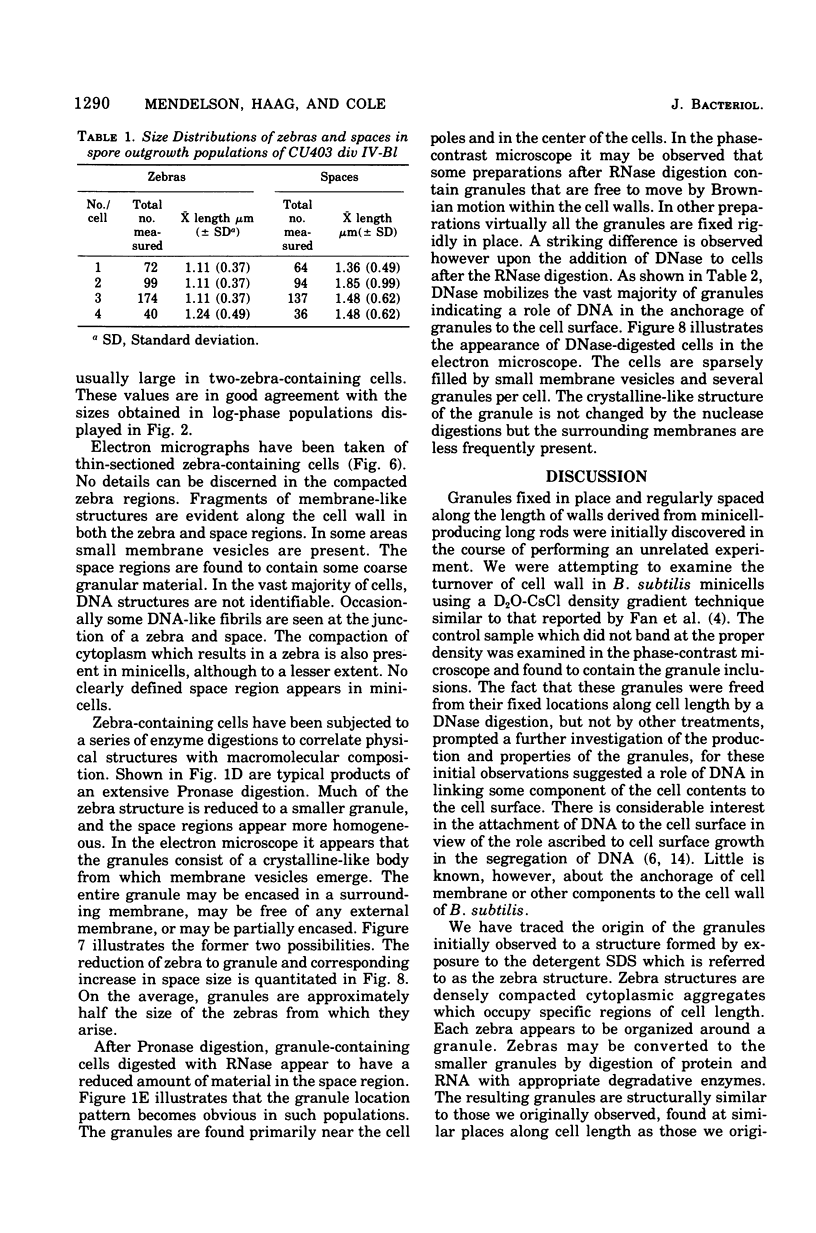
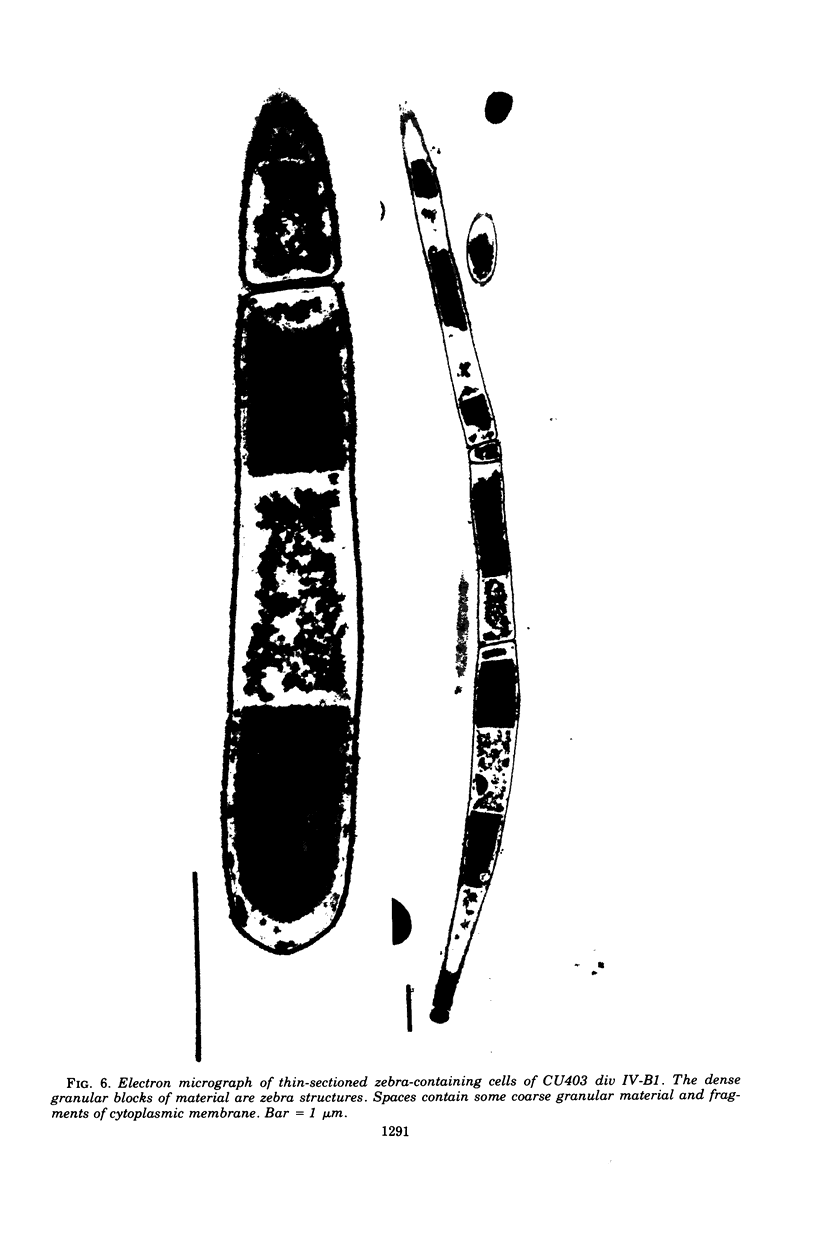
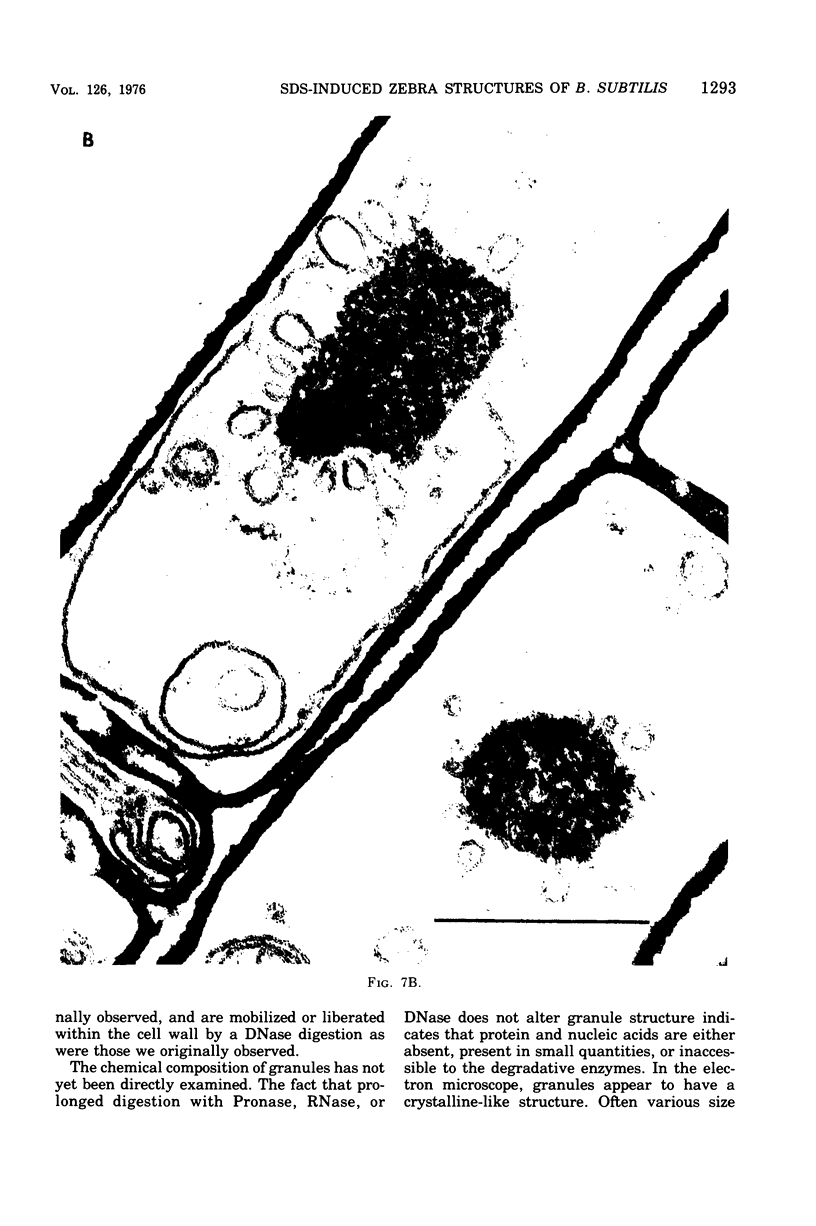
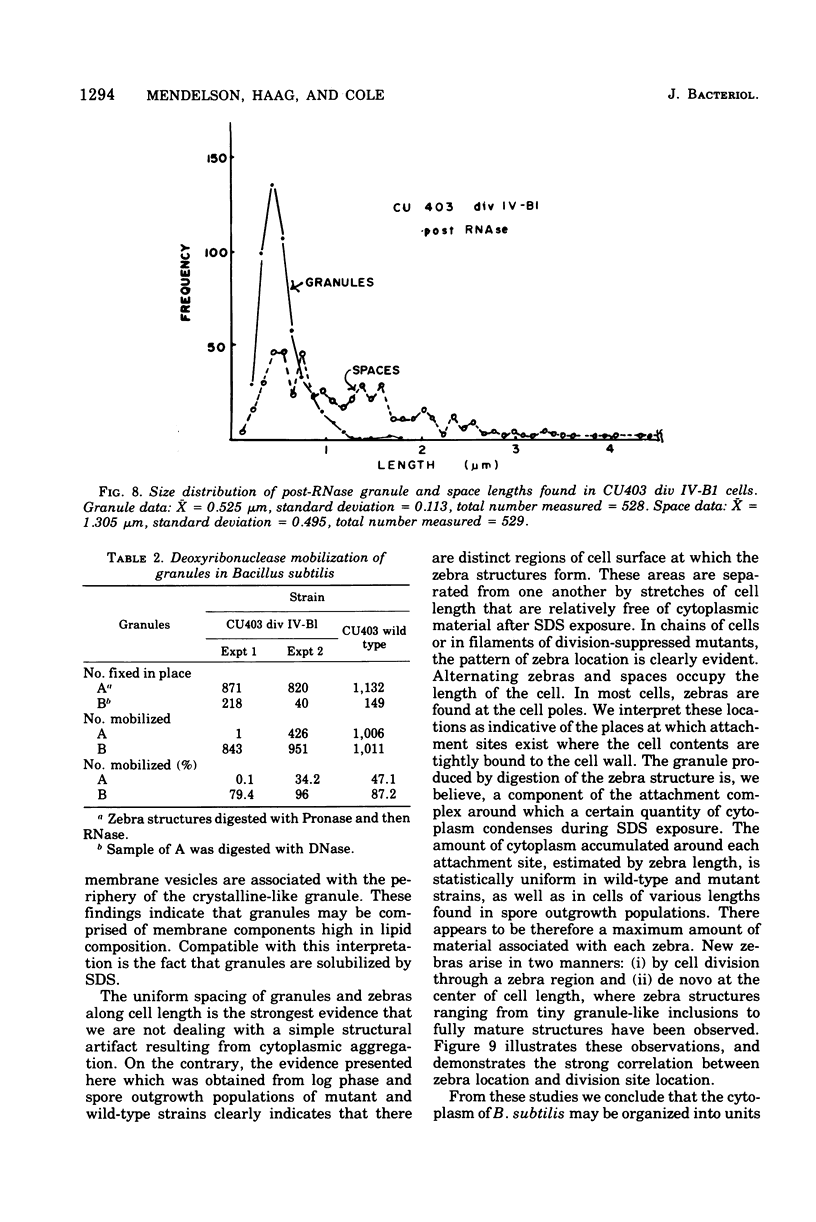
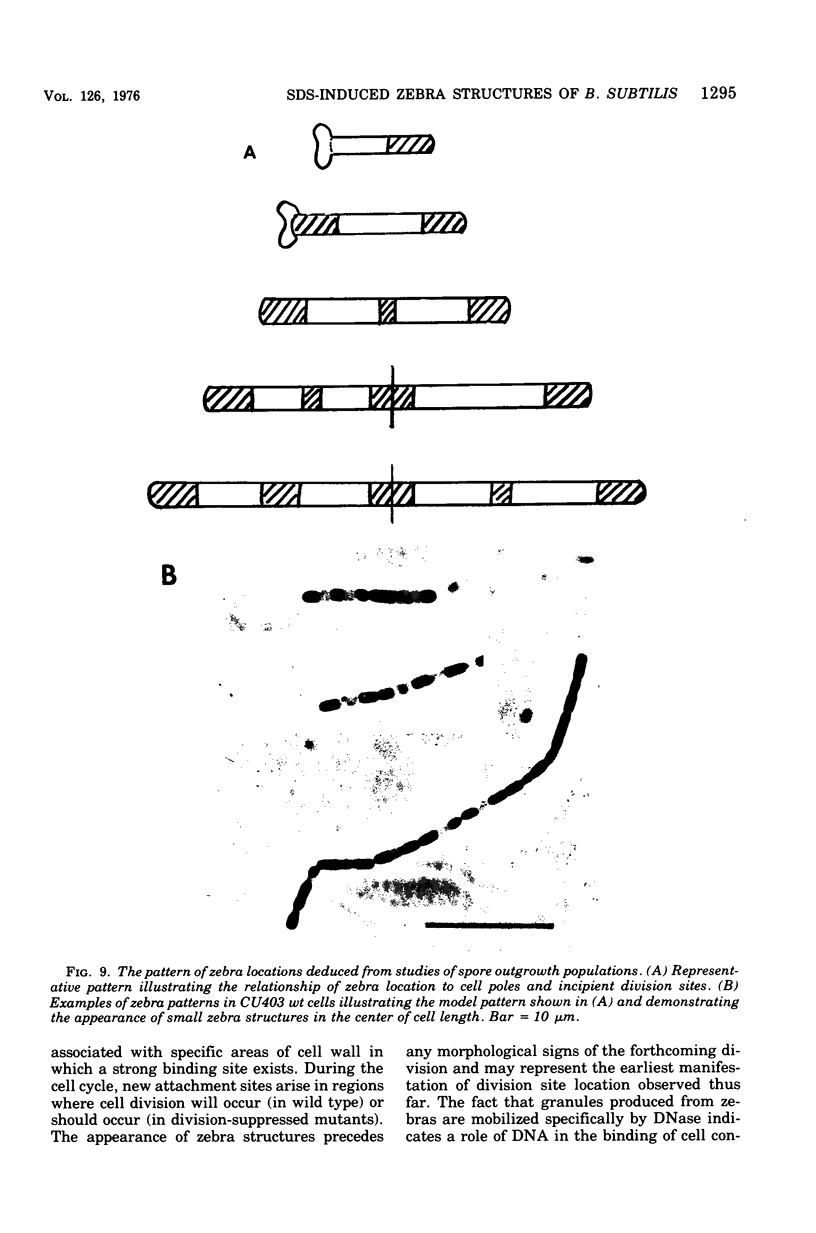
Cells of Bacillus subtilis heated in high concentrations of sodium dodecyl sulfate (5%) and then washed free of detergent with a hot salt solution (80 C) become structurally reorganized into regions of densely compacted cytoplasm (termed zebras) and regions of sparsely filled material (termed spaces). Size distribution studies of zebras indicate that division-suppressed mutants and wild-type cells both yield zebras of comparable length. Similarly the lengths of zebras found in populations emerging from spores are uniform in one-, two-, three-, and four-zebra-containing cells. In contrast, the length of spaces is slightly larger than that of zebras and is unusually large in two-zebra-containing cells. The locations of zebras and spaces along cell length have been studied in spore out-growth populations. A statistical procedure developed previously in genome location investigations was used to analyze the location of zebras along cell length. The data indicate that as cells elongate, new sites arise where the cell contents are strongly bound to the cell surface. Within filament populations produced by division-suppressed mutants there is a linear relationship of mean filament length and zebra number per filament. These data indicate that cytoplasm in filaments with no obvious structural compartmentalizations may be organized into units associated with particular regions of cell surface. The attachment of cell contents to the cell surface may involve deoxyribonucleic acid. Zebra-containing cells digested with proteolytic enzyme and ribonuclease are converted to cells that contain a crystalline-like granule fixed at the location of each zebra. Exposure to deoxyribonuclease mobilizes these granules within the cell wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne S. I., Mendelson N. H. Clonal analysis of cell division in the Bacillus subtilis div IV-B1 minicell-producing mutant. J Bacteriol. 1974 Apr;118(1):15–20. doi: 10.1128/jb.118.1.15-20.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E., Beckman M. M. Cell wall turnover at the hemispherical caps of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1330–1334. doi: 10.1128/jb.117.3.1330-1334.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck J., Martin J. P., Mock M. Action of lysozyme on penicillin-induced filaments of Proteus vulgaris. J Bacteriol. 1974 Nov;120(2):929–933. doi: 10.1128/jb.120.2.929-933.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Can defective segregation prevent initiation? Cold Spring Harb Symp Quant Biol. 1968;33:313–316. doi: 10.1101/sqb.1968.033.01.035. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Cole R. M. Genetic regulation of cell division initiation in Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):994–1003. doi: 10.1128/jb.112.2.994-1003.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Deoxyribonucleic acid distribution in Bacillus subtilis independent of cell elongation. J Bacteriol. 1972 Jul;111(1):156–162. doi: 10.1128/jb.111.1.156-162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Division site regulation in a temperature-sensitive mutant of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):298–300. doi: 10.1128/jb.111.1.298-300.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Nuclear segregation without DNA replication in Bacillus subtilis. Biochim Biophys Acta. 1969 Sep 17;190(1):132–138. doi: 10.1016/0005-2787(69)90162-2. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Reeve J. N. Growth of the Bacillus subtilis cell surface. Nat New Biol. 1973 May 9;243(123):62–64. [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Taylor C., Reeve J. N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974 Dec;85(2):335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Anucleate cell production and surface extension in a temperature-sensitive chromosome initiation mutant of Bacillus subtilis. J Bacteriol. 1975 Sep;123(3):1218–1234. doi: 10.1128/jb.123.3.1218-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., van Iterson W. Effects of treatment with sodium dodecyl sulfate on the ultrastructure of Escherichia coli. J Bacteriol. 1972 Sep;111(3):801–813. doi: 10.1128/jb.111.3.801-813.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]