Abstract
Highly purified preparations of cytoplasmic and outer membrane were isolated from aerobically grown Rhodospirillum rubrum lysed by sequential treatment with lysozyme, ethylenediaminetetraacetate, and Brij 58. The membranes were resolved and separated from other cellular constitutents by a combination of velocity and isopyknic sedimentation in sucrose density gradients. On the basis of their appearance in electron micrographs and their protein profiles in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, these preparations appear to be quite similar to those obtained from other gram-negative bacteria. The cytoplasmic membrane fraction contained the majority of the total membrane-bound succinic dehydrogenase activity and was 10-fold enriched in b- and c-type cytochrome with respect to the outer membrane. The latter fraction was characterized by a much greater carbohydrate content and the presence of arachidic acid, which is typical of R. rubrum lipopolysaccharide. Their protein fatty acid, and overall chemical compositions suggested that these preparations were freer from cross-contamination than those obtained from R. rubrum with currently available methods.
Full text
PDF


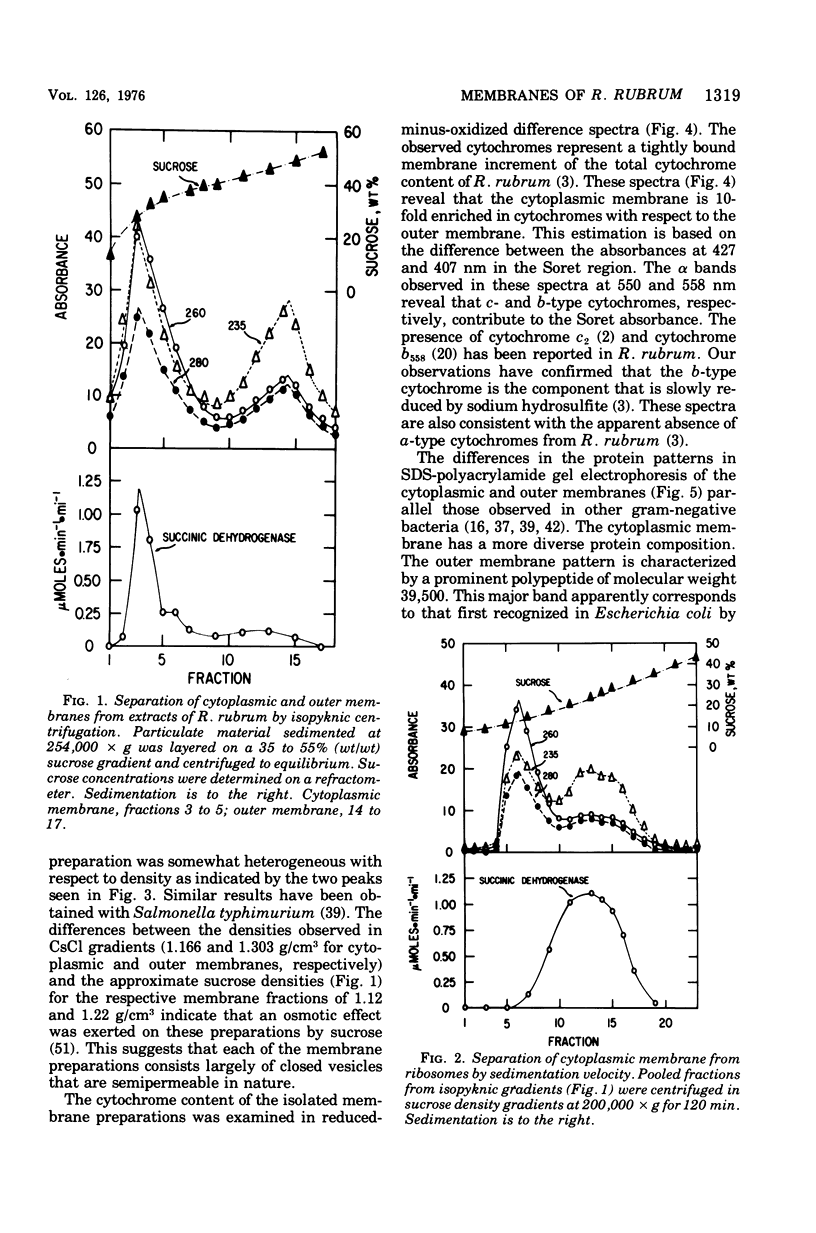
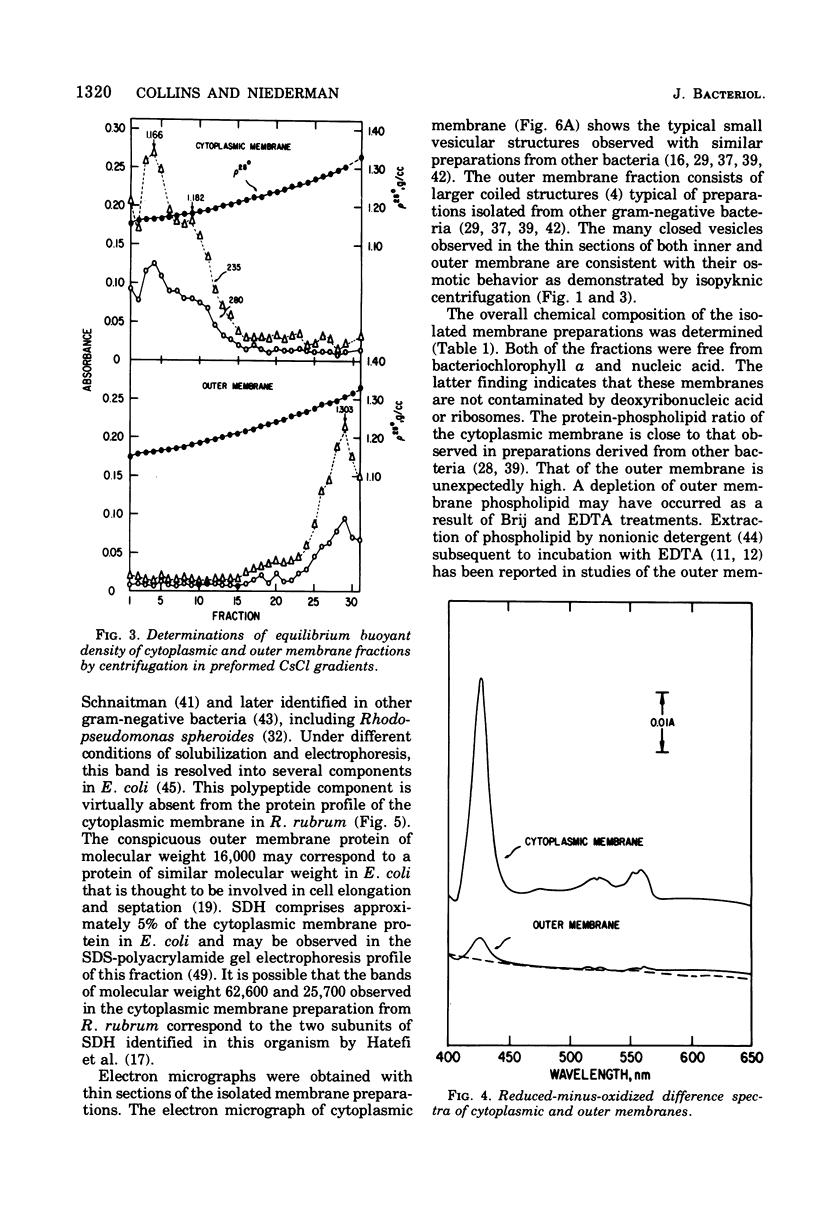





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG R. L., BOEZI J. A. STUDIES OF ESCHERICHIA COLI RIBONUCLEIC ACID-DEOXYRIBONUCLEIC ACID COMPLEX. Biochim Biophys Acta. 1965 May 11;103:60–69. doi: 10.1016/0005-2787(65)90541-1. [DOI] [PubMed] [Google Scholar]
- Bartsch R. G. Bacterial cytochromes. Annu Rev Microbiol. 1968;22:181–200. doi: 10.1146/annurev.mi.22.100168.001145. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., KUNISAWA R. The fine structure of Rhodospirillum rubrum. J Cell Biol. 1963 Feb;16:401–419. doi: 10.1083/jcb.16.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R. SOME OBSERVATIONS ON THE SYNTHESIS AND FUNCTION OF THE PHOTOSYNTHETIC APPARATUS IN RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1543–1553. doi: 10.1073/pnas.46.12.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: physicochemical properties of chromatophore fractions isolated from osmotically and mechanically disrupted cells. J Bacteriol. 1976 Jun;126(3):1326–1338. doi: 10.1128/jb.126.3.1326-1338.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971 Mar;105(3):1184–1199. doi: 10.1128/jb.105.3.1184-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and fractionation of the photosynthetic membranous organelles from Rhodopseudomonas spheroides. J Bacteriol. 1971 Oct;108(1):465–473. doi: 10.1128/jb.108.1.465-473.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Hasin M., Rottem S., Razin S. The outer membrane of Proteus mirabilis. I. Isolation and characterization of the outer and cytoplasmic membrane fractions. Biochim Biophys Acta. 1975 Feb 14;375(3):381–394. doi: 10.1016/0005-2736(75)90354-5. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Davis K. A., Baltscheffsky H., Baltscheffsky M., Johansson B. C. Isolation and properties of succinate dehydrogenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1972 Oct;152(2):613–618. doi: 10.1016/0003-9861(72)90257-3. [DOI] [PubMed] [Google Scholar]
- Irschik H., Oelze J. Membrane differentiation in phototrophically growing Rhodospirillum rubrum during transition from low to high light intensity. Biochim Biophys Acta. 1973 Nov 30;330(1):80–89. doi: 10.1016/0005-2736(73)90286-1. [DOI] [PubMed] [Google Scholar]
- James R. Identification of an outer membrane protein of Escherichia coli, with a role in the coordination of deoxyribonucleic acid replication and cell elongation. J Bacteriol. 1975 Nov;124(2):918–929. doi: 10.1128/jb.124.2.918-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Kamen M. D., Horio T. Bacterial cytochromes. I. Structural aspects. Annu Rev Biochem. 1970;39:673–700. doi: 10.1146/annurev.bi.39.070170.003325. [DOI] [PubMed] [Google Scholar]
- Ketchum P. A., Holt S. C. Isolation and characterization of the membranes from Rhodospirillum rubrum. Biochim Biophys Acta. 1970;196(2):141–161. doi: 10.1016/0005-2736(70)90002-7. [DOI] [PubMed] [Google Scholar]
- Konings W. N. Localization of membrane proteins in membrane vesicles of Bacillus subtilis. Arch Biochem Biophys. 1975 Apr;167(2):570–580. doi: 10.1016/0003-9861(75)90500-7. [DOI] [PubMed] [Google Scholar]
- Kosakowski M. H., Kaplan S. Topology and growth of the intracytoplasmic membrane system of Rhodopseudomonas spheroides: protein, chlorophyll, and phospholipid insertion into steady-state anaerobic cells. J Bacteriol. 1974 Jun;118(3):1144–1157. doi: 10.1128/jb.118.3.1144-1157.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Gibson K. D. The separation of chromatophores from the cell envelope in Rhodopseudomonas spheroides. Prep Biochem. 1971;1(2):141–150. doi: 10.1080/00327487108081935. [DOI] [PubMed] [Google Scholar]
- Niederman R. A. Membranes of Rhodopseudomonas spheroides: interactions of chromatophores with the cell envelope. J Bacteriol. 1974 Jan;117(1):19–28. doi: 10.1128/jb.117.1.19-28.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederman R. A., Segen B. J., Gibson K. D. Membranes of Rhodopseudomonas spheroides. I. Isolation and characterization of membrane fractions from extracts of aerobically and anaerobically grown cells. Arch Biochem Biophys. 1972 Oct;152(2):547–560. doi: 10.1016/0003-9861(72)90250-0. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Oelze J., Biedermann M., Freund-Mölbert E., Drews G. Bacteriochlorophyllgehalt und Proteinmuster der Thylakoide von Rhodospirillum rubrum während der Morphogenese des Photosynthese-Apparates. Arch Mikrobiol. 1969;66(2):154–165. [PubMed] [Google Scholar]
- Oelze J., Golecki J. R., Kleinig H., Weckesser J. Characterization of two cell-envelope fractions from chemotrophically grown Rhodospirillum rubrum. Antonie Van Leeuwenhoek. 1975;41(3):273–286. doi: 10.1007/BF02565063. [DOI] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltmann L. F., Stouthamer A. H. Purification of cytoplasmic membranes and outer membranes from Proteus mirabilis. Arch Mikrobiol. 1973 Nov 19;93(4):311–325. doi: 10.1007/BF00427928. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- SCHACHMAN H. K., PARDEE A. B., STANIER R. Y. Studies on the macro-molecular organization of microbial cells. Arch Biochem Biophys. 1952 Jul;38:245–260. doi: 10.1016/0003-9861(52)90029-5. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. Observations on the relationship between the formation of photopigments and the synthesis of protein in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:599–605. doi: 10.1099/00221287-28-4-599. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of the envelope protein compositions of several gram-negative bacteria. J Bacteriol. 1970 Dec;104(3):1404–1405. doi: 10.1128/jb.104.3.1404-1405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Biedermann M., Drews G. Die Fettsäuren in ganzen Zellen, Thylakoiden und Lipopolysacchariden von Rhodospirillum rubrum und Rhodopseudomonas capsulata. Arch Mikrobiol. 1969;66(3):273–280. [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: identification of succinate dehydrogenase by polyacrylamide gel electrophoresis with sdh amber mutants. J Bacteriol. 1974 Mar;117(3):947–953. doi: 10.1128/jb.117.3.947-953.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Straus J. H., Wallach D. F. A model for the behavior of vesicles in density gradients: implications for fractionation. Biochim Biophys Acta. 1970 Jun 2;203(3):385–393. doi: 10.1016/0005-2736(70)90179-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Kamen M. D. Observations on distribution of NADH oxidase in particles from dark-grown and light-grown Rhodospirillum rubrum. Biochem Biophys Res Commun. 1969 Feb 21;34(4):418–425. doi: 10.1016/0006-291x(69)90398-2. [DOI] [PubMed] [Google Scholar]




